Published online Oct 6, 2020. doi: 10.12998/wjcc.v8.i19.4280
Peer-review started: May 26, 2020
First decision: June 15, 2020
Revised: June 29, 2020
Accepted: September 8, 2020
Article in press: September 8, 2020
Published online: October 6, 2020
Currently clinicians all around the world are experiencing a pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The clinical presentation of this pathology includes fever, dry cough, fatigue and acute respiratory distress syndrome that can lead to death infected patients. Current studies on coronavirus disease 2019 (COVID-19) continue to highlight the urgent need for an effective therapy. Numerous therapeutic strategies have been used until now but, to date, there is no specific effective treatment for SARS-CoV-2 infection. Elevated inflammatory cytokines have been reported in patients with COVID-19. Evidence suggests that elevated cytokine levels, reflecting a hyperinflammatory response secondary to SARS-CoV-2 infection, are responsible for multi-organ damage in patients with COVID-19. For these reason, numerous randomized clinical trials are currently underway to explore the effectiveness of biopharmaceutical drugs, such as, interleukin-1 blockers, interleukin-6 inhibitors, Janus kinase inhibitors, in COVID-19. The aim of the present paper is to briefly summarize the pathogenetic rationale and the state of the art of therapeutic strategy blocking hyperinflammation.
Core Tip: Elevated inflammatory cytokines have been reported in patients with coronavirus disease 2019 (COVID-19). Evidence suggests that elevated cytokine levels and high levels in inflammatory markers are responsible for multi-organ damage in patients with COVID-19. Numerous randomized clinical trials are currently underway to explore the effectiveness of interleukin-1 blockers, interleukin-6 inhibitors. Or other strategies. For this reason it is necessary to make the point about using biopharmaceutical drugs in COVID-19.
- Citation: Ucciferri C, Vecchiet J, Falasca K. Role of monoclonal antibody drugs in the treatment of COVID-19. World J Clin Cases 2020; 8(19): 4280-4285
- URL: https://www.wjgnet.com/2307-8960/full/v8/i19/4280.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i19.4280
In the last decades, several viral epidemics has emerged in the world. In the last two decades three epidemics from coronoviruses appears: Severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002-2003 Middle East respiratory syndrome coronavirus in 2012-2013 and lately, the SARS-CoV-2[1]. Coronavirus is a large family of ribonucleic acid virus that can be isolated from humans and animals. To date coronavirus are responsible primary of acute respiratory infection. The most common manifestations due to these ribonucleic acid viruses are common cold, most interesting diseases are driven by other coronavirus (SARS-CoV, Middle East respiratory syndrome coronavirus, SARS-CoV-2) that cause epidemics disease with variable clinical severity and mortality rate range 2%-35%. In particular coronavirus disease 2019 (COVID-19) resulting from SARS-CoV-2 infection, is emerging as an unicum related to high infectivity and global diffusion, became a pandemic[2]. Despite a relatively long time since the COVID-19 outbreak has passed by, COVID-19 continue to threaten lives worldwide, and specific treatments besides supportive care are still lacking.
In humans, SARS-CoV-2 entry occurs via the host cell surface enzyme angiotensin-converting enzyme 2 (ACE2) receptor[3]. Specifically, downregulation of ACE2 leads to compensatory overproduction of angiotensin II by ACE. Angiotensin II in turn stimulates its 1a type receptor, which increases lung vascular permeability and potentiates lung pathology. Therefore, same viral protein (such as nonstructural protein) block the host innate immune response[4] and dysregulate the immune response. On the other hand, more histopathology data are emerging on COVID-19.
The clinical spectrum of SARS-CoV-2 infection ranges from asymptomatic to severe cases presenting with refractory hypoxemia requiring invasive mechanical ventilation. Evidence suggested that high levels of inflammatory biomarkes, reflecting an exaggerated immune host immune response, identify patients at high risk for diseases progression and unfavorable outcomes. This difference may be related to immune response in each patients and its immune damage to the cells. Same model from other infection, suggest that the viral escape of the innate immune response play a crucial rule[5], in fact the viral escape to the immune system cause an inadequate and delayed response. All this, implies the possibility for the virus to replicate without the immune control, resulting in a high spread of the virus in the body cells, while the delayed immune response results in a hyper-activated proinflammatory response secondary to the previous spread of the virus. A recent data corroborated this hypothesis, in SARS-CoV-2 infected cytokines related genes are upregolated and chemokine are predominant[6]. These chemokines are thought to be critical in the recruitment of neutrophils and monocyte in the lungs and other tissues (i.e. heart, vasculature). Moreover, interleukin-1 (IL-1) genes are significantly upregulated in SARS-CoV-2 infection. Therefore data suggested that higher virus replication results in a hyperinflammatory response[6].
