- 1Department of Environmental and Prevention Sciences, University of Ferrara, Ferrara, Italy
- 2Local Health Unit of Pescara, Pescara, Italy
- 3Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy
Current data suggest that SARS-CoV-2 reinfections are rare. Uncertainties remain, however, on the duration of the natural immunity, its protection against Omicron variant, and on the impact of vaccination to reduce reinfection rates. In this retrospective cohort analysis of the entire population of an Italian region, we followed 1,293,941 subjects from the beginning of the pandemic to the current scenario of Omicron predominance (up to mid-February 2022). After an average of 277 days, we recorded 729 reinfections among 119,266 previously infected subjects (overall rate: 6.1‰), eight COVID-19-related hospitalizations (7/100,000), and two deaths. Importantly, the incidence of reinfection did not vary substantially over time: after 18–22 months from the primary infection, the reinfection rate was still 6.7‰, suggesting that protection conferred by natural immunity may last beyond 12 months. The risk of reinfection was significantly higher among females, unvaccinated subjects, and during the Omicron wave.
Introduction
After the first documented case in August 2020 in Hong Kong (1), a number of field studies estimated the rate of SARS-CoV-2 reinfections after a primary episode (2, 3). Although the reported rates have been consistently low, the results differed according to the adopted definition of reinfection, setting, pandemic period, and follow-up duration, and uncertainties remain on the duration of the natural immunity and the impact of vaccination to decrease reinfection rates (4–6). In fact, some evidence on the degree of protection exerted by the existing vaccines against the BA.1/B.1.1.529 (Omicron) variant is available, but still preliminary (3, 7). We performed a retrospective cohort study on the entire population of an Italian region in order to estimate the incidence of SARS-CoV-2 reinfections and COVID-19 according to vaccination status, predominant viral strain, and time after primary infection.
Methods
We included all residents in the Abruzzo Region, Italy with ≥1 positive nasopharyngeal swab detected through RT-PCR by the regional-accredited laboratories, from the start of the pandemic (March 2, 2020) up to January 4, 2022. On February 18, 2022 (to allow ≥45 days of follow-up), we extracted all data of the official vaccination (Regional database “Vaccinazioni Anti-COVID-19”), COVID-19 (“Surveillance COVID-19 Platform”), demographic (Italian “Anagrafica sanitaria”), hospital (Italian “File A - SDO”), and co-pay exemption (Italian “Esenzioni Ticket”) datasets of the National Healthcare System, merging individual information through encrypted fiscal code (8). Since the high-quality National Tax Registry has been recently used for fiscal codes generation and input in the main above databases, we used a deterministic linkage.
A reinfection was defined by the presence of two positive RT-PCR samples detected ≥45 days apart with ≥1 intermediate negative RT-PCR test (9, 10). Subjects were classified as “vaccinated” if they received ≥1 dose of BNT162b2, ChAdOx1 nCoV-19, mRNA-1273 or JNJ-78436735, ≥14 days before the reinfection. The follow-up started from the date of the first positive swab and ended the 1st day of reinfection, or was censored on February 18, 2022.
To account for some of the main potential confounders of the association between vaccination and COVID-19 or death (11), we used (a) the COVID-19 database, (b) the co-pay exemption database, and (c) the administrative discharge abstracts of the last 10 years to extract the following conditions of each resident—diabetes (ICD-9-CM codes in any diagnosis field-−250.xx); hypertension (401.xx-405.xx); major cardiovascular or cerebrovascular diseases (410.xx-412.xx; 414.xx-415.xx; 428.xx or 433.xx-436.xx); chronic obstructive pulmonary diseases—COPD (491.xx-493.xx); kidney diseases (580.xx-589.xx); cancer (140.xx-172.xx or 174.xx-208.xx).
