Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6663
Peer-review started: March 17, 2021
First decision: April 4, 2021
Revised: April 16, 2021
Accepted: July 2, 2021
Article in press: July 2, 2021
Published online: August 16, 2021
At present, over 180 million people have been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) worldwide and there have been more than 3.8 million deaths due to the virus. However, specific effective antiviral treatment for this infectious disease is absent. At the beginning of the epidemic, relevant cellular and animal experiments of antiviral treatment for SARS-CoV-2 were conducted based on the prior studies of SARS-CoV and Middle East respiratory syndrome coronavirus. Some antivirals were preliminarily validated to be potentially effective in the clinical settings. But as the epidemic continued and more studies were carried out, the efficacy of these antiviral drugs became controversial. This paper reviews the pharmacology and application of interferon, lopinavir/ritonavir, ribavirin, chloroquine, arbidol, favipiravir, remdesivir, and thymosin α1 in coronavirus disease 2019. The actual effect of these drugs remains controversial. Meanwhile, the efficacy and safety of these drugs for patients with coronavirus disease 2019 still need to be explored.
Core Tip: This paper reviews the pharmacology and application of interferon, lopinavir/ritonavir, ribavirin, chloroquine, arbidol, favipiravir, remdesivir, and thymosin α1 in coronavirus disease 2019 (COVID-19). The actual effect of these drugs remains controversial. Meanwhile, the efficacy and safety of these drugs for patients with COVID-19 still need to be explored.
- Citation: Zhao B, Yang TF, Zheng R. Theory and reality of antivirals against SARS-CoV-2. World J Clin Cases 2021; 9(23): 6663-6673
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6663.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6663
Similar to severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), the SARS-CoV-2 belongs to the genus Betacoronavirus and is genetically close to a novel type of bat-derived coronavirus. The SARS-CoV-2 genome shares 79.5% homology with the SARS-CoV genome. Further, SARS-CoV-2 shares a common cell entry receptor with SARS-CoV[1]. Currently, SARS-CoV-2 has had a higher infectivity but a lower fatality rate than severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS)[1]. At the beginning of the epidemic, researchers across the globe have examined prior reports of SARS-CoV and MERS-CoV and conducted relevant cellular and animal experiments, and several antivirals were preliminarily validated to be potentially effective in the clinical settings. However, in the actual treatment process, effective antivirals are still absent. In the present paper, we seek to summary the potential antivirals against SARS-CoV-2.
Interferons (IFNs) are a class of proteins that exhibit broad-spectrum antiviral, antitumoral, and immunomodulatory effects. IFNs can be classified into type I (such as α and β), type II (γ), and type III (λ) based on the cell surface receptors, acid stability, initiation sequence, and chromosomal location[2]. The interaction between IFN and the target cells leads to the production of antiviral proteins that interfere with mRNA transcription. This hinders viral nucleic acid synthesis and inhibits viral replication[3]. Previous studies have demonstrated that high-dose IFN (types I and III) administration exhibits significant inhibitory effects on SARS-CoV and MERS-CoV in cell culture, animal studies, and patients. In particular, MERS-CoV was found to be more sensitive to IFNs than SARS-CoV in cell culture experiments[4]. The study by Omrani et al[5] showed that treating MERS with IFNs prolonged the survival by 14 d in 70% of patients. Conversely, in the report by Al-Tawfiq et al[6], all five patients eventually died. However, the authors of both the studies agreed that severe infections and comorbidities were the main reasons for poor efficacy. Al-Tawfiq et al[6] suggested that delayed treatment was a prognostic factor, and initiating treatment within 48 h of hospitalization or immediately after diagnosis could lead to better outcomes. Three types of IFN have been used to treat coronavirus infections. A study has suggested that IFN-β displays the most prominent therapeutic effect[7]. The recommended route of administration for IFN-α is inhalation of nebulized solution in novel coronavirus pneumonia diagnosis and treatment plan of China[8]. According to the autopsy report of the first patient who died from pneumonia caused by SARS-CoV-2, the lesions were found to be concentrated in the lungs, whereas evidence of damage in other organs was insufficient[9]. Nebulizer inhalation therapy can thus be directly delivered to the target organs, namely, the respiratory tract and the lungs, resulting in rapid onset of drug action and high local drug concentrations. Other advantages of inhalation therapy include low dosage, convenient administration, and few systemic adverse reactions[10]. However, all IFNs currently available in the market are injectable formulations. Intravenous formulations often contain preservatives such as phenol and nitrite, which can induce asthma attacks upon inhalation. Non-nebulized inhalation formulations do not meet the requirements for effective nebulization of particles. Further, they cannot be eliminated by the respiratory tract and may deposit in the lungs, increasing the incidence of lung infections[9]. Previous clinical studies on SARS and MERS mainly utilized subcutaneous or intramuscular injections. Further, the efficacy of the inhaled nebulizer lacks supportive evidence. If the nebulizer is used in a relatively closed environment, patients will be at risk of contracting the infection through aerosol transmission[8]. Therefore, the optimal IFN subtype and drug delivery route have not been confirmed through large-scale, standardized clinical studies. Early administration may lead to better outcomes in patients with mild-to-moderate disease.