Indeed, SARS-Cov-2 patients display increased levels of pro-inflammatory cytokines, such as IL1-β, IFNγ, MCP, TNFα, and VEGF; these may be employed as biomarkers to identify patients at risk for unfavorable prognosis, and druggable targets to resolve the hyperinflammatory response secondary to SARS-CoV-2 infection[7,8].
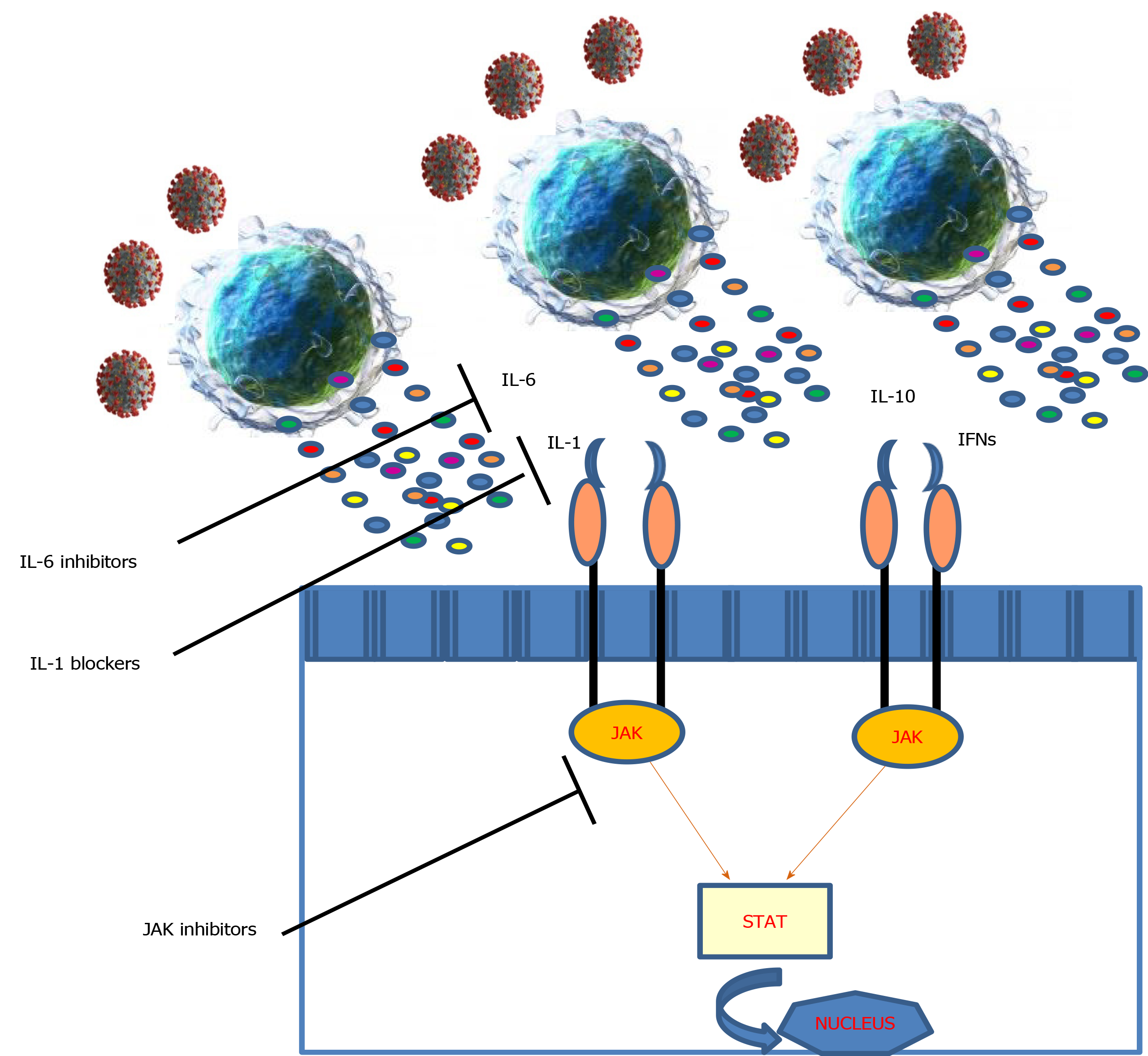
In SARS-CoV-2 infection we have an upregulation of a plethora of proinflammatory cytokines, suggesting the pathogenic role of hypercytokinemia in infection-related damage. The cytokine storms mediated by overproduction of proinflammatory cytokines have been observed in COVID-19 patients[9]. Of note, among pro-inflammatory cytokines, markedly elevated levels of IL-1 and IL-6 correlate with clinical outcomes. IL-1 and IL-6 levels are typically elevated in cytokine release syndrome, suggesting a mechanistic parallelism between the latter and COVID-19. For this reason, drugs that block the biological activity of IL-1 and its downstream product IL-6 may prove to be beneficial in the treatment of SARS-CoV-2 infection, in particularly in COVID-19 (Table 1). Several randomized controlled trials exploring this therapeutic strategy in mild to severe COVID-19 patients are ongoing (Table 2).
| Target | Drug type | Drugs |
| IL 6 signaling | Anti-IL 6 | Clazakizumab, Siltuximab |
| Anti-IL6 receptor | Sarilumab, Tocilizumab | |
| IL 1 signaling | Anti-IL1β | Canakinumab |
| Anti-IL1 repector | Anakinra | |
| JAK-STAT signaling | JAK1/JAK2 inhibitors | Baricitinib, Ruxolitinib |
| JAK1/JAK3 inhibitors | Tofacitinib |
| Target | Drugs | Clinical trial |
| IL 6 signaling | Clazakizumab | NCT04348500, NCT04363502, NCT04343989 |
| Siltuximab | NCT04329650, NCT04322188, NCT04330638 | |
| Sarilumab | NCT04359901, NCT04324073, NCT04357808, NCT04315298, NCT04327388, NCT04345289, NCT04380519, NCT04322773 | |
| Tocilizumab | NCT04317092, NCT04435717, NCT04331795, NCT04412772, NCT04377750, NCT04332094, NCT04377659, NCT04346355, NCT04335071, NCT04403685, NCT04372186, NCT04356937, NCT04320615, NCT04363736, NCT04332913, NCT04363853, NCT04306705, NCT04370834, NCT04339712, NCT04315480, NCT04330638, NCT04322773 | |
