- Department of Neurosurgery, Max Hospital, Patparganj, New Delhi, India.
Correspondence Address:
Hershdeep Singh, Department of Neurosurgery, Max Hospital, Patparganj, New Delhi, India.
DOI:10.25259/SNI_772_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hershdeep Singh, Sanjeev Dua, Amitabh Goel, Anil Dhar, Vikram Bhadauria, Amit Garg, Vikrant Katyar, Sumit Sharma, Aditi Shukla. Rhino-orbital-cerebral mucormycosis in times of COVID-19: A neurosurgical experience. 25-Oct-2021;12:538
How to cite this URL: Hershdeep Singh, Sanjeev Dua, Amitabh Goel, Anil Dhar, Vikram Bhadauria, Amit Garg, Vikrant Katyar, Sumit Sharma, Aditi Shukla. Rhino-orbital-cerebral mucormycosis in times of COVID-19: A neurosurgical experience. 25-Oct-2021;12:538. Available from: https://surgicalneurologyint.com/surgicalint-articles/11199/
Abstract
Background: The gravity of “second wave” of COVID-19 has effaced many new challenges in India; mucormycosis being a recent one. Diabetes mellitus (DM) is a known significant risk factor for mucormycosis. Here, we present our experience with rhino-orbital-cerebral mucormycosis (ROCM) during the “second wave of COVID-19” at a tertiary health care centre in North India.
Methods: This case series includes four cases of ROCM that were managed by our neurosurgical team in view ofcerebral involvement.
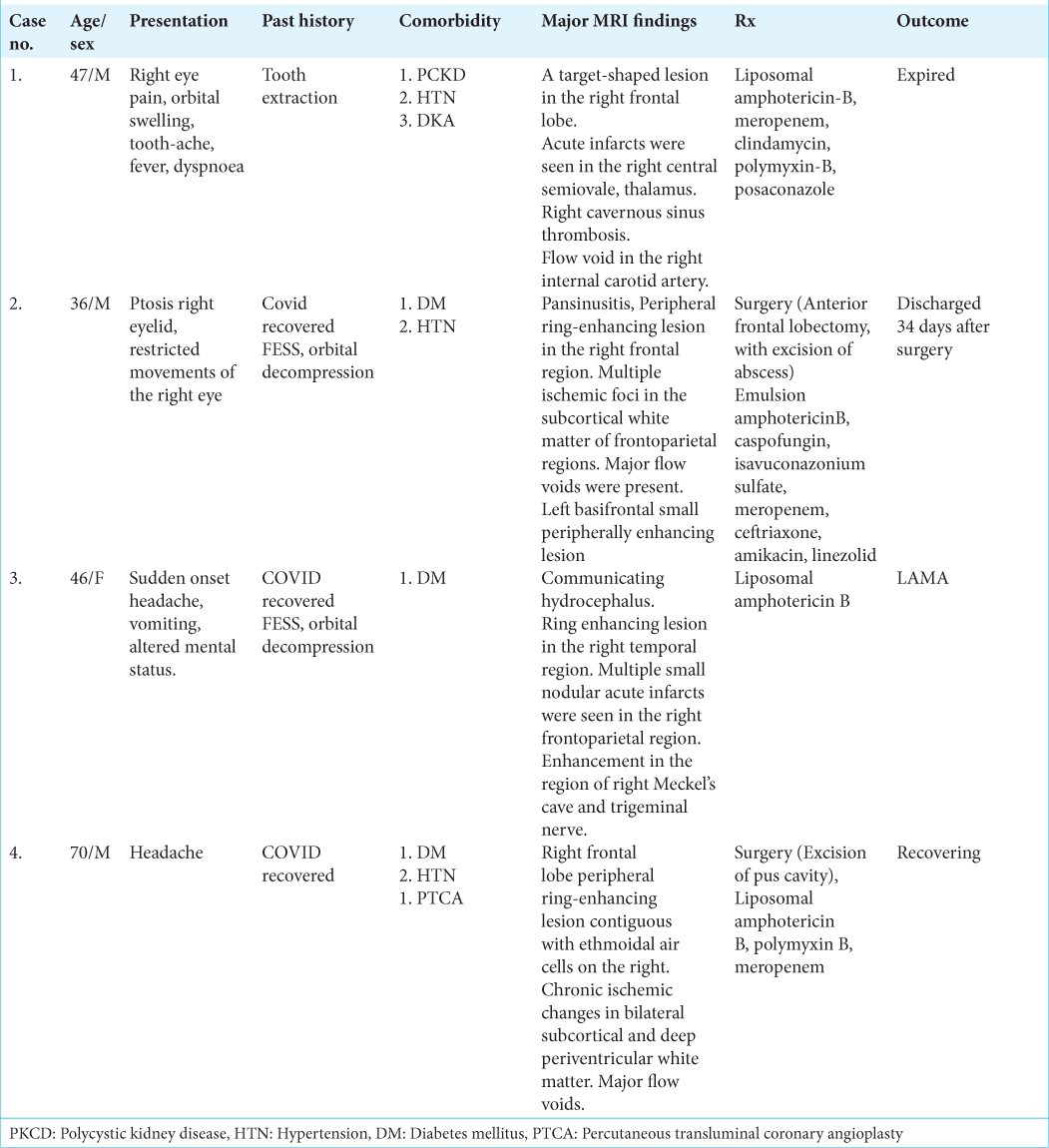
Results: All the cases with an exception of one (Case 1), had a history of treatment for COVID-19 pneumonia. Case 2, 3 had undergone functional endoscopic sinus surgery (FESS) and orbital decompression before the onset of cerebral involvement; Case 4 underwent FESS and cranial surgery in the same sitting. All the patients had a history of DM and all the cases treated for COVID-19 pneumonia had a history of treatment with corticosteroids. Two patients underwent surgery with the exception of one patient, who did not provide consent for the same. One patient expired before surgical excision could be attempted.
Conclusion: Regular and intensive follow-up is the key in swift detection and management of ROCM in post-COVID patients. While surgical excision is advisable in the fungal lesion, it must be borne in mind that radical excision of cerebral lesions is associated with morbidity, delayed recovery, and prolonged ICU stay. Culture and sensitivity-based antibiotics should be used judiciously as fever is a common postoperative complication. Blood sugar monitoring and control of DM are paramount in this condition. Steroids should be avoided in the management of cerebral edema with judicious use of hypertonic saline or mannitol.