Lopinavir/ritonavir tablet is a compound formulation consisting of lopinavir and ritonavir that is used as a second-line treatment for HIV[11]. In particular, lopinavir is an HIV-1 protease inhibitor that blocks the cleavage of the Gag-Pol polyprotein, leading to the production of immature, non-infectious viral particles while ritonavir inhibits the CYP3A-mediated metabolism of lopinavir, resulting in increased lopinavir concentrations[12,13]. During the SARS outbreak, researchers from Hong Kong, China conducted in vitro and in vivo studies[14]. The in vitro studies showed that lopinavir and ribavirin at concentrations of 4 mg/mL and 50 mg/mL, respectively, exhibited antiviral activity against SARS-CoV after 48 h[14]. A controlled clinical study revealed that the addition of lopinavir/ritonavir to the previously used ribavirin and hormonal regimens lowered the incidence of acute respiratory distress syndrome or death and reduced hormonal use and nosocomial infections. Additionally, reduced viral loads and increased peripheral lymphocyte counts were observed in patients[14]. In vitro cellular experiments on MERS demonstrated that lopinavir/ritonavir exhibited anti-MERS-CoV activity[15]. Through animal models, the combination was demonstrated to exhibit a significant therapeutic effect relative to the control[16]. Lopinavir/ritonavir has been reported to display considerable efficacy when used as a combination therapy in the clinical setting[17]. Based on research on SARS and MERS to date, lopinavir/ritonavir has been often used in the SARS-CoV-2 treatment across the globe. A study from South Korea reported that in a patient with severe coronavirus disease 2019 (COVID-19) administered with lopinavir/ritonavir on the 10th day of disease onset, the β-coronavirus load began to decrease on the following day[18]. Certainly, the potential of natural disease outcome could not be ruled out. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with COVID-19 was held by Cao et al[19]. The results showed that there was no significant benefit of lopinavir/ritonavir compared with conventional treatment in the hospitalized severe patients with COVID-19. Although it was a negative conclusion, we can still explore the effect of lopinavir/ritonavir in the treatment of mild-to-moderate disease, and observe its reduction in the occurrence of severe disease. With the wider adoption of lopinavir/ritonavir in clinics, adverse reactions such as diarrhea, nausea, vomiting, and liver damage are common, which need to be constantly vigilant.
Ribavirin is a synthetic nucleoside analog that can inhibit a multitude of RNA and DNA viruses. Upon entry into cells, ribavirin is phosphorylated. Thereafter, it competitively inhibits the biosynthesis of guanosine triphosphate, which is required by the virus. This interferes with the synthesis of viral RNAs and proteins, thereby inhibiting viral replication and transmission[20]. Owing to the lack of other effective treatments in 2003, ribavirin was widely used to treat SARS[21]. However, during the course of application, the efficacy of ribavirin in patients with SARS was not definitive. Retrospective studies suggested that SARS-CoV was resistant to ribavirin[22]. Further, cohort studies failed to draw definitive conclusions regarding its efficacy owing to the study designs or the inability to distinguish its therapeutic effects from those of other drugs[23]. In vitro studies revealed that ribavirin exhibits detectable antiviral activity. However, this activity was low or decreased in cell lines after 48 h of incubation. The combination of ribavirin and IFN has been demonstrated to display a synergistic antiviral effect[24]. In the MERS epidemic, ribavirin was administered as a treatment regimen. Many studies have adopted the combined regimen of ribavirin and IFN and attained success in in vitro models[25]. However, the efficacy of the combined regimen in clinical practice remains inconclusive owing to the occurrence of sporadic cases and the heterogeneity of studies in different regions[6]. Based on the SARS or MERS studies, an effective concentration cannot be attained with the conventional dosage of ribavirin; however, the increased dosage will lead to a multitude of adverse reactions, primarily hemolytic anemia and abnormal liver function. Ribavirin alone might not exert a significant clinical effect; however, its early use in combination with IFN or lopinavir/ritonavir may result in a certain degree of clinical effect. The eighth version of the diagnosis and treatment plan of China specified the usage and dosage of ribavirin, and recommended "500 mg/administration, 2 to 3 intravenous drips per day in adults" and "the duration of treatment should not exceed 10 d" was specified[8]. It still requires confirmation in larger studies to prove the therapeutic effect on mild-to-moderate and severe patients with COVID-19.
Chloroquine is an antimalarial drug that has been on the market for many years. Chloroquine is also used to treat autoimmune rheumatic diseases. In vitro cellular experiments showed that chloroquine effectively blocked infections owing to SARS-CoV-2 at low concentrations and exhibited a high selectivity[26]. Further, the outcomes of more than 100 patients administered with chloroquine phosphate were superior to those administered with the control drug[27]. Chloroquine was found to improve the lung imaging findings, promote a virus-negative conversion, and shorten the disease course[27]. Chloroquine was studied during the SARS epidemic. Further, it was demonstrated to effectively prevent the spread of SARS-CoV in the cell culture. Treatment of cells with chloroquine prior to or after SARS-CoV infection effectively inhibited the spread of the virus[28]. Chloroquine is a weakly basic drug that can alter the pH of endosomes, thereby blocking pH-dependent viral replication. Meanwhile, chloroquine can interfere with the glycosylation of viral cell receptors and inhibit viral replication. Chloroquine also acts as a potent autophagy inhibitor and interferes with viral infection and replication via affecting autophagy[29]. The replacement of an ethyl group by hydroxyethyl in chloroquine will result in the formation of hydroxychloroquine. Hydroxychloroquine and chloroquine have similar pharmacokinetics, indications, and associated adverse reactions. However, compared to chloroquine, the overall incidence of adverse reactions associated with hydroxychloroquine is lower and is mainly reflected in the lower incidence of ocular adverse reactions[30]. Conceptually, hydroxychloroquine should be effective and might serve as a better option from the perspective of drug safety. The study by Gautret et al[31] showed that 20 cases treated with hydroxychloroquine had a significant reduction of the viral carriage and much lower average carrying duration compared to the 16 controls. Especially, six patients who used hydroxychloroquine in combination with azithromycin had a 100% negative rate of nasopharynx swab virus on the sixth day. Despite its small sample size, the study brings great hope that hydroxychloroquine treatment is significantly associated with a viral load reduction in COVID-19 patients and its effect is reinforced by azithromycin. Owing to the above antiviral mechanisms and previous research and application outcomes, chloroquine and hydroxychloroquine, with or without azithromycin, have been studied in multiple clinical trials for the treatment of COVID-19. Subsequent analyses confirmed that hydroxychloroquine alone was not effective in the treatment of SARS-CoV-2, and it increased the risk of death when combined with azithromycin[32]. Hydroxychloroquine did not show any new coronavirus clearance or clinical benefit. On the other hand, it brought additional side effects[33]. The use of chloroquine or hydroxychloroquine with or without azithromycin for the treatment of COVID-19 was not recommended. However, many clinical trials are still in progress. An editorial suggested that hydroxychloroquine might have a preventive effect, and its potential preventive benefit remains to be determined[34].