| IL 1 signaling | Anakinra | NCT04362943, NCT04364009, NCT04324021, NCT04357366, NCT04339712, NCT04330638 |
| Canakinumab | NCT04362813, NCT04365153 | |
| JAK-STAT signaling | Baricitinib | NCT04358614, NCT04373044, NCT04390464, NCT04362943, NCT04401579, NCT04346147, NCT04321993, NCT04345289 |
| Ruxolitinib | NCT04377620, NCT04362137, NCT04334044, NCT04338958, NCT04403243, NCT04348695 | |
| Tofacitinib | NCT04332042 |
Most data are available for tocilizumab, a humanized monoclonal antibody that inhibits both membrane-bound and soluble IL-6 receptors. Initial clinical data from China have shown an improvement in pneumonia and associated symptoms in patients with COVID-19 treated with tocilizumab[10]. Subsequently, several retrospective cohort studies have focused on the efficacy and safety of this treatment. Few studies showed a rapid, sustained, and significant clinical improvement in patients taking tocilizumab[11,12], on the other hand same studies failed to demonstrate the superiority of tocilizumab strategy[13,14]. These studies suggest that tocilizumab may be a candidate to improve the outcome of patients with severe COVID-19 infections. However, these results need confirmation by randomized controlled trials before this treatment can be advocated. Genentech has recently announced a phase III randomized controlled clinical trial with tocilizumab for severe COVID-19. Studies with other IL-6 receptor blockers are underway, but the available data are currently limited. Sarilumab, a Phase II/III trial are ongoing the early results, from a press relase of Sanofi, announced the discontinuing of 200 mg dose arm and restricting future enrollment to critical patients only. For this reason, Sarilumab seem to show that its utility may be limited to critically ill patients. Another anti-IL-6 drug, Siltuximab, is undergoing a Phase II open-label study comparing the efficacy and safety versus methylprednisolone in hospitalized patients with COVID-19 pneumonia, but data are not available yet. Similarly, Clazakizumab is being tested in clinical studies.