Keywords: Abscess, COVID-19, Diabetes, Mucormycosis, Rhino-orbital-cerebral mucormycosis, Steroids
INTRODUCTION
COVID-19 has affected the global population physically, mentally, and economically. India is one of the worst affected countries in the world. The “second wave” has been especially severe, causing much morbidity and mortality.
With an increased number of patients, newer data on complications after recovery from COVID-19 pneumonia have come forth. A few days after being discharged, the COVID-19 recovered patients are being brought to the emergency room with orbital swelling, discoloration, nasal discharge, headache, and fever, among other symptoms. We are treating such cases of mucormycosis with cerebral extension in our daily practice.
We share our experience, a series of four cases, with rhino-orbital-cerebral mucormycosis (ROCM) and the challenges it offers, especially concerning a COVID-19 recovered patient from a neurosurgical standpoint.
CASE DESCRIPTION
Case 1
A 47-year-old man, a known case of polycystic kidney disease (PCKD) and hypertension (HTN), was admitted with complaints of toothache, right eye pain, orbital swelling for 3 weeks, fever, and dyspnea of 1-day duration. The patient had a history of tooth extraction 10 days before the onset of symptoms. The patient presented with GCS of E4V5M6 with no focal neurological deficit. CBNAAT examination done at admission was negative for COVID-19.
CE-MRI done 6 days before admission was suggestive of mucormycosis involving right paranasal sinus extending to right orbit, with a black turbinate sign along with cerebral atrophy with white matter changes. His right eye vision deteriorated rapidly; hence functional endoscopic sinus surgery (FESS) with orbital decompression was done on an emergency basis. Histopathology thus obtained was suggestive of mucormycosis, which was concurrent with the findings of the nasal swab.
The patient had one episode of secondarily generalized seizures, following which the patient became drowsy (GCSE3V4M6) and developed left-sided weakness power (upper limb 1/5, lower limb 3/5). Restricted movement of the right eye was also observed. Fundus examination revealed temporal disc pallor with featureless retina.