Arbidol is a small indole-derivative molecule developed by the former Soviet Union. It is primarily indicated in flu caused by influenza A and B viruses. Arbidol has been used in Russia for more than 20 years and has exhibited good safety and tolerability[35]. Further, it was approved for marketing in China in 2006[35]. As a hemagglutinin inhibitor, an IFN inducer, and an immunostimulating agent, arbidol can bind to lipid and protein residues of the viruses and prevent cell entry and fusion by locally impairing viral attachment to the cell plasma membrane. In addition, it inhibits viral replication, assembly, and budding[35,36]. Thus, arbidol demonstrates remarkable potential as a broad-spectrum antiviral agent. In vivo and in vitro experiments have confirmed that it exerts antiviral activity against many RNA or DNA viruses, such as adenovirus, respiratory syncytial virus, hantaan virus, hepatitis B virus, hepatitis C viruses, SARS-CoV, and MERS-CoV[35,37]. However, it is a relatively new drug for the treatment of influenza A and B viruses. Accordingly, earlier work had mainly focused on in vitro experiments, and clinical data supporting its use for SARS-CoV and MERS-CoV are lacking. For the current SARS-CoV-2 epidemic, arbidol was mentioned in the novel coronavirus pneumonia diagnosis and treatment plan of China[8]. According to media reports, in vitro cellular experiments showed that arbidol at 10-30 μm was 60 times more efficacious at inhibiting coronavirus than the control drug. Further, it significantly inhibited the cytopathic effect of the virus[38]. Although in vitro experiments have demonstrated its efficacy, whether the standard dosage can result in an effective concentration in vivo remains unknown. Clinical studies with arbidol have been reported; however, patients with favorable outcomes often received a combination with other drugs instead of arbidol alone. In addition, the sample sizes of the studies were small[39]. The early epidemic was identified to coincide with the flu season. The use of arbidol might be beneficial to flu patients who cannot be screened in time.
Favipiravir is a novel type of RNA polymerase inhibitor that has obtained marketing approval for the treatment of novel and re-emerging influenza. A study has shown that in addition to those on influenza viruses, favipiravir exhibits robust antiviral effects on different RNA viruses, such as the Ebola virus, arenavirus, bunyavirus, and rabies virus[40]. Favipiravir is converted intracellularly into favipiravir ribofuranosyl-5'-triphosphate, which is structurally similar to purine and can compete with purine for viral RNA polymerase. This hinders the replication and transcription of viral RNA strands and increases the rate of mutation during viral genome replication, leading to a significant reduction in virus-specific infectivity and to virus elimination[41]. As SARS-CoV-2 is an RNA virus, favipiravir should, in principle, be effective against the virus. However, since its launch in 2014, only few clinical studies with favipiravir have been conducted on coronavirus. A study in Vero E6 cells infected with SARS-CoV-2 showed that favipiravir was not an ideal drug, with a half maximal effective concentration (EC50) of 61.88 μmol/L, 50% cytotoxic concentration of > 400 μmol/L, and selectivity index of > 6.46. Although its EC50 value in the Ebola-infected Vero E6 cells reached 67 μmol/L, it was demonstrated to exert 100% protection against Ebola infection in mice. Thus, different conclusions could be derived from the in vitro experiments and in vivo studies[26]. Further, the clinical trial is currently underway in Shenzhen. Preliminarily results of this trial have shown promising efficacy and a low incidence of adverse reactions. After 3 to 4 d of treatment, the negative conversion rate of the viral nucleic acids was significantly higher in the treatment group than the control group[42]. Media reports generally assert optimism; however, they are not based on rigorous scientific evidence. In a small trial of SARS-CoV-2, 120 patients were treated with favipiravir. Although favipiravir improved the latency to relief for pyrexia and cough, it did not significantly improve the clinical recovery rate on day 7[43]. Therefore, conclusions from authentic and validated studies are still needed to further delineate the efficacy and tolerability of the drug.