Nevertheless, the use of IL-1 blockers appears to be pathogenetically more promising than IL-6 blockers. The network of mediators orchestrating inflammatory responses to tissue damage includes IL-1α and IL-1β: Specifically, IL-1α is released by dying epithelial and endothelial cells, whereas IL-1β is produced by infiltrating macrophages, monocytes and neutrophils[15]. For this reason data on Anakinra, an IL-1 receptor antagonist that blocks activity of the proinflammatory cytokines IL-1α and IL-1β and is used to treat autoinflammatory disorders, and Canakinumab, a human monoclonal antibody to IL-1 beta are extremely interesting. Recent data on anakinra showed that, in a cohort of patients with COVID-19 and Acute respiratory distress syndrome managed with non-invasive mechanical ventilation, treatment with high-dose anakinra was safe and associated with clinical improvement[16,17]. Similar data are available on canakinumab in adult patients with COVID-19 and respiratory failure, not requiring mechanical ventilation, that was associated with rapid reduction of systemic inflammatory response, and improvement of oxygenation[18].
Finally, Janus kinase (JAK) inhibitors, which may have a role in blocking the fibrotic evolution in some patients, are being evaluated[19]. The JAK/signal transducers and activators of transcription STAT-pathway mediates the signaling of multiple cytokines, therefore interrupting this pathway may be an attractive strategy to modulate the immunopathology observed in SARS-CoV-2 infection (Figure 1). Besides, many JAK inhibitors exhibit antiviral effects when administered at therapeutic doses, by targeting host factors that viruses use for cell entry[20]. Baricitinib is an oral JAK inhibitor, that inhibits the JAK signal transducer and activator of transcription pathway. It is under investigation in some clinical trials on COVID-19 patients. A pilot study from Italy showed a significantly improvement in clinical and laboratory parameters in patients treated with baricinib[21]. Ruxolitinib, another JAK-kinase inhibitor, showed in COVID-19 patients with hyperinflammation, to be safe and prevent multiorgan failure[22]. However, at the moment, no data are available on different JAK inhibitors.
Currently available data on SARS-CoV-2 infection show that the extent of the inflammatory response correlates with disease progression and subsequent organ damage. Hyperinflammation contributes to disease severity and death in these patients. Using drugs that act on the inflammatory process appears to be increasingly crucial in the treatment of COVID-19. A better understanding of which subgroups of patients are at higher risk for severe disease, and which major inflammatory pathways sustain diseases progression is crucial to develop targeted therapeutic strategies.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koustas E S-Editor: Zhang L L-Editor: A P-Editor: Wang LL
| 1. | Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1873] [Cited by in F6Publishing: 1743] [Article Influence: 435.8] [Reference Citation Analysis (1)] |
| 2. | Helmy YA, Fawzy M, Elaswad A, Sobieh A, Kenney SP, Shehata AA. The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 434] [Cited by in F6Publishing: 349] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 3. | Barone M, Ucciferri C, Cipollone G, Mucilli F. Recombinant Human Angiotensin-Converting Enzyme 2 and COVID-19 Acute Respiratory Distress Syndrome: A Theoretical or a Real Resource? EJMO. 2020;4:139-140. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Lei J, Kusov Y, Hilgenfeld R. Nsp3 of coronaviruses: Structures and functions of a large multi-domain protein. Antiviral Res. 2018;149:58-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 415] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 5. | D'Elia RV, Harrison K, Oyston PC, Lukaszewski RA, Clark GC. Targeting the "cytokine storm" for therapeutic benefit. Clin Vaccine Immunol. 2013;20:319-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 6. | Zhou Z, Ren L, Zhang L, Zhong J, Xiao Y, Jia Z, Guo L, Yang J, Wang C, Jiang S, Yang D, Zhang G, Li H, Chen F, Xu Y, Chen M, Gao Z, Yang J, Dong J, Liu B, Zhang X, Wang W, He K, Jin Q, Li M, Wang J. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe. 2020;27:883-890.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 666] [Article Influence: 166.5] [Reference Citation Analysis (0)] |
