Abstract
Background:
Respiratory viruses play important roles in respiratory tract infections; they are the major cause of diseases such as the common cold, bronchiolitis, pneumonia, etc., in humans that circulate more often in the cold seasons. During the COVID-19 pandemic, many strict public health measures, such as hand hygiene, the use of face masks, social distancing, and quarantines, were implemented worldwide to control the pandemic. Besides controlling the COVID-19 pandemic, these introduced measures might change the spread of other common respiratory viruses. Moreover, with COVID-19 vaccination and reducing public health protocols, the circulation of other respiratory viruses probably increases in the community.Objectives:
This study aims to explore changes in the circulation pattern of common respiratory viruses during the COVID-19 pandemic.Methods:
In the present study, we evaluated the circulation of seven common respiratory viruses (influenza viruses A and B, rhinovirus, and seasonal human coronaviruses (229E, NL63, OC43, and HKU1) and their co-infection with SARS-CoV-2 in suspected cases of COVID-19 in two time periods before and after COVID-19 vaccination. Clinical nasopharyngeal swabs of 400 suspected cases of COVID-19 were tested for SARS-CoV-2 and seven common respiratory viruses by reverse transcription real-time polymerase chain reaction.Results:
Our results showed common respiratory viruses were detected only in 10% and 8% of SARS-CoV-2-positive samples before and after vaccination, respectively, in which there were not any significant differences between them (P-value = 0.14). Moreover, common viral respiratory infections were found only in 12% and 32% of SARS-CoV-2-negative specimens before and after vaccination, respectively, in which there was a significant difference between them (P-value = 0.041).Conclusions:
Our data showed a low rate of co-infection of other respiratory viruses with SARS-CoV-2 at both durations, before and after COVID-19 vaccination. Moreover, the circulation of common respiratory viruses before the COVID-19 vaccination was lower, probably due to non-pharmaceutical interventions (NPI), while virus activity (especially influenza virus A) was significantly increased after COVID-19 vaccination with reducing strict public health measures.Keywords
1. Background
Acute respiratory infections are a serious health problem due to their high mortality and high prevalence in the community. Several bacteria and viruses such as Staphylococcus spp., Enterococcus spp., Klebsiella pneumoniae, Enterobacter spp., influenza viruses A and B, respiratory syncytial virus (RSV), human rhinovirus (HRV), human metapneumovirus (hMPV), and human coronavirus (hCoV) are by far the most common causative pathogens of respiratory tract infection (1-3). Among various microorganisms, respiratory viruses are associated with the highest rate of hospitalization and deaths annually, which often occur during the fall and winter seasons. These viruses invade the upper and lower respiratory tracts and lead to a wide spectrum of infections, from mild to severe or even fatal. Some respiratory viruses, such as rhinovirus, metapneumovirus, and seasonal human coronaviruses (sHCoVs), can cause mild upper respiratory infections such as the common cold, otitis media, and sinusitis, while others, such as influenza viruses, RSV, and SARS and MERS can cause severe lower respiratory infections as bronchiolitis, bronchitis, pneumonia and acute respiratory distress syndrome (ARDS) (1-3).
Recently, the world has faced a new respiratory disease called COVID-19. It has been nearly two years since the onset of the COVID-19 pandemic by a new coronavirus called SARS-CoV-2 that belongs to the Coronaviridae family. SARS-CoV-2 infection can cause a wide range of illnesses, from asymptomatic, mild symptoms such as fever, fatigue, dry cough, pain and bruising, nasal congestion, runny nose, sore throat, or some gastrointestinal manifestations, diarrhea, to serious problems such as pneumonia, and multiple organ dysfunction and even death (4, 5). Since the onset of the pandemic in December 2020, approximately 5.5 million people have died of COVID-19, and more than 350 million infected cases have been reported (6). Owing to the severity and rapid spread of SARS-CoV-2 worldwide, it has become one of the most serious global health threats. Therefore, different approaches have been adopted all over the world to control the disease spread, including travel restrictions (global), partial or complete lockdown, the wearing of masks, frequent hand-washing, more ventilation, less gathering, and efforts to produce different vaccines and antiviral drugs (4, 6). Vaccinating against SARS-CoV-2 started early in December 2020 and has raised hopes for limiting COVID-19 and ending the pandemic.