Remdesivir is a novel nucleoside analog and a broad-spectrum antiviral agent. It binds to the nascent viral RNA strands, leading to the premature termination of viral replication[44]. The results of in vitro cellular experiments and animal studies have demonstrated that it exerts extremely potent in vitro antiviral activities against coronaviruses that infect human and different bat-derived coronaviruses[45]. In a mouse model of SARS-CoV, prophylactic and early treatment with remdesivir significantly reduced the viral load in the lungs of mice and improved the clinical symptoms and respiratory functions[46]. Additionally, remdesivir effectively reduced the viral titer in the lung tissue of mice infected with MERS-CoV and improved lung tissue injury. Its efficacy was superior to that of the combination of lopinavir/ritonavir and IFN-β[17]. In Vero E6 cells, the EC50 and EC90 of remdesivir for SARS-CoV-2 are 0.77 μM and 1.76 μM, respectively[26]. Remdesivir has been studied in several clinical trials for the treatment of SARS-CoV-2, and it has been approved by the Food and Drug Administration for the treatment of COVID-19 in hospitalized patients[47]. Wang et al[48] had published a randomised, double-blind, placebo-controlled, multicentre trial about remdesivir in adults with severe COVID-19. Compared with the placebo group, the clinical improvement time of the remdesivir group was shortened by an average of 2 d and the invasive mechanical ventilation time was shortened by an average of 4 d, but the above differences were not statistically significant. Positive data was published by the American Institute of Allergy and Infectious Diseases at almost the same time. Preliminary data analysis from a randomized controlled trial of 1063 patients showed that COVID-19 patients who had progression had lung involvement recovered faster than similar patients who had received placebo[49]. The median time to recovery was 11 d in patients receiving remdesivir and 15 d in patients receiving placebo, but the fatality rate did not improve. Two studies showed that remdesivir did not reduce the mortality, but can shorten the course of disease. In another cohort of patients hospitalized for severe COVID-19, clinical benefit was observed in 36 (68%) of 53 patients who were treated with remdesivir[50], but there was no control group for this study. There is no sufficient evidence to prove that remdesivir can improve the survival rate, so it is not recommended by the guideline of World Health Organization[51]. It still requires confirmation in larger studies to prove the therapeutic effect on mild-to-moderate and severe patients with COVID-19.