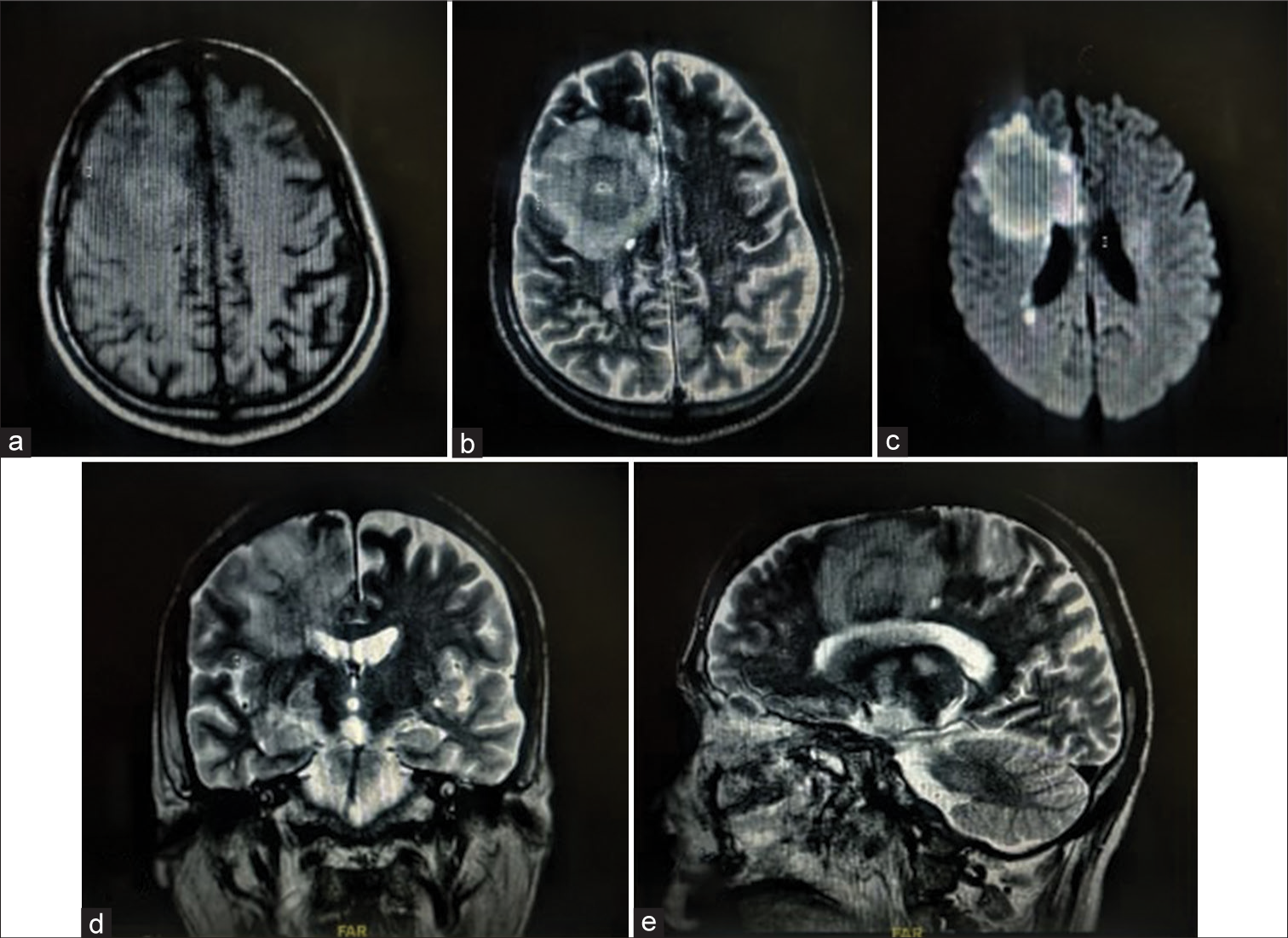
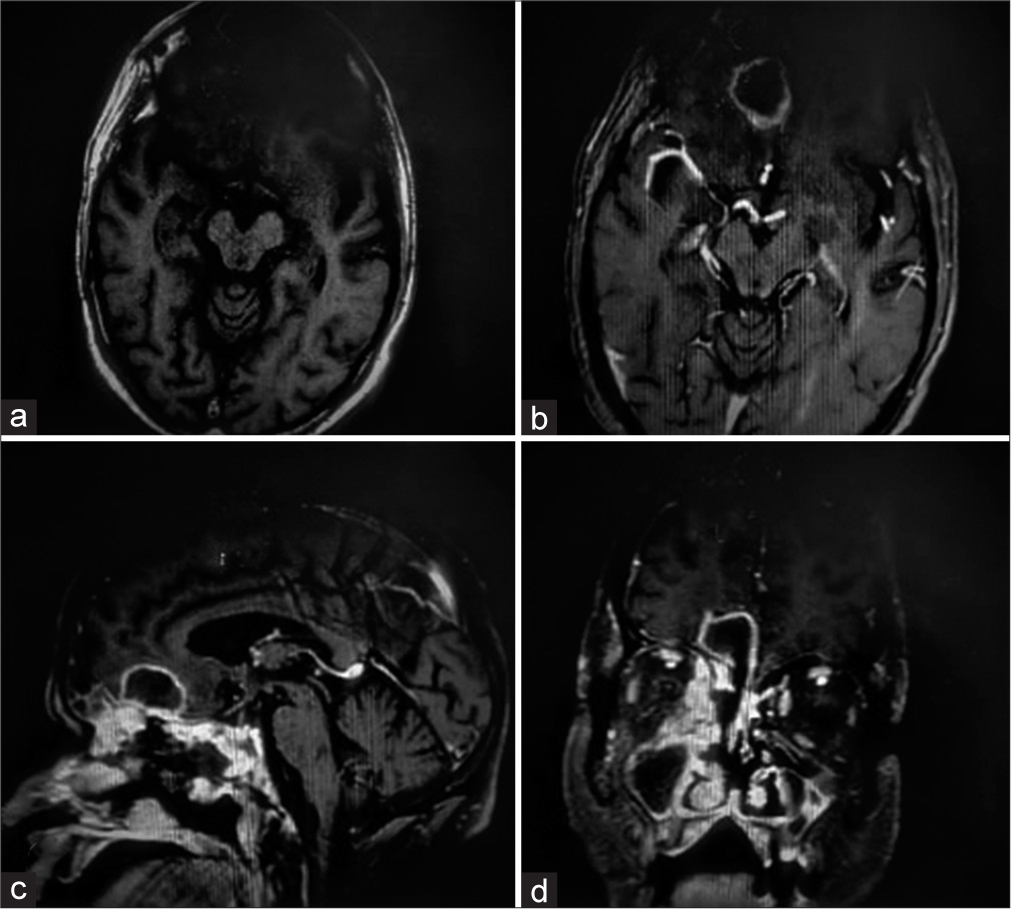
Contrast MRI brain was planned but could not be obtained due to poor renal function (dialyzed thrice). Non-Contrast MRI brain [
During hospitalization, the patient developed sepsis with blood cultures revealing the growth of Kluyvera intermedia and Pseudomonas aeruginosa. After blood culture analysis, he was treated with amphotericin-B, meropenem, clindamycin, polymyxin-B, and posaconazole. The patient’s general condition deteriorated rapidly thus, he was taken up on supportive measures, including mechanical ventilation and inotropes. He expired soon before surgical excision of the intracranial lesion could be performed. (Refer to [
Case 2
A 36-year-old male, with a history of diabetes mellitus (DM) and HTN, post COVID-19 pneumonia, was readmitted 10 days after discharge with complaints of ptosis and restricted movements of the right eye. The patient was conscious and oriented to time, place, and person with no motor deficits, however, the light reaction of the right eye was sluggish.
The patient had undergone bilateral FESS and orbital decompression during the previous admission.