| 7. | Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2713] [Cited by in F6Publishing: 2658] [Article Influence: 664.5] [Reference Citation Analysis (0)] |
| 8. | Verhagen J, Vliegenthart JF, Boldingh J. Micelle and acid-soap formation of linoleic acid and 13-L-hydroperoxylinoleic acid being substrates of lipoxygenase-1. Chem Phys Lipids. 1978;22:255-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5228] [Cited by in F6Publishing: 5526] [Article Influence: 1381.5] [Reference Citation Analysis (2)] |
| 10. | Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92:814-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 834] [Cited by in F6Publishing: 854] [Article Influence: 213.5] [Reference Citation Analysis (0)] |
| 11. | Di Giambenedetto S, Ciccullo A, Borghetti A, Gambassi G, Landi F, Visconti E, Zileri Dal Verme L, Bernabei R, Tamburrini E, Cauda R, Gasbarrini A. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J Med Virol. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 12. | Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, Franceschini F, Airò P, Bazzani C, Beindorf EA, Berlendis M, Bezzi M, Bossini N, Castellano M, Cattaneo S, Cavazzana I, Contessi GB, Crippa M, Delbarba A, De Peri E, Faletti A, Filippini M, Filippini M, Frassi M, Gaggiotti M, Gorla R, Lanspa M, Lorenzotti S, Marino R, Maroldi R, Metra M, Matteelli A, Modina D, Moioli G, Montani G, Muiesan ML, Odolini S, Peli E, Pesenti S, Pezzoli MC, Pirola I, Pozzi A, Proto A, Rasulo FA, Renisi G, Ricci C, Rizzoni D, Romanelli G, Rossi M, Salvetti M, Scolari F, Signorini L, Taglietti M, Tomasoni G, Tomasoni LR, Turla F, Valsecchi A, Zani D, Zuccalà F, Zunica F, Focà E, Andreoli L, Latronico N. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19:102568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 565] [Cited by in F6Publishing: 541] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 13. | Quartuccio L, Sonaglia A, McGonagle D, Fabris M, Peghin M, Pecori D, De Monte A, Bove T, Curcio F, Bassi F, De Vita S, Tascini C. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: Results from a single Italian Centre study on tocilizumab versus standard of care. J Clin Virol. 2020;129:104444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 14. | Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, Tomelleri A, Baldissera E, Rovere-Querini P, Ruggeri A, Monti G, De Cobelli F, Zangrillo A, Tresoldi M, Castagna A, Dagna L; TOCI-RAF Study Group. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 287] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 15. | Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720-3732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1309] [Cited by in F6Publishing: 1434] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 16. | Aouba A, Baldolli A, Geffray L, Verdon R, Bergot E, Martin-Silva N, Justet A. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 17. | Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, Oltolini C, Castiglioni B, Tassan Din C, Boffini N, Tomelleri A, Farina N, Ruggeri A, Rovere-Querini P, Di Lucca G, Martinenghi S, Scotti R, Tresoldi M, Ciceri F, Landoni G, Zangrillo A, Scarpellini P, Dagna L. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325-e331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 744] [Cited by in F6Publishing: 693] [Article Influence: 173.3] [Reference Citation Analysis (0)] |
| 18. | Ucciferri C, Auricchio A, Di Nicola M, Potere N, Abbate A, Cipollone F, Vecchiet J, Falasca K. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020;. [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 19. | Caocci G, La Nasa G. Could ruxolitinib be effective in patients with COVID-19 infection at risk of acute respiratory distress syndrome (ARDS)? Ann Hematol. 2020;99:1675-1676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Jorgensen SCJ, Tse CLY, Burry L, Dresser LD. Baricitinib: A Review of Pharmacology, Safety, and Emerging Clinical Experience in COVID-19. Pharmacotherapy. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 21. | Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J Infect. 2020;81:318-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 300] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 22. | La Rosée F, Bremer HC, Gehrke I, Kehr A, Hochhaus A, Birndt S, Fellhauer M, Henkes M, Kumle B, Russo SG, La Rosée P. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. 2020;34:1805-1815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 163] [Article Influence: 40.8] [Reference Citation Analysis (0)] |