Thymosin α1 is a group of immunoactive peptides that can induce T-cell differentiation and maturation and regulate T-cell functions. Thymosin α1 is a broad-spectrum antiviral biologic that is clinically used as a primary or adjuvant treatment for chronic hepatitis, acquired immunodeficiency syndrome, other viral infections, and tumors. Most patients with COVID-19 exhibit reduced lymphocyte counts, suggesting that SARS-CoV-2 may primarily affect lymphocytes, particularly T cells[52]. As thymosin α1 can promote T-cell development, differentiation, and maturation, it can be employed as an adjuvant antiviral therapy to enhance the immune response to the virus in immunocompromised patients. A prior case report described the use of thymosin α1 as part of a combination therapy in the treatment of MERS-nCoV infection[53]. The autopsy report of a patient with COVID-19 revealed the occurrence of alveolar septal congestion, edema, and the infiltration of mononuclear cells and lymphocytes[9]. Lymphocyte infiltration is the immune response elicited by the host to eliminate the virus. However, an overactive response may eliminate the virus while concurrently inducing local inflammatory response in the lung tissue or uncontrolled systemic inflammatory response. Whether the use of thymosin α1 will aggravate the immune response and lead to immune-mediated damage should be elucidated. For healthy individuals with normal immunity, the preventive effect of thymosin remains unclear. Therefore, additional research is required to determine the stage of the infection that is most suited for the use of thymosin[54]. As thymosin can enhance the body’s immune response, some hospitals have initiated clinical studies of antiviral agents in combination with thymosin α1. Data from these studies are expected to serve as useful references[54].
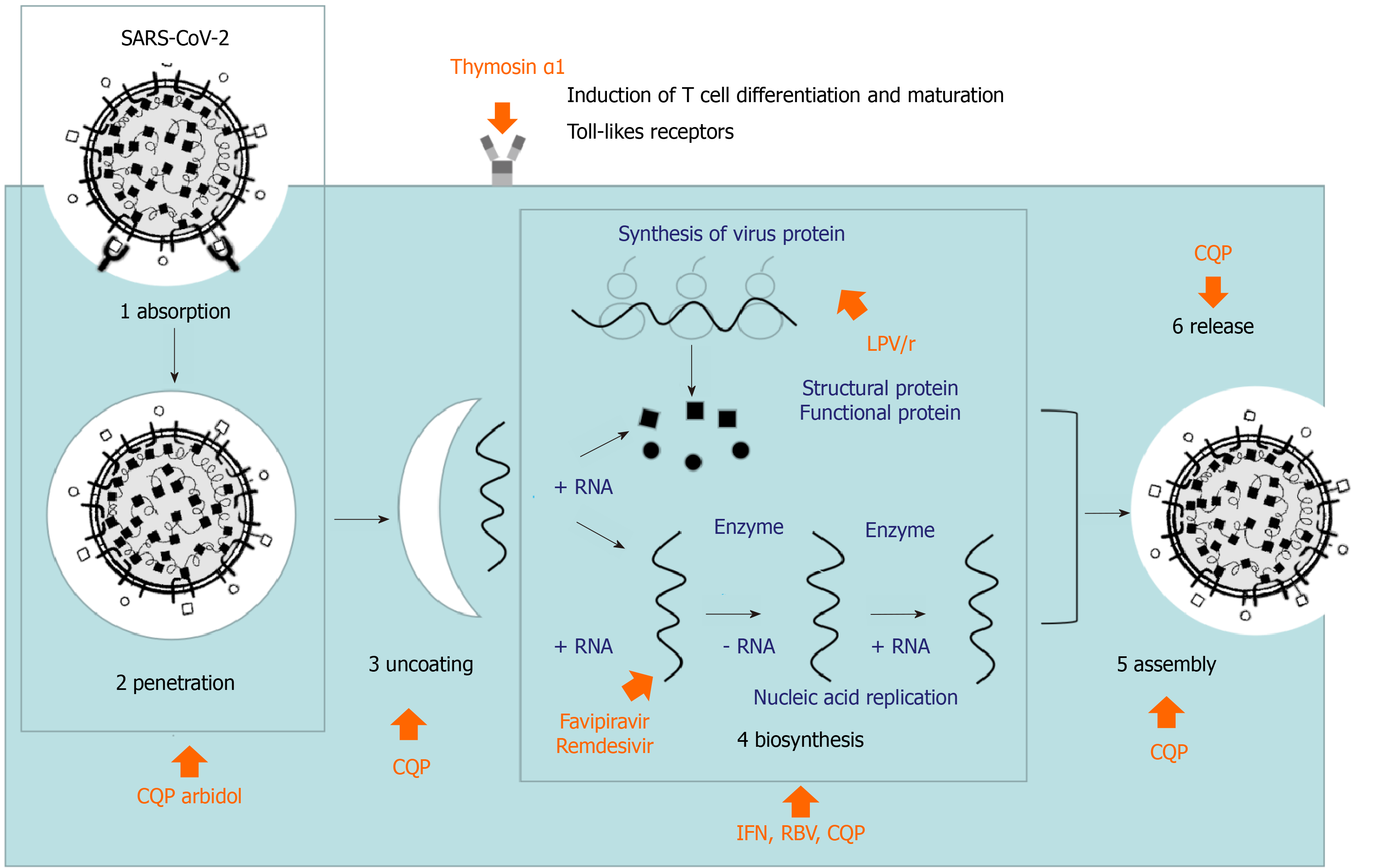
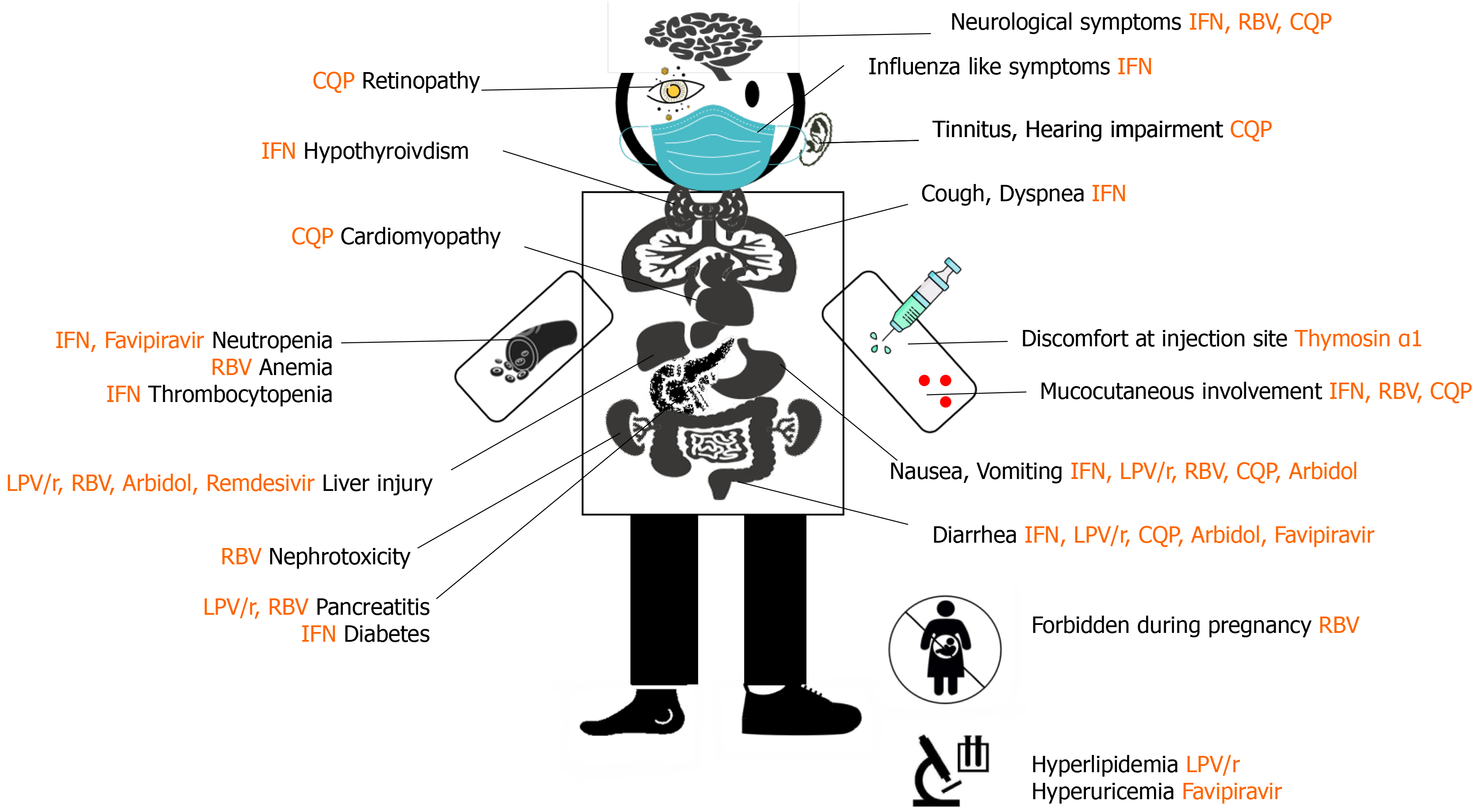
The drugs mentioned in this paper have certain theoretical basis, but the actual research results are not completely satisfactory, especially for severe patients (Figures 1 and 2, Table 1). There is a distance between theory and practice. The efficacy and safety of these drugs need to be verified in future clinical research. We still have to be optimistic. In the early stage, we did not know about this disease, but now we have enough experience to diagnose it. It will achieve better results if having early diagnosis and early treatment. A clearer picture is expected in the near future to ensure that safe and effective drugs can be utilized and the overuse of ineffective drugs can be avoided.