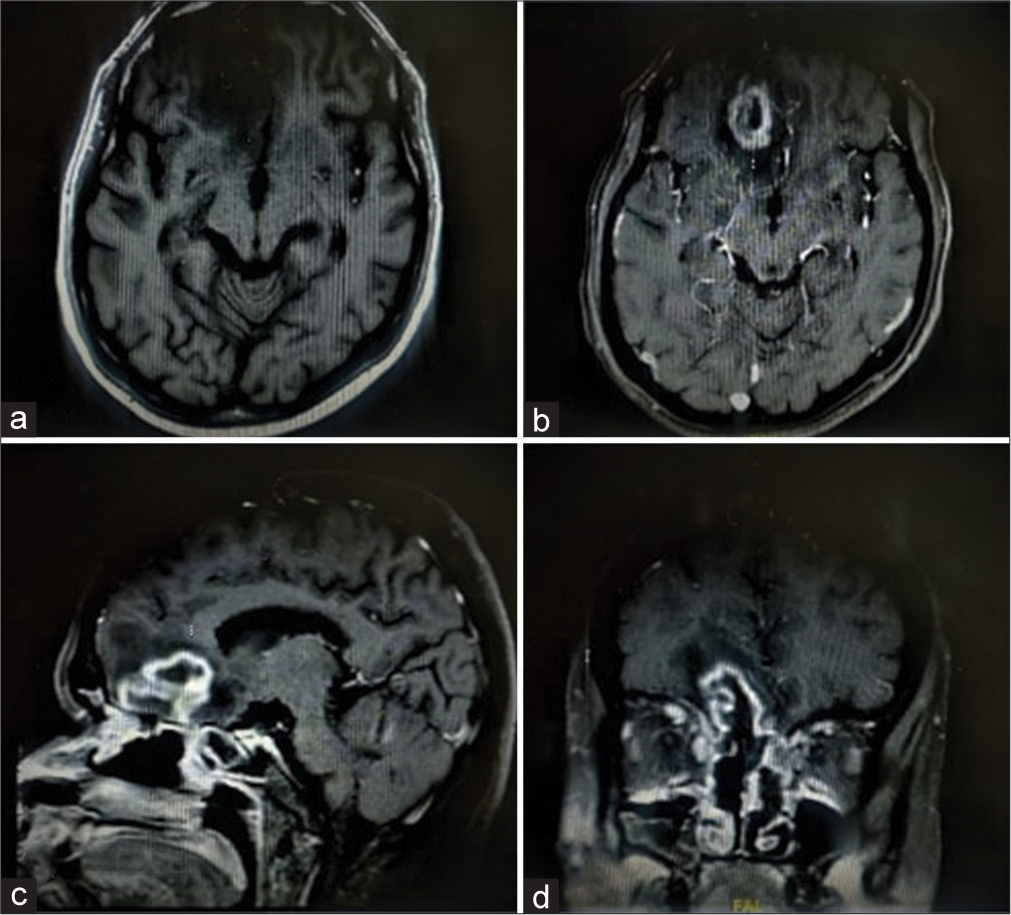
CE-MRI brain [
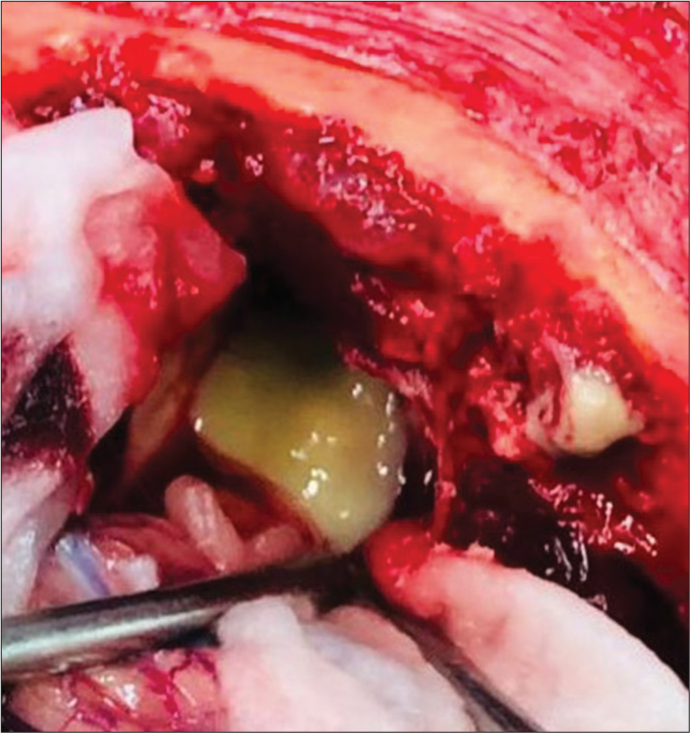
The patient was taken up for surgery, and a right frontal craniotomy was done. Lobectomy was performed in the anterior frontal area containing the abscess formed of thick greenish pus. All pus and necrotic tissue material was excised and sent for biopsy, which consequently, on histopathological examination was suggestive of mucormycosis.
During the immediate postoperative period, patient developed left-sided weakness (power 2/5), which gradually improved throughout treatment. It was noted that the patient was allergic to liposomal amphotericin B, therefore, he was started on emulsion Amphotericin B, to which he responded well. Urine culture done for fever episodes revealed Enterococcus fecium, which was managed by upgrading antibiotics based on culture sensitivity reports.