As mentioned above, COVID-19 leads to various clinical manifestations ranging from asymptomatic to severe. Several risk factors for severe COVID-19 disease have been demonstrated, such as age, sex, and obesity. Moreover, co-infection with other pathogens, such as bacteria and other viruses, may impact disease severity (7-11). Before the COVID-19 pandemic, the prevalence of different respiratory viruses (influenza viruses A and B, respiratory syncytial virus, parainfluenza viruses 1, 2, 3, and 4, rhinovirus, adenovirus, metapneumovirus, and human coronaviruses) usually occurred in different seasons, especially in the fall and winter. The SARS-CoV-2 infection has common characteristics with other respiratory viruses, including respiratory system involvement, clinical features, and similar modes of transmission.
Given the similar transmission and circulation of other respiratory viruses in the winter season, it is not surprising that individuals are co-infected with these viruses and the SARS-CoV-2 virus during the COVID-19 pandemic, which may predispose patients to secondary pneumonia with a higher mortality rate. Indeed co-infection with other respiratory viruses with SARS-CoV-2 could affect the severity of COVID-19 and also the complexity of its diagnosis and treatment (12-15). Therefore, understanding the epidemiology of different respiratory viruses during the COVID-19 pandemic is essential to improve the clinical care and control of COVID-19. Early reports from China indicated that co-infection of COVID-19 with other respiratory pathogens was rare and uncommon (12), while various reports have demonstrated co-infection of SARS-CoV-2 with other respiratory viruses during the COVID-19 pandemic (7, 9, 10, 14). Furthermore, different studies show a decrease in the circulation of other respiratory viruses during the COVID-19 pandemic before COVID-19 vaccination, probably caused by the lockdown and quarantine, using masks and social distancing (16, 17). Recently, several studies indicated the circulation of other respiratory viruses after COVID-19 vaccination and an increase in the circulation of other respiratory viruses, probably due to the reduction of social restrictions after vaccination (16-18).
2. Objectives
In this study, we reported and discussed the circulation of common respiratory viruses and their co-infection with COVID-19 in two periods before and after COVID-19 vaccination. Moreover, we evaluated if the relaxation of restrictions could affect the circulation of common respiratory viruses.
3. Methods
3.1. Study Design and Population
To determine the circulation of common respiratory viruses in the COVID-19 pandemic and their co-infection with SARS-CoV-2, specimens were collected from suspected cases of COVID-19, with symptoms such as fever, runny nose, cough, myalgia, nasal congestion, headache, and sore throat, who were referred to Khatamolanbia Hospital, Shoushtar city, Iran. The specimens were randomly selected in two time periods before vaccination for COVID-19 (between December 1, 2020, and February 1, 2021) and after vaccination for COVID-19 (at the same time between December 1, 2021, and February 1, 2022). In each period, 100 SARS CoV-2 positive samples and 100 SARS CoV-2 negative samples (200 samples in each period) were collected. The exclusion criteria for this study were: (1) patients younger than 18 years of age; (2) patients with malignancies. The variables of each patient, including demographic and clinical features, were recorded.
3.2. Real-Time PCR for COVID-19 Detection
To detect SARS-CoV-2 via RT-PCR, nasopharyngeal samples were obtained using a specific swab. The swabs were immediately placed into sterile tubes containing 2 mL of viral transport media (VTM) and consequently sent to the Khatamolanbia Hospital Lab. Viral RNA was extracted from samples using the ROJE TECHNOLOGIE RNA extraction kit (Pishgam, Iran). The extracted RNAs were amplified using the COVID-19 one-step RT-PCR kit (Pishtaz Teb Zaman Iran), which targets the RdRp and N genes of SARS-CoV-2. PCR amplification was performed via Step One plus a real-time PCR system thermal cycler (Applied Biosystems, USA). The COVID-19 one-step RT-PCR kit has high sensitivity with a minimum detection limit (LOD) of 200 copies/mL. The test sensitivity and specificity of this kit are 100%.
3.3. Real-Time PCR for the Detection of Other Respiratory Viruses
The presence of seven common respiratory viruses, such as influenza virus A/B, rhinovirus, and four species of seasonal coronaviruses-NL63, 229E, OC43, and HKU1, was screened in all specimens using a real-time RT-PCR assay. The expression of these viruses was evaluated using specific probes and primers (listed in Table 1) with One-Step Add-Probe RT-PCR Kit (Addbio, KOREA) through Step One plus real-time PCR system thermal cycler (Applied Biosystems, USA).