| Antiviral | Notes | Usage and dosage (adults) |
| IFN | (1) Not recommended for the treatment of patients with severe or critical COVID-19, except in a clinical trial; (2) Patients with early (i.e., < 7 d from symptom onset) mild and moderate disease may benefit; and (3) IFN-α has primarily been used as nebulization and usually as part of a combination regimen | (1) Nebulized INF-α: 5 million units, add 2 mL of sterile water for injection, twice daily. Preferably less than 10 d; and (2) Subcutaneous IFN-β: 8 million international units every other day. Preferably less than 10 d |
| LPV/r | (1) Not recommended to treat patients with COVID-19 at any severity; (2) Not recommended to use alone; and (3) The plasma drug concentrations of typical doses are far below the levels that may be needed | Lopinavir 400 mg/ritonavir 100 mg orally twice daily. Preferably less than 10 d |
| RBV | (1) There is not enough sample size, scientific and objective clinical data to prove effective; and (2) Not recommended to use alone. Usually in combination with IFN and/or LPV/r | 500 mg per time, inject 2-3 times per day intravenously. Preferably less than 10 d |
| CQ | (1) Not recommended to treat patients with COVID-19 at any severity; and (2) Recommendation against CQ with or without azithromycin | (1) CQ 600 mg twice daily for 10 d (high dose, serious side effect). CQ 450 mg twice daily for 1 d, then CQ 450 mg for 4 d (low dose, mild side effect); (2) HCQ 800 to 1600 mg orally on the first day, 1 to 3 divided doses. 200 to 800 mg orally daily for 5 to 21 d, 1 to 2 divided doses; and (3) CQP 500 mg twice daily for 10 d |
| Arbidol | There is not enough sample size, scientific and objective clinical data to prove effective | 100 mg orally twice daily for 5 d. Preferably less than 10 d |
| Favipiravir | There is not enough sample size, scientific and objective clinical data to prove effective | 1600 mg for the first dose, then 600 mg orally twice daily for 5 d |
| Remdesivir | (1) Remdesivir is the only Food and Drug Administration-approved drug for the treatment of COVID-19; and (2) Consider remdesivir for hospitalized patients with COVID-19 who require supplemental oxygen but who do not require oxygen delivery through a high-flow device, noninvasive ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation | 200 mg on day 1, then 100 mg injected intravenously once daily for 5-7 d |
| Thymosin α1 | There is not enough sample size, scientific and objective clinical data to prove effective | 1.6 mg subcutaneous injection, twice a week, each time 3-4 d apart. Preferably more than 4 wk |
Manuscript source: Invited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mocan T S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Li JH
| 1. | Del Rio C, Malani PN. 2019 Novel Coronavirus-Important Information for Clinicians. JAMA. 2020;323:1039-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 198] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 2. | George PM, Badiger R, Alazawi W, Foster GR, Mitchell JA. Pharmacology and therapeutic potential of interferons. Pharmacol Ther. 2012;135:44-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Bandurska K, Król I, Myga-Nowak M. [Interferons: between structure and function]. Postepy Hig Med Dosw (Online). 2014;68:428-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Kindler E, Thiel V, Weber F. Interaction of SARS and MERS Coronaviruses with the Antiviral Interferon Response. Adv Virus Res. 2016;96:219-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 204] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 5. | Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY, Almakhlafi GA, Albarrak MM, Memish ZA, Albarrak AM. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 363] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 6. | Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 7. | Hart BJ, Dyall J, Postnikova E, Zhou H, Kindrachuk J, Johnson RF, Olinger GG, Frieman MB, Holbrook MR, Jahrling PB, Hensley L. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol. 2014;95:571-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 8. | Central People's Government of the People's Republic of China. General Office of the national health and Health Commission. Novel coronavirus pneumonia diagnosis and treatment plan (trial version 8). August 18, 2020. [cited 10 March 2021]. Available from: http://www.gov.cn/zhengce/zhengceku/2020-08/19/content_5535757.htm. [Cited in This Article: ] |
| 9. | Liu Q, Wang RS, Qu GQ, Wang YY, Liu P, Zhu YZ, Fei G, Ren L, Zhou YW. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36:19-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 134] [Reference Citation Analysis (0)] |
| 10. | Clinical Pharmacy Branch of Chinese Medical Association. Expert consensus on rational use of aerosol inhalation therapy (2019 version). Yixue Daobao. 2019;38:135-146. [Cited in This Article: ] |
| 11. | Su B, Wang Y, Zhou R, Jiang T, Zhang H, Li Z, Liu A, Shao Y, Hua W, Zhang T, Wu H, He S, Dai L, Sun L. Efficacy and Tolerability of Lopinavir/Ritonavir- and Efavirenz-Based Initial Antiretroviral Therapy in HIV-1-Infected Patients in a Tertiary Care Hospital in Beijing, China. Front Pharmacol. 2019;10:1472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Flexner C. HIV-protease inhibitors. N Engl J Med. 1998;338:1281-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 653] [Cited by in F6Publishing: 597] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 13. | Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag. 2008;4:1023-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY; HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1080] [Cited by in F6Publishing: 1092] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 15. | Chan JF, Chan KH, Kao RY, To KK, Zheng BJ, Li CP, Li PT, Dai J, Mok FK, Chen H, Hayden FG, Yuen KY. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67:606-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 16. | Chan JF, Yao Y, Yeung ML, Deng W, Bao L, Jia L, Li F, Xiao C, Gao H, Yu P, Cai JP, Chu H, Zhou J, Chen H, Qin C, Yuen KY. Treatment With Lopinavir/Ritonavir or Interferon-β1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset. J Infect Dis. 2015;212:1904-1913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 494] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 17. | Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1216] [Cited by in F6Publishing: 1109] [Article Influence: 277.3] [Reference Citation Analysis (0)] |