The proportion of reinfections was computed in the total sample, and by demographic and clinical characteristics, time after primary infection (0–5, 6–11, 12–17, 18–22 months), and predominant circulating variant (pre-Omicron vs. Omicron; from December 28, 2021 on). Cox proportional hazard analysis was then used to compute the relative hazards of reinfection, after adjusting for age, gender, vaccine status (zero, one, two or more doses), severe COVID-19 after the first infection, and comorbidities, all included a priori. Schoenfeld's test was used to assess the validity of proportional hazards assumption. A two-sided p-value < 0.05 was considered significant. Stata, version 13.1 (Stata Corp., College Station, TX, 2014) was used for all analyses.
Results
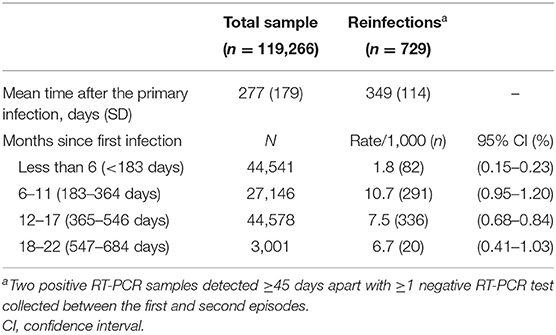
From the start of the pandemic, a total of 251,047 infections were detected among the 1,293,941 residents in the Abruzzo Region. After the exclusions of the subjects with a follow-up <45 days, or lacking negative intermediate swabs, a total of 119,266 subjects with primary infection were included in the analysis. The average time after the primary infection was 277 days (for a total of 90,557 person-years of follow-up); 3,001 subjects had a follow-up longer than 18 months (Table 1).
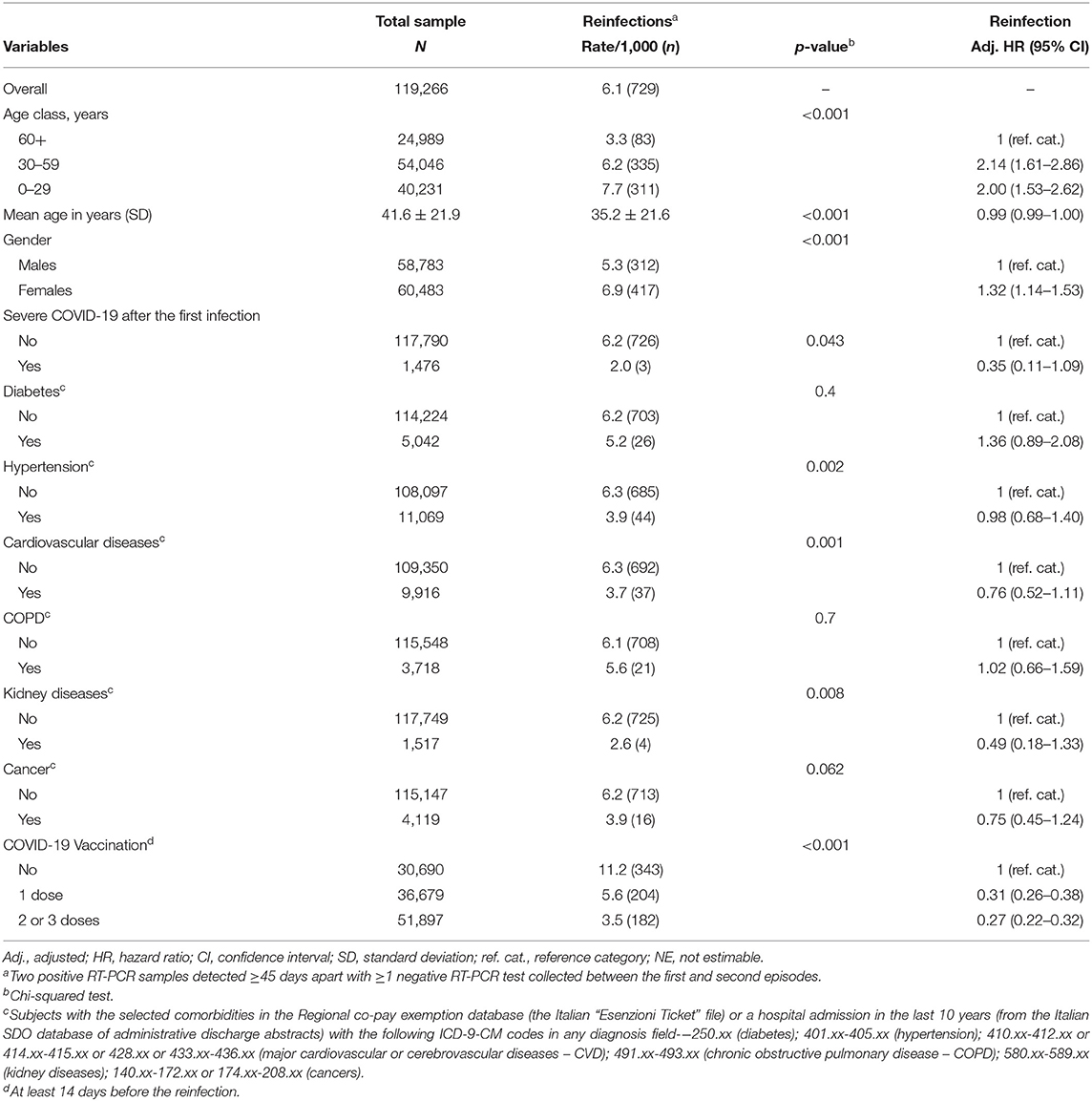
Overall, the incidence of reinfection was 6.1‰ (n = 729). Eight subjects were hospitalized due to COVID-19 (8/119,266: 7/100,000; none among the youngest), and two died (a 73-year old female, with major cardiovascular disease and diabetes, and a 79-year old male, with cancer, COPD, and kidney disease).