The patient was managed in ICU for 23 days and was discharged 34 days after surgery. At discharge patient was E4V5M6, with left-sided power of 3/5 and grip weakness. (Refer to [
Case 3
A 46-year-old female, known case of DM, recovered from COVID-19 pneumonia was readmitted a week after discharge with complaints of sudden onset headache, vomiting and altered mental status. FESS and orbital decompression had been performed for fungal sinusitis during the previous admission. The histopathology examination revealed fungal elements indicative of mucormycosis. The patient was discharged 8 days after surgery.
On presentation to the hospital, she was drowsy with GCS of E2V4M6 and had a left-sided weakness (power of 3/5).
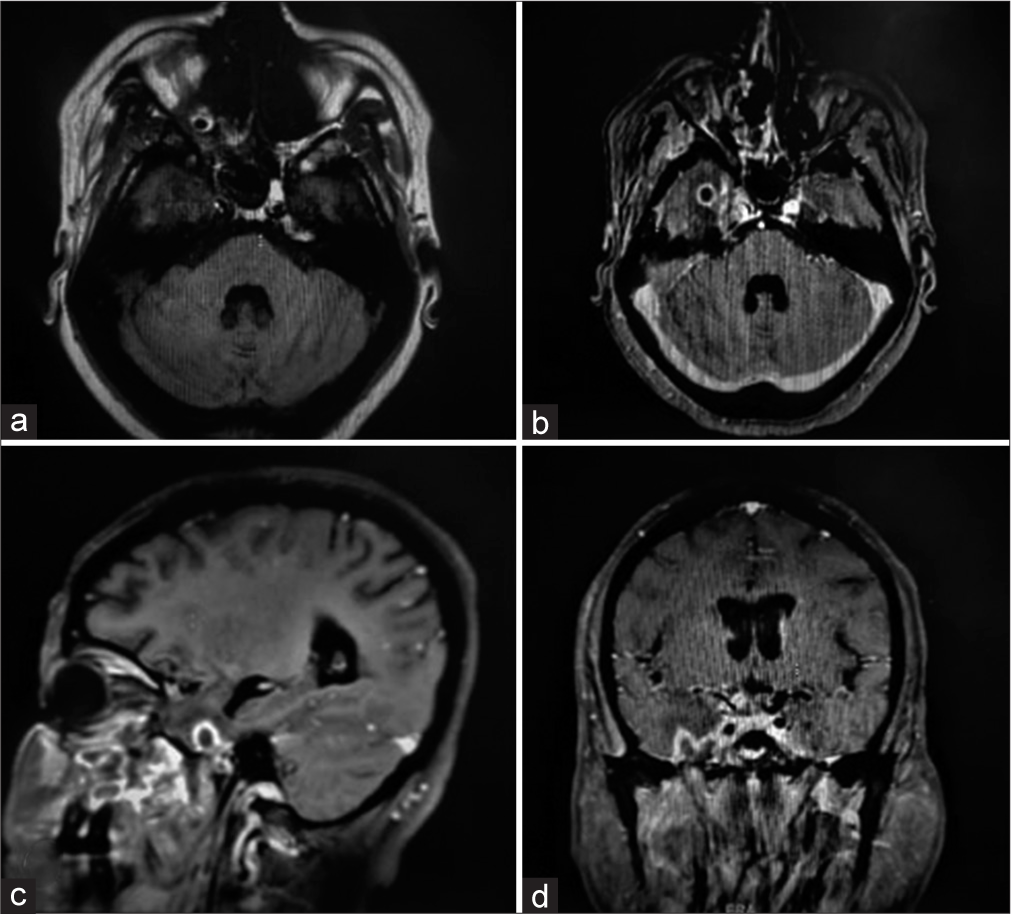
CE-MRI brain [
Surgical excision of the lesion was planned; however, consent wasn’t provided by patient as well as the attendants, hence leaving the hospital against medical advice. (Refer to [
Case 4
A 70-year-old male, recovered from COVID pneumonia, was admitted with chief complaints of headache for a duration of 2 weeks. On examination, green nasal discharge was seen in the right nasal cavity. The patient had a history of DM, HTN and post percutaneous transluminal coronary angioplasty.
The patient had been discharged after undergoing treatment for COVID-19 pneumonia 2 months back. He was treated with corticosteroids in his weeklong hospitalization and was prescribed Dexamethasone in tapering doses over a period of 6 days.