Specific Probes & Primers Used for PCR Amplification of Respiratory Viruses
| Virus | Probe | Sense Primer | Antisense Primer | References |
|---|---|---|---|---|
| Influenza A | FAM-CTGGGCACGGTGAGC-BHQ | TCTYATGGAATGGCTAAAGACAAGAC | SCGTCTACGCTGCAGTCCTC | (19) |
| Influenza B | FAM-CTTTGCCTTCTCCATCTT-BHQ | CACAATTGCCTACCTGCTTTCA | CCAACAGTGTAATTTTTCTGCTAGTTCT | (19) |
| Rhinovirus | FAM-CTC CGG CCC CTG AAT GYG GCT AA-BHQ | AGC CYG CGT GGT GCC C | GAA ACA CGG ACA CCC AAA GTA GT | (20) |
| HCoV-OC43 | FAM-TCC GCC TGG CAC GGT ACT CCC T-BHQ | CGA TGA GGC TAT TCC GAC TAG GT | CCT TCC TGA GCC TTC AAT ATA GTA ACC | (21) |
| HCoV-NL63 | FAM-ATAATCCCAACCCATRAG-BHQ | TTTATGGTGGTTGGAATAATATGTTG | GGCAAAGCTCTATCACATTTGG | (19) |
| HCoV-229E | FAM-CCACACTTCAATCAAAAGCTCCCAAATG-BHQ | CGCAAGAATTCAGAACCAGAG | GGCAGTCAGGTTCTTCAACAA | (22) |
| HCoV-HKU1 | FAM-TGTGTGGCGGTTGCTATTATGTTAAGCCTG-BHQ | CCTTGCGAATGAATGTGCT | TTGCATCACCACTGCTAGTACCAC | (21) |
3.4. Statistical Analysis
A chi-square test was performed to compare differences between categorical variables. The differences were regarded as statistically significant when *P < 0.05. Statistical analyses were conducted in the SPSS software 19.0 version.
4. Results
The population under investigation were suspected COVID-19 patients randomly selected in two periods (before and after COVID-19 vaccination). Then, 200 samples were tested for other respiratory viruses in each period (100 patients were positive for SARS-CoV-2, while 100 patients were negative for SARS-CoV-2). Demographic information of patients, including sex, age, inpatient/outpatient status, and the presence or absence of underlying diseases such as kidney disease, diabetes, hypertension, or heart diseases, is given in Table 2.
The Distribution of Respiratory Viruses in SARS-CoV-2 Positive and Negative Patients and Their Demographic Information
| Characteristics | SARS-CoV-2 Positive Before Vaccination | SARS-CoV-2 Negative Before Vaccination | SARS-CoV-2 Positive After Vaccination | SARS-CoV-2 Negative After Vaccination |
|---|---|---|---|---|
| Sex | ||||
| Male | 54 | 49 | 56 | 61 |
| Female | 46 | 51 | 44 | 39 |
| Age (y) | ||||
| < 50 | 64 | 45 | 52 | 60 |
| ≥ 50 | 36 | 55 | 48 | 40 |
| Chronic disease | ||||
| Diabetes | 5 | 3 | 7 | 3 |
| Kidney disease | 2 | 1 | 3 | 2 |
| Hypertension | 5 | 6 | 8 | 5 |
| Heart diseases | 4 | 3 | 3 | 3 |
| Hospital status | ||||
| ICU admission | 20 | 15 | 25 | 32 |
| Hospitalized patients | 40 | 37 | 35 | 30 |
| Outpatients | 40 | 48 | 40 | 38 |
| Other viruses | ||||
| Influenza A | 0 | 1 | 3 | 19 |
| Influenza B | 0 | 0 | 1 | 1 |
| HCoV-NL63 | 3 | 2 | 0 | 4 |
| HCoV-OC43 | 2 | 3 | 1 | 3 |
| HCoV-229E | 1 | 2 | 1 | 2 |
| HCoV- HKU1 | 0 | 1 | 0 | 0 |