As shown in Table 2, the reinfection rate was significantly higher among females, younger subjects, and unvaccinated individuals (11.2 vs. 3.5‰ among those who received ≥2 vaccine doses). Moreover, a markedly higher rate of reinfections was recorded during the first 54 days of the Omicron wave (n = 613; 11.4 per day) than during the 317 days of the pre-Omicron period (n = 116; 0.4 per day). In contrast, the incidence of reinfection did not vary substantially by baseline comorbidities and over time: after 18 or more months from the primary infection (up to 22 months), the reinfection rate was still 6.7‰. The multivariable analysis (the Cox proportional hazards model adjusted for all the recorded variables, included a priori) confirmed univariate results (Table 2). When the analysis was stratified by circulating variant, the effectiveness of ≥2 vaccine doses was slightly higher in pre-Omicron (adjusted hazard ratio—HR vs. unvaccinated: 0.20; 95% confidence interval: 0.12–0.33) than the Omicron wave (HR: 0.28; 0.23–0.35 – data not shown). Although the incidence of reinfection was lower among those with a history of severe COVID-19 manifestation, the numbers were too scarce to detect a significant difference at multivariable analysis.
Discussion
This study confirms and expands previous findings reporting a low risk of SARS-CoV-2 reinfection, and a very low risk of severe or lethal COVID-19 for those who recovered from primary infection (3, 12, 13), suggesting that the protection conferred by the natural immunity lasts beyond 12 months. Although the marked increase of the reinfection rates during the Omicron wave is concerning, the risk of a secondary severe disease or death remained close to zero. Therefore, despite the vaccines were able to significantly reduce the likelihood of reinfection in both pre-Omicron and Omicron waves, the risk-benefit profile of multiple vaccine doses for this population should be carefully evaluated.
Interestingly, reinfection seemed to be more frequent among females, which might be a consequence of the lower attitude to routine diagnostic testing of the males (14), as well as the higher work-related exposure to the contagion of the females (15).