CE- MRI brain [
Neurosurgical and ENT surgeons teamed up to take up the surgical challenge. Abscess excision with FESS and orbital decompression was done in a single sitting.
Right Frontal craniotomy was performed and abscess cavity was accessed via sub-frontal approach. After the evacuation of greenis pus [
Throat culture done for intermittent fever revealed Klebsiella pneumoniae. Culture-appropriate antibiotics were started, and the patient responded positively to the treatment. (Refer to [
DISCUSSION
Mucormycosis is an invasive infection caused by fungi of the order Mucorales, which includes Rhizopus, Mucor, Rhizomucor, Cunninghamella, and Absidia[
Epidemiologically, this disease is 70 times more common in India than rest of the world.[
DM is the most significant risk factor in developing countries like India, while in the developed world, malignancy is a huge predisposing factor to mucormycosis. These predisposing factors weaken the immunity, thereby making the patient susceptible. According to some studies, the risk factors may also determine the clinical manifestation and systemic involvement, with DM more likely to predispose to ROCM and malignancy associated with pulmonary involvement.[
Angioinvasion is the hallmark of mucormycosis[
The initial paranasal sinus involvement is not characteristically distinguishable from a more common sinusitis, with usual symptoms of pain, congestion, and altered sense of smell. The disease is notoriously rapid in its progression, advancing to involve the orbit which can clinically present as swelling, restricted eye movements, and discoloration. Deteriorating vision is a significant sign of involvement of the optic nerve. Cerebral involvement can present clinically with focal seizures, neurological deficits, and altered mental status however, it is important to know that the cavernous sinus is the first structure to be involved in CNS, manifesting with the decreasing function of its constituent structures (i.e.,CN III, IV, VI, V1, and V2).[
Radioimaging such as MRI usually reveals infarcts and abscesses that frequently involve basal ganglia.[
A review study done by Singh et al. in May 2021 reported a total of 101 cases of mucormycosis with COVID-19 globally, out of which 82 were from Indian strata. As per the review, 22 patients had CNS involvement. Diabetes and steroid administration were associated with mucormycosis in 80% and 76.3% cases, respectively.[
Our experience
We encountered four patients of ROCM with cerebral involvement at our institution. ([
The other cases (2, 3, and 4) had been under hospital care for COVID-19 treatment. Case 2 and 3 had undergone FESS and orbital decompression for rhino-orbital mucormycosis during the previous admissions and Case 4 underwent craniotomy for abscess excision and FESS in the same sitting.
With a history of 2 previous admissions, Case 2 was initially discharged after treatment for COVID-19; however, on the 3rd day of discharge, he was admitted again with features of ROCM, for which FESS and orbital decompression were done. He was discharged on the 4th post-operative day but had to be readmitted, on the 6th day of discharge in view of progression of ROCM with the involvement of the right frontal lobe.
Case 3 had a history of a single previous admission where initially, she was treated at another facility for 16 days followed by a referral to our institution. The management plan included treatment of COVID-19 pneumonia and FESS with orbital decompression. She was discharged on the 8th postoperative day but returned a week later with altered mental status.
Case 4 was treated at another facility for COVID around 2 months back for around a week and discharged following which e patient developed symptoms of ROCM in 2 months.
It is interesting that unlike cases 2 and 3, patient 4 had a relatively more extended, uneventful period of approximately 2 months. It alerts us to be cautious in following up on post-COVID patients, especially with predisposing factors, even during a later stage.
All patients in our study had a history of DM. While case 1 had controlled DM (Hb A1c - 5.6%), patients 2, 3, and 4 had poorly controlled diabetes with Hb A1C of 10.4%, 9.7%, and 8.0%, respectively. Thus, impaired blood sugar control is paramount during management, requiring specialist guidance.
Among other comorbidities, patient 1 was also known to have PCKD, making treatment trickier. The patient needed regular dialysis, and a more comprehensive CE-MRI brain could not be performed. Patients 3 and 4 were known cases of HTN, which was controlled on medical treatment. Intraoperative and postoperative maintenance of blood pressure in an ideal range is a must to attain a positive clinical outcome.
Common findings included target-shaped, right-sided lesions, areas of infarct, and flow voids. in all cases. The frontal lobe was the commonly involved region except for case 3, where the lesion was located in the right temporal lobe. Cavernous sinus thrombosis was observed in one case (Case 1). Only in case 3, Meckel’s cave and trigeminal nerve involvement could be appreciated on MRI. On histopathology, in all four patients, nonseptate fungal hyphae were seen on PAS and silver stain, while fungal culture showed growth of R. oryzae in only cases 1 and 3. Intravenous steroids had been administered to Cases 2, 3, and 4 during their treatment for COVID-19 pneumonia. Upon discharge, oral steroids had been prescribed, tapered typically over 1 week period.