| Rhinovirus | 4 | 3 | 2 | 3 |
| 10% | 12% | 8% | 32% |
4.1. Circulation of Common Respiratory Viruses Among SARS-CoV-2-Positive and Negative Patients Before COVID-19 Vaccination
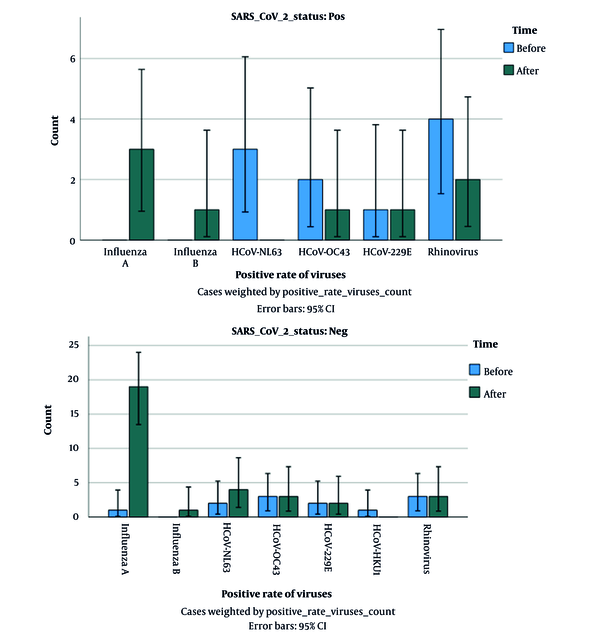
Before COVID-19 vaccination, out of 100 SARS-CoV-2 positive patients, concurrent infection was found only in 10 patients (10%). The frequency of viruses among SARS-CoV-2 positive patients was as follows: Influenza A, n = 0 (0.00%), influenza B, n = 0 (0.00%), rhinovirus, n = 4 (4%), coronavirus OC43, n = 2 (2%), coronavirus NL63, n = 3 (3%), coronavirus 229E, n = 1 (1%), coronavirus HKU1, n = 0 (0%). Also, out of 100 suspected COVID-19 patients who were negative for SARS-CoV-2 before COVID-19 vaccination, other respiratory viruses were detected in 12% of patients. The frequency of viruses among patients who were negative for COVID-19 was as follows: Influenza A, n = 1 (1%), influenza B, n = 0 (0.00%), rhinovirus, n = 3 (3%), coronavirus OC43, n = 3 (3%), coronavirus NL63, n = 2 (2%), coronavirus 229E, n = 2 (2%), coronavirus HKU1, n = 1 (1%).
4.2. Circulation of Common Respiratory Viruses Among SARS-Cov-2-Positive and Negative Patients After COVID-19 Vaccination
Moreover, after COVID-19 vaccination, out of 100 SARS-CoV-2 positive patients only 8 patients (8%) had co-infection: 3 with influenza A (3%), 1 with influenza B (1%), 2 with rhinovirus (2%), 1 with coronavirus OC43 (1%), 0 with coronavirus NL63 (0%), 1 with coronavirus 229E (1%), 0 with coronavirus HKU1 (0%). Also, out of 100 SARS-CoV-2 negative patients after vaccination, other respiratory infections were found only in 32 cases (32%). The prevalence of viruses in SARS-CoV-2 negative patients after COVID-19 vaccination was as follows: Influenza A, n = 19 (19%), influenza B, n = 1 (1%), rhinovirus, n = 3 (3%), coronavirus OC43, n = 3 (3%), coronavirus NL63, n = 4 (4%), coronavirus 229E, n = 2 (2%), coronavirus HKU1, n = 0 (0%). According to the results of Fisher's exact test, the rate of positivity of other respiratory viruses in SARS-CoV-2-positive patients (co-infection) was not statistically significant during the two periods (before and after vaccination) (P-value = 0.14), while this rate in SARS-CoV-2- negative patients, was statistically significant during the two periods (before and after vaccination) (P-value = 0.041, Figure 1).
Comparison of positivity rates of respiratory viruses in suspected cases of COVID-19 before and after COVID-19 vaccination
4.3. Comparison of Demographic and Clinical Characteristics
Demographic characteristics showed no statistically significant differences in gender, age, chronic disease, and inpatient/outpatient status of patients among positive and negative subjects during the two periods (data not shown).