Our estimates might be limited by a lack of viral genotype data, which are the gold standard diagnostic method for SARS-CoV-2 reinfections (5). Also, to reduce the likelihood of defining as reinfected the subjects with prolonged viral shedding, we followed CDC criteria (9) and classified as reinfections only the cases with ≥1 intermediate negative swab between the two positive tests. Thus, we may have missed a reinfection case if an infected subject did not have a negative swab after healing. However, according to the Italian legislation, during the pandemic, a negative swab was requested to all positive subjects to end the mandatory quarantine (16). Thus, the number of subjects who healed and did not have a negative test is likely to be low. Indeed, in this sample, only 14 subjects had two positive tests 45 days apart and no negative swab. Also, the adoption of the CDC criteria to define reinfection allowed the comparison with several previous studies on the topic (17), including two Italian field studies, which showed similar results (13, 18).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Emilia-Romagna Region (protocol code 2525/21). The Ethics Committee waived the requirement of written informed consent for participation.
Author Contributions
MF, GS, CAM, and LM: concept and design. GS, GDM, RC, and LM: acquisition, analysis, or interpretation of data. MF, CAM, and LM: drafting of the manuscript. GS, GDM, AC, and LM: critical revision of the manuscript for important intellectual content. MF and LM: statistical analysis. GS, GDM, RC, and AC: administrative, technical, or material support. AC and LM: supervision. LM: had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. To KK, Hung IF, Ip JD, Chu AW, Chan WM, Tam AR, et al. Coronavirus disease 2019(COVID-19) re-infection by a phylogenetically distinct severe acute respiratory syndrome coronavirus 2 strain confirmed by whole genome sequencing. Clin Infect Dis. (2021) 73:e2946–51. doi: 10.1093/cid/ciaa1275
2. Boyton RJ, Altmann DM. Risk of SARS-CoV-2 reinfection after natural infection. Lancet. (2021) 397:1161–3. doi: 10.1016/S0140-6736(21)00662-0
3. Helfand M, Fiordalisi C, Wiedrick J, Ramsey KL, Armstrong C, Gean E, et al. Risk for reinfection after SARS-CoV-2: a living, rapid review for american college of physicians practice points on the role of the antibody response in conferring immunity following SARS-CoV-2 infection. Ann Intern Med. (2022) 175:547–55. doi: 10.7326/M21-4245
4. Krutikov M, Palmer T, Tut G, Fuller C, Shrotri M, Williams H, et al. Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 long-term care facilities (VIVALDI): a prospective cohort study. Lancet Healthy Longev. (2021) 2:e362–70. doi: 10.1016/S2666-7568(21)00093-3
5. Peltan ID, Beesley SJ, Webb BJ, Lopansri BK, Sinclair W, Jacobs JR, et al. Evaluation of potential COVID-19 recurrence in patients with late repeat positive SARS-CoV-2 testing. PLoS ONE. (2021) 16:e0251214. doi: 10.1371/journal.pone.0251214
6. Yahav D, Yelin D, Eckerle I, Eberhardt CS, Wang J, Cao B, et al. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin Microbiol Infect. (2021) 27:315–8. doi: 10.1016/j.cmi.2020.11.028
7. Shrestha NK, Burke PC, Nowacki AS, Terpeluk P, Gordon SM. Necessity of COVID-19 vaccination in persons who have already had COVID-19. Clin Infect Dis. (2022) ciac022. doi: 10.1101/2021.06.01.21258176. [Epub ahead of print].
8. Flacco ME, Soldato G, Acuti Martellucci C, Carota R, Di Luzio R, Caponetti A, et al. Interim estimates of COVID-19 Vaccine effectiveness in a mass vaccination setting: data from an Italian Province. Vaccines. (2021) 9:628. doi: 10.3390/vaccines9060628
9. Center for Disease Control. Common Investigation Protocol for Investigating Suspected SARS-CoV-2 Reinfection (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/php/reinfection.html (accessed April 19, 2022).