Amphotericin B is the drug of choice for mucormycosis, liposomal amphotericin B being the favored injectable formulation. In our experience, one of the patients (case 2) had a hypersensitive reaction to it; hence amphotericin B emulsion was administered, which was tolerated well by the patient. It is crucial to bore in mind here that antibiotics need to be used judiciously in such cases, based strictly on cultures.
Surgical excision of the infected tissue remains the primary treatment of choice for mucormycosis.[
CONCLUSION
Treating COVID-19 pneumonia patients is a challenge, especially with a background of DM which is a strong predisposing factor for mucormycosis. Despite recovery, follow-up visits of such patients should be comprehensive with a watchfulness over, even a trivial complaint such as toothache. This can help clinicians in early detection and proactive treatment of the infection. The authors recommend expedited surgical excision of a localized fungal lesion while keeping in mind that radical excision of cerebral lesions is associated with morbidity, delayed recovery, and prolonged ICU stay. Culture and sensitivity-based antibiotics should be used judiciously and steroids must be used with reasonable caution, especially in people with diabetes while keeping the diabetes under control. Cerebral edema, if present, should be managed with hypertonic saline or mannitol with avoidance of steroids. Postoperative fever is a common occurrence but overall recovery is uneventful.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
References
1. Ben-Ami R, Luna M, Lewis RE, Walsh TJ, Kontoyiannis DP. A clinicopathological study of pulmonary mucormycosis in cancer patients: Extensive angioinvasion but limited inflammatory response. J Infect. 2009. 59: 134-8
2. Chakrabarti ASood PDenning D. Estimating Fungal Infection Burden in India: Mucormycosis Burden as a Case Study. Available from: https://www.gaffi.org/wp-content/uploads/p1044.pdf [Last accessed on 2021 Aug 12].
3. Jimenez C, Lumbreras C, Aguado JM. Successful treatment of Mucor infection after liver or pancreas-kidney transplantation. Transplantation. 2002. 73: 476-80
4. Kathuria MK, Gupta RK, Gupta RK, Lufkin RB.editors. Fungal infections. MR Imaging and Spectroscopy of Central Nervous System Infections. New York: Kluwer Press; 2001. p. 177-203
5. Kontoyiannis DP, Wessel VC, Bodey GP, Rolston KV. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis. 2000. 30: 851-6
6. Mantadakis E, Samonis G. Clinical presentation of zygomycosis. Clin Microbiol Infect. 2009. 15: 15-20
7. Melsom SM, Khangure MS. Craniofacial mucormycosis following assault: An unusual presentation of an unusual disease. Australas Radiol. 2000. 44: 104-6
8. Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: Analysis of multicenter prospective antifungal therapy (PATH) alliance registry. Clin Infect Dis. 2009. 48: 265-73
9. Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi (Basel). 2019. 5: 26
10. Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin Infect Dis. 2005. 41: 634-53
11. Rumboldt Z, Castillo M. Indolent intracranial mucormycosis: Case report. J Neuroradiol. 2002. 23: 932-4
12. Siegal JA, Cacayorinb ED, Nassif AS, Rizk D, Galambos C, Levy B. Cerebral mucormycosis: Proton MR spectroscopy and MR imaging. Magn Reson Imaging. 2000. 18: 915-20
13. Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021. 15: 102146
14. Singh V, Singh M, Joshi C, Sangwan J. Rhinocerebral mucormycosis in a patient with Type 1 diabetes presenting as toothache: A case report from Himalayan region of India. BMJ Case Rep. 2013. p.
15. Spellberg B, Edwards J, Ibrahim A. Novel per-spectives on mucormycosis: Pathophysiology presentation, and management. Clin Microbiol Rev. 2005. 18: 556-69
16. Sugar AM, Mandell GL, Bennett JE, Dolin R.editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. New York, United States: Churchill Livingstone; 2000. p.
17. Turner JH, Soudry E, Nayak JV, Hwang PH. Survival outcomes in acute invasive fungal sinusitis: A systematic review and quantitative synthesis of published evidence. Laryngoscope. 2013. 123: 1112-8