| 18. | Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ, Choe KW, Kang YM, Lee B, Park SJ. The Author's Response: Case of the Index Patient Who Caused Tertiary Transmission of Coronavirus Disease 2019 in Korea: the Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Pneumonia Monitored by Quantitative RT-PCR. J Korean Med Sci. 2020;35:e89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3386] [Cited by in F6Publishing: 3503] [Article Influence: 875.8] [Reference Citation Analysis (0)] |
| 20. | Cameron CE, Castro C. The mechanism of action of ribavirin: lethal mutagenesis of RNA virus genomes mediated by the viral RNA-dependent RNA polymerase. Curr Opin Infect Dis. 2001;14:757-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Lai ST. Treatment of severe acute respiratory syndrome. Eur J Clin Microbiol Infect Dis. 2005;24:583-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Smith EC, Blanc H, Surdel MC, Vignuzzi M, Denison MR. Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics. PLoS Pathog. 2013;9:e1003565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 322] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 23. | Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 861] [Cited by in F6Publishing: 841] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 24. | Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, Cheng VC, Tsui WH, Hung IF, Lee TS, Guan Y, Peiris JS, Yuen KY. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 370] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 25. | Momattin H, Al-Ali AY, Al-Tawfiq JA. A Systematic Review of therapeutic agents for the treatment of the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Travel Med Infect Dis. 2019;30:9-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 26. | Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4289] [Cited by in F6Publishing: 4373] [Article Influence: 1093.3] [Reference Citation Analysis (0)] |
| 27. | Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1597] [Cited by in F6Publishing: 1561] [Article Influence: 390.3] [Reference Citation Analysis (0)] |
| 28. | Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1198] [Cited by in F6Publishing: 1186] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 29. | Multicenter Collaboration Group of the Science and Technology Department of Guangdong Province and the Health Commission of Guangdong Province for the Treatment of COVID-19 by Chloroquine Phosphate. Expert consensus on novel coronavirus pneumonia treated by chloroquine phosphate. Zhonghua Jiehe He Huxi Zazhi. 2020;43:185-188. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 131] [Reference Citation Analysis (0)] |
| 30. | Romanelli F, Smith KM, Hoven AD. Chloroquine and hydroxychloroquine as inhibitors of human immunodeficiency virus (HIV-1) activity. Curr Pharm Des. 2004;10:2643-2648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3242] [Cited by in F6Publishing: 3180] [Article Influence: 795.0] [Reference Citation Analysis (0)] |
| 32. | Fiolet T, Guihur A, Rebeaud ME, Mulot M, Peiffer-Smadja N, Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:19-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 190] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 33. | Kim MS, An MH, Kim WJ, Hwang TH. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis. PLoS Med. 2020;17:e1003501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 34. | Cohen MS. Hydroxychloroquine for the Prevention of Covid-19 - Searching for Evidence. N Engl J Med. 2020;383:585-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 35. | Blaising J, Polyak SJ, Pécheur EI. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 36. | Beigel JH, Nam HH, Adams PL, Krafft A, Ince WL, El-Kamary SS, Sims AC. Advances in respiratory virus therapeutics - A meeting report from the 6th isirv Antiviral Group conference. Antiviral Res. 2019;167:45-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 37. | Guan WD, Du QL, Jiang HM, Zhao JC, Yang ZF. Comparison of inhibitory effects of arbidol and Lianhuaqingwen Capsules on Middle East respiratory syndrome coronavirus in vitro and in vivo. Guangdong Yixue. 2018;39: 3454-3458. [DOI] [Cited in This Article: ] |
| 38. | China News Network. Li Lanjuan's team: Abidor and darunavir can effectively inhibit coronavirus. February 11, 2020. [cited 10 March 2021]. Available from: http://www.sd.chinanews.com/2/2020/0205/70145.html. [Cited in This Article: ] |
| 39. | Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 326] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 40. | Wang Y, Fan G, Salam A, Horby P, Hayden FG, Chen C, Pan J, Zheng J, Lu B, Guo L, Wang C, Cao B. Comparative Effectiveness of Combined Favipiravir and Oseltamivir Therapy Versus Oseltamivir Monotherapy in Critically Ill Patients With Influenza Virus Infection. J Infect Dis. 2020;221:1688-1698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 41. | Delang L, Abdelnabi R, Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res. 2018;153:85-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 42. | China News Network. Ministry of science and technology: some therapeutic drugs have initially shown good efficacy. February 15, 2020. [cited 10 March 2021]. Available from: https://www.sohu.com/a/373304259_123753. [Cited in This Article: ] |
| 43. | Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, Chen S, Chen B, Lu M, Luo Y, Ju L, Zhang J, Wang X. Favipiravir vs Arbidol for COVID-19: A Randomized Clinical Trial. 2020 Preprint. Available from: medRxiv:20037432. [DOI] [Cited in This Article: ] |