10. Flacco ME, Acuti Martellucci C, Soldato G, Carota R, Fazii P, Caponetti A, et al. Rate of reinfections after SARS-CoV-2 primary infection in the population of an Italian province: a cohort study. J Public Health. (2021). doi: 10.1093/pubmed/fdab346. [Epub ahead of print].
11. Bravi F, Flacco ME, Carradori T, Volta CA, Cosenza G, De Togni A, et al. Predictors of severe or lethal COVID-19, including Angiotensin Converting Enzyme inhibitors and Angiotensin II Receptor Blockers, in a sample of infected Italian citizens. PLoS ONE. (2020) 15:e0235248. doi: 10.1371/journal.pone.0235248
12. Coppola A, Buonerba C, Cardinale D, Lo Conte G, Sansone D, Rofrano G, et al. Durability of humoral immune responses to SARS-CoV-2 in citizens of Ariano Irpino (Campania, Italy): a longitudinal observational study with an 11.5-month follow-up. Front Public Health. (2021) 9:801609. doi: 10.3389/fpubh.2021.801609
13. Vitale J, Mumoli N, Clerici P, De Paschale M, Evangelista I, Cei M, et al. Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy, Italy. JAMA Intern Med. (2021) 181:1407–8. doi: 10.1001/jamainternmed.2021.2959
14. Ballering AV, Oertelt-Prigione S, Olde Hartman TC, Rosmalen JGM. Sex and gender-related differences in COVID-19 diagnoses and SARS-CoV-2 testing practices during the first wave of the pandemic: the Dutch lifelines COVID-19 cohort study. J Womens Health. (2021) 30:1686–92. doi: 10.1089/jwh.2021.0226
15. Marconi M. Gender differences in Covid-19: the importance of sex-disaggregated data. Ital J Gender-Specific Med. (2021) 7:4–6. doi: 10.1723/3528.35160
16. Italian Government. Misure urgenti per il contenimento della diffusione dell'epidemia da COVID-19 e disposizioni in materia di sorveglianza sanitaria. Law Decree n. 229, December 30, 2021. Italian Government, Gazzetta Ufficiale, Serie Generale, Roma, Italy. (2021). Available online at: https://www.gazzettaufficiale.it/eli/id/2021/12/30/21G00258/sg (accessed April 19, 2022).
17. Flacco ME, Acuti Martellucci C, Baccolini VCDV, Renzi E, Villari P, et al. Risk of reinfection and disease after SARS-CoV-2 primary infection: meta-analysis (2022).
Keywords: SARS-CoV-2, COVID-19, reinfection, Omicron variant, cohort study, Italy
Citation: Flacco ME, Soldato G, Acuti Martellucci C, Di Martino G, Carota R, Caponetti A and Manzoli L (2022) Risk of SARS-CoV-2 Reinfection 18 Months After Primary Infection: Population-Level Observational Study. Front. Public Health 10:884121. doi: 10.3389/fpubh.2022.884121
Received: 25 February 2022; Accepted: 04 April 2022;
Published: 02 May 2022.
Edited by:
Chiara de Waure, University of Perugia, ItalyReviewed by:
Andreia Leite, New University of Lisbon, PortugalEfrén Murillo-Zamora, Mexican Social Security Institute (IMSS), Mexico
Daihai He, Hong Kong Polytechnic University, Hong Kong SAR, China
Copyright © 2022 Flacco, Soldato, Acuti Martellucci, Di Martino, Carota, Caponetti and Manzoli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lamberto Manzoli, lmanzoli@post.harvard.edu
 Maria Elena Flacco
Maria Elena Flacco Graziella Soldato2
Graziella Soldato2 Cecilia Acuti Martellucci
Cecilia Acuti Martellucci Giuseppe Di Martino
Giuseppe Di Martino Lamberto Manzoli
Lamberto Manzoli