5. Discussion
Every year, as winter approaches, we face a rise in respiratory infections, including viral respiratory pathogens. Viral respiratory infections are one of the most common and important factors leading to hospitalization and death, especially in children and the elderly, and those with immune system deficiencies. Until before December 2019, that SARS-CoV-2 became the main circulating severe acute respiratory syndrome in the world, several respiratory viruses were circulated in human populations every year with a distinct seasonal pattern. For example, some respiratory viruses, such as human influenza viruses, respiratory syncytial virus (RSV), and human coronaviruses, circulated during the winter season, and other human respiratory viruses, such as parainfluenza viruses, human metapneumoviruses, and rhinoviruses circulated in the whole year (1-3). Since the beginning of the COVID-19 pandemic, one of the most important early questions has been whether co-infection of other respiratory viruses with SARS-CoV-2 would occur and whether SARS-CoV-2 could affect the circulation of other respiratory viruses. Various studies have reported the co-infection of SARS-CoV-2 with other respiratory viruses (7-9, 14, 23).
Although the co-infection rate reported by different studies varies, the majority of studies have shown a low co-infection rate. Additionally, researchers revealed that widespread protective strategies which have been exploited to control COVID-19 could also reduce the circulation rate of other respiratory viruses during the COVID-19 pandemic; therefore, it can be inferred that with COVID-19 vaccination and reducing public health protocols, the circulation of other respiratory viruses can increase and recent studies have highlighted this issue. There are limited data about the prevalence of other respiratory viruses during the COVID-19 pandemic in Iran. This study described the circulation of common respiratory viruses and their co-infection with SARS-CoV-2 in two consecutive years during the pandemic (before and after COVID-19 vaccination) in Shoushtar, a county in Khuzestan province in Iran. Our results showed that co-infection of common respiratory viruses and SARS-CoV-2 (in SARS-CoV-2-positive patients) was observed only in 10% and 8% of cases before and after COVID-19 vaccination, respectively.
In line with our results, several studies reported a low rate of respiratory viral co-infections in COVID-19 patients. For example, Garcia-Vidal et al., Nowak et al., and Castillo et al. demonstrated low rates (0.6%), (2.99%), and (1.9%) of co-infection of respiratory viruses in COVID-19 patients, respectively. However, Kim et al., Zhu et al., Veisi et al., and Hashemi et al., reported relatively high rates of respiratory viral co-infection in COVID-19 patients (8, 9, 15, 24-27). Several reasons, such as viral interference in the established infection and competition among co-infecting viruses, as well as social distancing and wearing a mask in public and decreased circulation of other respiratory viruses, might lead to low rates of viral respiratory co-infections. On the other hand, the high rate of co-infection in some studies may be due to different laboratory methods, different types of viruses being evaluated, and the different climate conditions under which the investigations have been carried out. Additionally, to evaluate the prevalence of common respiratory viruses during the COVID-19 pandemic, the above-mentioned viruses were tested in SARS-CoV-2-negative patients in two periods (before and after COVID-19 vaccination).
Common respiratory viruses were found in 12% and 32% of SARS-CoV-2-negative patients before and after COVID-19 vaccination, respectively. In agreement with our report, several studies showed preventive strategies for controlling the COVID-19 pandemic significantly affected the prevalence pattern of other common respiratory viruses. For example, Ye and Wang showed there was a marked reduction in the positive rate of four respiratory viruses (including influenza A, influenza B, adenovirus, and respiratory syncytial virus) in 2020 (during the pandemic) compared with those in 2019 (pre-pandemic) (28). Also, De Francesco et al. reported that the test positivity for respiratory viruses influenza A and B, metapneumovirus, parainfluenza virus, respiratory syncytial virus, and human coronaviruses reduced significantly during the pandemic (16). It seems multiple factors such as higher R0 of SARS-CoV-2 (compared to other respiratory viruses) and stringent NPIs (e.g., hand hygiene, the use of face masks, social distancing, and quarantines) significantly reduced the frequency of influenza and other respiratory viruses for several consecutive months during COVID-19 pandemic (before vaccination) while, SARS-CoV-2 remained the predominant respiratory virus. On the other hand, our result showed a significant increase in the circulation of common respiratory viruses in SARS-CoV-2-negative patients, which seems to be the result of the relaxation of NPIs after COVID-19 vaccination.