| 44. | Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, Van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, McMullan LK, Chen SS, Fearns R, Swaminathan S, Mayers DL, Spiropoulou CF, Lee WA, Nichol ST, Cihlar T, Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 965] [Cited by in F6Publishing: 1012] [Article Influence: 126.5] [Reference Citation Analysis (0)] |
| 45. | Li H, Wang YM, Xu JY, Cao B. [Potential antiviral therapeutics for 2019 Novel Coronavirus]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:170-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 44] [Reference Citation Analysis (0)] |
| 46. | Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1011] [Cited by in F6Publishing: 1065] [Article Influence: 177.5] [Reference Citation Analysis (0)] |
| 47. | Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana DK, Rodriguez-Morales AJ. Coronavirus Disease 2019-COVID-19. Clin Microbiol Rev. 2020;33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 650] [Cited by in F6Publishing: 525] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 48. | Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2337] [Cited by in F6Publishing: 2357] [Article Influence: 589.3] [Reference Citation Analysis (0)] |
| 49. | Gilead. Gilead Sciences Statement on Positive Data Emerging From National Institute of Allergy and Infectious Diseases’ Study of Investigational Antiviral Remdesivir for COVID-19. April 29, 2020. [cited 10 March 2021]. Available from: https://www.gilead.com/news-and-press/press-room/press-releases/2020/4/gilead-sciences-statement-on-positive-data-emerging-from-national-institute-of-allergy-and-infectious-diseases-study-of-investigational-antiviral-rem. [Cited in This Article: ] |
| 50. | Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382:2327-2336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1800] [Cited by in F6Publishing: 1809] [Article Influence: 452.3] [Reference Citation Analysis (0)] |
| 51. | World Health Organization. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: Interim Guidance. [cited 10 March 2021]. Available from: https://apps.who.int/iris/handle/10665/331446. [Cited in This Article: ] |
| 52. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13871] [Cited by in F6Publishing: 12365] [Article Influence: 3091.3] [Reference Citation Analysis (1)] |
| 53. | Guan WD, Mok CK, Chen ZL, Feng LQ, Li ZT, Huang JC, Ke CW, Deng X, Ling Y, Wu SG, Niu XF, Perera RA, Da Xu Y, Zhao J, Zhang LQ, Li YM, Chen RC, Peiris M, Chen L, Zhong NS. Characteristics of Traveler with Middle East Respiratory Syndrome, China, 2015. Emerg Infect Dis. 2015;21:2278-2280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Liu XX, Shen PX, Du S, Yang Y, Shu YQ, Bian Y, Tong RS, Yan JF, He L, Long EW, Chen M. Rational Use and Pharmaceutical Care of Thymosin Immunomodulators for Novel Coronavirus Pneumonia. Yixue Daobao. 2020;39:451-458. [Cited in This Article: ] |
| 55. | Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3:722-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 840] [Cited by in F6Publishing: 867] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 56. | Yang BF, Chen JG. Pharmacology. 9th ed. People's Health Press, 2018. [Cited in This Article: ] |
| 57. | Li MH, Xie Y. Expert consensus on clinical treatment of adverse reactions in patients with chronic viral hepatitis treated with interferon α. Zhonghua Shiyan He Linchuang Ganranbing Zazhi. 2014;8:108-113. [Cited in This Article: ] |
| 58. | Nijland HM, L'homme RF, Rongen GA, van Uden P, van Crevel R, Boeree MJ, Aarnoutse RE, Koopmans PP, Burger DM. High incidence of adverse events in healthy volunteers receiving rifampicin and adjusted doses of lopinavir/ritonavir tablets. AIDS. 2008;22:931-935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 59. | Greffrath WP, du Plessis JM, Viljoen M, Cockeran M. Hypertriglyceridaemia and the risk of pancreatitis six months post lopinavir/ritonavir initiation. South Afr J HIV Med. 2018;19:766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Gross AE, Bryson ML. Oral Ribavirin for the Treatment of Noninfluenza Respiratory Viral Infections: A Systematic Review. Ann Pharmacother. 2015;49:1125-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Kiselev OI, Maleev VV, Deeva EG, Leneva IA, Selkova EP, Osipova EA, Obukhov AA, Nadorov SA, Kulikova EV. [Clinical efficacy of arbidol (umifenovir) in the therapy of influenza in adults: preliminary results of the multicenter double-blind randomized placebo-controlled study ARBITR]. Ter Arkh. 2015;87:88-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Xu Z, Xinbo Z, Wu Z, Zhou LX. A new antiviral drug-Favipiravir. Linchuang Yiyao Zazhi. 2015;13:16-20. [DOI] [Cited in This Article: ] |
| 63. | Kan R, Zhang JT, Wang YH, Liu SM, Zuo H. A case of adverse reaction of subcutaneous injection of thymosin α. Yixue Lilun Yu Shijian. 2010;23:1449. [DOI] [Cited in This Article: ] |
| 64. | National Institutes of Health. COVID-19 Treatment Guidelines. [cited 10 March 2021]. Available from: https://www.covid19treatmentguidelines.nih.gov/. [Cited in This Article: ] |
| 65. | National Health Commission. National Administration of Traditional Chinese Medicine of the P. Guidance for Corona Virus Disease 2019: Prevention, Control, Diagnosis and Management. People's Medical Publishing House, 2020. [Cited in This Article: ] |
| 66. | NICE. COVID-19 rapid guideline: managing COVID-19. [cited 10 March 2021]. Available from: https://www.nice.org.uk/guidance/ng191. [Cited in This Article: ] |
| 67. | Rochwerg B, Siemieniuk RA, Agoritsas T, Lamontagne F, Askie L, Lytvyn L, Agarwal A, Leo YS, Macdonald H, Zeng L, Amin W, Burhan E, Bausch FJ, Calfee CS, Cecconi M, Chanda D, Du B, Geduld H, Gee P, Harley N, Hashimi M, Hunt B, Kabra SK, Kanda S, Kawano-Dourado L, Kim YJ, Kissoon N, Kwizera A, Mahaka I, Manai H, Mino G, Nsutebu E, Pshenichnaya N, Qadir N, Sabzwari S, Sarin R, Shankar-Hari M, Sharland M, Shen Y, Ranganathan SS, Souza JP, Stegemann M, De Sutter A, Ugarte S, Venkatapuram S, Dat VQ, Vuyiseka D, Wijewickrama A, Maguire B, Zeraatkar D, Bartoszko JJ, Ge L, Brignardello-Petersen R, Owen A, Guyatt G, Diaz J, Jacobs M, Vandvik PO. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 472] [Article Influence: 118.0] [Reference Citation Analysis (0)] |