There is little data about the period following the relaxation of NPIs and its impact on the pattern of common respiratory viruses. A noteworthy point in our study was the higher percentage of respiratory viruses, especially influenza A, in individuals with negative COVID-19 tests after COVID-19 vaccination. In agreement with our result, several recent studies have shown that once the COVID-19 outbreak became relatively under control, influenza activity increased again. For instance, an increase in influenza cases was reported in Australia in June 2022 after the Omicron wave. Also, several reports demonstrated an increase in influenza activity in South America in July 2022 (29). Another study in Finland reported with reducing the restrictions in September 2021, RSV and influenza returned in Finland (30). As mentioned before, the circulation of influenza viruses (predominantly influenza A virus subtypes H1N1 and H3N2) occurs every year from late fall to early spring. Influenza viruses lead to 3 to 5 million cases of severe illness, with around 250,000 - 500,000 deaths globally each. Annual vaccination is the most effective way to prevent influenza and reduce the mortality rate in high-risk groups (1, 3). Unfortunately, during the COVID-19 pandemic, vaccine programs for other diseases, including influenza, were neglected (31), which may lead to increased population susceptibility.
It appears that after the beginning of COVID-19 vaccination and reduced public health protocols, as well as the reduced circulation of other respiratory viruses for several consecutive months and the absence of natural exposure to these viruses, especially influenza (consequently, decrease in herd immunity and increased population susceptibility) the prevalence of circulating respiratory viruses, particularly influenza viruses, in the community significantly increased. Therefore, understanding how the circulation of common respiratory viruses changed following the relaxation of NPIs is of great importance for public health in the post‐COVID‐19 era. The limitations of this study are its small sample size and also miss detection of other respiratory viruses such as RSV, HMPV, HBoV, and HAdVs due to lack of budget.
5.1. Conclusions
This study indicated that the strict public health measures during the COVID-19 pandemic have a great effect on the circulating of other common respiratory viruses; otherwise, with the ending of social restrictions, it seems we have to wait for a possible resurgence of some respiratory viruses.
References
-
1.
Boncristiani HF, Criado MF, Arruda E. Respiratory viruses. Encyclopedia of microbiology. 2009. p. 500-18. https://doi.org/10.1016/b978-012373944-5.00314-x.
-
2.
Goka EA, Vallely PJ, Mutton KJ, Klapper PE. Single, dual and multiple respiratory virus infections and risk of hospitalization and mortality. Epidemiol Infect. 2015;143(1):37-47. [PubMed ID: 24568719]. [PubMed Central ID: PMC9206808]. https://doi.org/10.1017/S0950268814000302.
-
3.
Neumann G, Kawaoka Y. Seasonality of influenza and other respiratory viruses. EMBO Mol Med. 2022;14(4). e15352. [PubMed ID: 35157360]. [PubMed Central ID: PMC8988196]. https://doi.org/10.15252/emmm.202115352.
-
4.
Ciotti M, Angeletti S, Minieri M, Giovannetti M, Benvenuto D, Pascarella S, et al. COVID-19 outbreak: An overview. Chemotherapy. 2019;64(5-6):215-23. [PubMed ID: 32259829]. [PubMed Central ID: PMC7179549]. https://doi.org/10.1159/000507423.
-
5.
Faezeh S, Noorimotlagh Z, Mirzaee SA, Kalantar M, Barati B, Fard ME, et al. The SARS-CoV-2 (COVID-19) pandemic in hospital: An insight into environmental surfaces contamination, disinfectants' efficiency, and estimation of plastic waste production. Environ Res. 2021;202:111809. [PubMed ID: 34333010]. [PubMed Central ID: PMC8320441]. https://doi.org/10.1016/j.envres.2021.111809.
-
6.
World Health Organization. Coronavirus disease (COVID-19). Geneva, Switzerland: World Health Organization; 2020. Available from: https://apps.who.int/iris/bitstream/handle/10665/336034/nCoV-weekly-sitrep11Oct20-eng.pdf?sequence=1&isAllowed=y.
-
7.
Calcagno A, Ghisetti V, Burdino E, Trunfio M, Allice T, Boglione L, et al. Co-infection with other respiratory pathogens in COVID-19 patients. Clin Microbiol Infect. 2021;27(2):297-8. [PubMed ID: 32822882]. [PubMed Central ID: PMC7434691]. https://doi.org/10.1016/j.cmi.2020.08.012.
-
8.
Castillo EM, Coyne CJ, Brennan JJ, Tomaszewski CA. Rates of coinfection with other respiratory pathogens in patients positive for coronavirus disease 2019 (COVID-19). J Am Coll Emerg Physicians Open. 2020;1(4):592-6. [PubMed ID: 32838387]. [PubMed Central ID: PMC7361860]. https://doi.org/10.1002/emp2.12172.
-
9.
Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085-6. [PubMed ID: 32293646]. [PubMed Central ID: PMC7160748]. https://doi.org/10.1001/jama.2020.6266.
-
10.
Lin D, Liu L, Zhang M, Hu Y, Yang Q, Guo J, et al. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci China Life Sci. 2020;63(4):606-9. [PubMed ID: 32170625]. [PubMed Central ID: PMC7089461]. https://doi.org/10.1007/s11427-020-1668-5.
-
11.
Wu X, Cai Y, Huang X, Yu X, Zhao L, Wang F, et al. Co-infection with SARS-CoV-2 and influenza A virus in patient with Pneumonia, China. Emerg Infect Dis. 2020;26(6):1324-6. [PubMed ID: 32160148]. [PubMed Central ID: PMC7258479]. https://doi.org/10.3201/eid2606.200299.
-
12.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395(10223):507-13. [PubMed ID: 32007143]. [PubMed Central ID: PMC7135076]. https://doi.org/10.1016/S0140-6736(20)30211-7.
-
13.
Guo L, Wei D, Zhang X, Wu Y, Li Q, Zhou M, et al. Clinical Features predicting mortality risk in patients with viral Pneumonia: The MuLBSTA score. Front Microbiol. 2019;10:2752. [PubMed ID: 31849894]. [PubMed Central ID: PMC6901688]. https://doi.org/10.3389/fmicb.2019.02752.
-
14.
Massey BW, Jayathilake K, Meltzer HY. Respiratory microbial co-infection with SARS-CoV-2. Front Microbiol. 2020;11:2079. [PubMed ID: 32983056]. [PubMed Central ID: PMC7477285]. https://doi.org/10.3389/fmicb.2020.02079.
-
15.
Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B, et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285:198005. [PubMed ID: 32408156]. [PubMed Central ID: PMC7213959]. https://doi.org/10.1016/j.virusres.2020.198005.
-
16.
De Francesco MA, Pollara C, Gargiulo F, Giacomelli M, Caruso A. Circulation of respiratory viruses in hospitalized adults before and during the COVID-19 pandemic in Brescia, Italy: A retrospective study. Int J Environ Res Public Health. 2021;18(18). [PubMed ID: 34574450]. [PubMed Central ID: PMC8469422]. https://doi.org/10.3390/ijerph18189525.
-
17.
Wu D, Lu J, Liu Y, Zhang Z, Luo L. Positive effects of COVID-19 control measures on influenza prevention. Int J Infect Dis. 2020;95:345-6. [PubMed ID: 32283283]. [PubMed Central ID: PMC7151395]. https://doi.org/10.1016/j.ijid.2020.04.009.
-
18.
Liu P, Xu M, Cao L, Su L, Lu L, Dong N, et al. Impact of COVID-19 pandemic on the prevalence of respiratory viruses in children with lower respiratory tract infections in China. Virol J. 2021;18(1):159. [PubMed ID: 34344406]. [PubMed Central ID: PMC8329611]. https://doi.org/10.1186/s12985-021-01627-8.
-
19.
Neisi N, Abbasi S, Makvandi M, Salmanzadeh S, Biparva S, Nahidsamiei R, et al. Detection of common respiratory viruses in patients with acute respiratory infections using multiplex real-time RT-PCR. Jundishapur J Microbiol. 2019;12(11). e96513. https://doi.org/10.5812/jjm.96513.
-
20.
Brahim Belhaouari D, Wurtz N, Grimaldier C, Lacoste A, Pires de Souza GA, Penant G, et al. Microscopic observation of SARS-like particles in RT-qPCR SARS-CoV-2 positive sewage samples. Pathogens. 2021;10(5). [PubMed ID: 33923138]. [PubMed Central ID: PMC8146039]. https://doi.org/10.3390/pathogens10050516.
-
21.
Dare RK, Fry AM, Chittaganpitch M, Sawanpanyalert P, Olsen SJ, Erdman DD. Human coronavirus infections in rural Thailand: A comprehensive study using real-time reverse-transcription polymerase chain reaction assays. J Infect Dis. 2007;196(9):1321-8. [PubMed ID: 17922396]. [PubMed Central ID: PMC7109921]. https://doi.org/10.1086/521308.
-
22.
Jeong K, Chang J, Park SM, Kim J, Jeon S, Kim DH, et al. Rapid discovery and classification of inhibitors of coronavirus infection by pseudovirus screen and amplified luminescence proximity homogeneous assay. Antiviral Res. 2023;209:105473. [PubMed ID: 36435212]. [PubMed Central ID: PMC9682871]. https://doi.org/10.1016/j.antiviral.2022.105473.
-
23.
Marshall NC, Kariyawasam RM, Zelyas N, Kanji JN, Diggle MA. Broad respiratory testing to identify SARS-CoV-2 viral co-circulation and inform diagnostic stewardship in the COVID-19 pandemic. Virol J. 2021;18(1):93. [PubMed ID: 33933115]. [PubMed Central ID: PMC8087885]. https://doi.org/10.1186/s12985-021-01545-9.
-
24.
Garcia-Vidal C, Sanjuan G, Moreno-Garcia E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83-8. [PubMed ID: 32745596]. [PubMed Central ID: PMC7836762]. https://doi.org/10.1016/j.cmi.2020.07.041.
-
25.
Hashemi SA, Safamanesh S, Ghasemzadeh-Moghaddam H, Ghafouri M, Azimian A. High prevalence of SARS-CoV-2 and influenza A virus (H1N1) coinfection in dead patients in Northeastern Iran. J Med Virol. 2021;93(2):1008-12. [PubMed ID: 32720703]. https://doi.org/10.1002/jmv.26364.
-
26.
Nowak MD, Sordillo EM, Gitman MR, Paniz Mondolfi AE. Coinfection in SARS-CoV-2 infected patients: Where are influenza virus and rhinovirus/enterovirus? J Med Virol. 2020;92(10):1699-700. [PubMed ID: 32352574]. [PubMed Central ID: PMC7267652]. https://doi.org/10.1002/jmv.25953.
-
27.
Veisi P, Shatizadeh Malekshahi S, Choobin H, Jabbari MR, Mohammadi Torbati P. Simultaneous detection of multiple respiratory viruses among SARS-CoV-2-positive and negative patients by multiplex TaqMan one-step real-time PCR. Jundishapur J Microbiol. 2022;15(1). e122090. https://doi.org/10.5812/jjm.122090.
-
28.
Ye Q, Wang D. Epidemiological changes of common respiratory viruses in children during the COVID-19 pandemic. J Med Virol. 2022;94(5):1990-7. [PubMed ID: 34981839]. [PubMed Central ID: PMC9015628]. https://doi.org/10.1002/jmv.27570.
-
29.
Lee SS, Viboud C, Petersen E. Understanding the rebound of influenza in the post COVID-19 pandemic period holds important clues for epidemiology and control. Int J Infect Dis. 2022;122:1002-4. [PubMed ID: 35932966]. [PubMed Central ID: PMC9349026]. https://doi.org/10.1016/j.ijid.2022.08.002.
-
30.
Kuitunen I, Artama M, Haapanen M, Renko M. Respiratory virus circulation in children after relaxation of COVID-19 restrictions in fall 2021-A nationwide register study in Finland. J Med Virol. 2022;94(9):4528-32. [PubMed ID: 35577532]. [PubMed Central ID: PMC9347728]. https://doi.org/10.1002/jmv.27857.
-
31.
Leuchter RK, Jackson NJ, Mafi JN, Sarkisian CA. Association between Covid-19 Vaccination and Influenza Vaccination Rates. N Engl J Med. 2022;386(26):2531-2. [PubMed ID: 35704429]. [PubMed Central ID: PMC9258773]. https://doi.org/10.1056/NEJMc2204560.