Vitamin D Metabolites and Clinical Outcome in Hospitalized COVID-19 Patients
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Laboratory Analysis
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D.; et al. Genetic mechanisms of critical illness in COVID-19. Nature 2021, 591, 92–98. [Google Scholar] [CrossRef]
- Alguwaihes, A.M.; Sabico, S.; Hasanato, R.; Al-Sofiani, M.E.; Megdad, M.; Albader, S.S.; Alsari, M.H.; Alelayan, A.; Alyusuf, E.Y.; Alzahrani, S.H.; et al. Severe vitamin D deficiency is not related to SARS-CoV-2 infection but may increase mortality risk in hospitalized adults: A retrospective case-control study in an Arab Gulf country. Aging Clin. Exp. Res. 2021, 33, 1415–1422. [Google Scholar] [CrossRef]
- Bennouar, S.; Cherif, A.B.; Kessira, A.; Bennouar, D.E.; Abdi, S. Vitamin D Deficiency and Low Serum Calcium as Predictors of Poor Prognosis in Patients with Severe COVID-19. J. Am. Coll. Nutr. 2021, 40, 104–110. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Penna, G.; Amuchastegui, S.; Cossetti, C.; Aquilano, F.; Mariani, R.; Sanvito, F.; Doglioni, C.; Adorini, L. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J. Immunol. 2006, 177, 8504–8511. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Pantalena, L.C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-dihydroxyvitamin D3 ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol. Cell. Biol. 2011, 31, 3653–3669. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.H.; Cha, H.R.; Lee, D.S.; Seo, K.Y.; Kweon, M.N. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS ONE 2010, 5, e12925. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Baeke, F.; Korf, H.; Overbergh, L.; Verstuyf, A.; Thorrez, L.; Van Lommel, L.; Waer, M.; Schuit, F.; Gysemans, C.; Mathieu, C. The vitamin D analog, TX527, promotes a human CD4+CD25highCD127low regulatory T cell profile and induces a migratory signature specific for homing to sites of inflammation. J. Immunol. 2011, 186, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Baeke, F.; Korf, H.; Overbergh, L.; van Etten, E.; Verstuyf, A.; Gysemans, C.; Mathieu, C. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J. Steroid Biochem. Mol. Biol. 2010, 121, 221–227. [Google Scholar] [CrossRef]
- Baeke, F.; Gysemans, C.; Korf, H.; Mathieu, C. Vitamin D insufficiency: Implications for the immune system. Pediatr. Nephrol. 2010, 25, 1597–1606. [Google Scholar] [CrossRef]
- De Haan, K.; Groeneveld, A.B.; de Geus, H.R.; Egal, M.; Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis. Crit. Care 2014, 18, 660. [Google Scholar] [CrossRef] [Green Version]
- Thickett, D.R.; Moromizato, T.; Litonjua, A.A.; Amrein, K.; Quraishi, S.A.; Lee-Sarwar, K.A.; Mogensen, K.M.; Purtle, S.W.; Gibbons, F.K.; Camargo, C.A.; et al. Association between prehospital vitamin D status and incident acute respiratory failure in critically ill patients: A retrospective cohort study. BMJ Open Respir. Res. 2015, 2, e000074. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef]
- Yisak, H.; Ewunetei, A.; Kefale, B.; Mamuye, M.; Teshome, F.; Ambaw, B.; Yitbarek, G.Y. Effects of Vitamin D on COVID-19 Infection and Prognosis: A Systematic Review. Risk Manag. Healthc. Policy 2021, 14, 31–38. [Google Scholar] [CrossRef]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Hanotte, B.; Grandin de l’Eprevier, C.; Sabatier, J.M.; Lafaie, L.; Celarier, T. Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J. Steroid Biochem. Mol. Biol. 2020, 204, 105771. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimenez, V.M.M.; Sanz, R.L.; Maron, F.J.M.; Ferder, L.; Manucha, W. Vitamin D-RAAS Connection: An Integrative Standpoint into Cardiovascular and Neuroinflammatory Disorders. Curr. Protein Pept. Sci. 2020, 21, 948–954. [Google Scholar] [CrossRef]
- Cereda, E.; Bogliolo, L.; Lobascio, F.; Barichella, M.; Zecchinelli, A.L.; Pezzoli, G.; Caccialanza, R. Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy. Nutrition 2021, 82, 111055. [Google Scholar] [CrossRef]
- Ali, N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health 2020, 13, 1373–1380. [Google Scholar] [CrossRef]
- Farrell, C.; Soldo, J.; Williams, P.; Herrmann, M. 25-Hydroxyvitamin D testing: Challenging the performance of current automated immunoassays. Clin. Chem. Lab Med. 2012, 50, 1953–1963. [Google Scholar] [CrossRef]
- Enko, D.; Kriegshauser, G.; Stolba, R.; Worf, E.; Halwachs-Baumann, G. Method evaluation study of a new generation of vitamin D assays. Biochem. Med. 2015, 25, 203–212. [Google Scholar] [CrossRef]
- Cavalier, E.; Fraser, C.G.; Bhattoa, H.P.; Heijboer, A.C.; Makris, K.; Ulmer, C.Z.; Vesper, H.W.; Vasikaran, S.; Lukas, P.; Delanaye, P.; et al. Analytical Performance Specifications for 25-Hydroxyvitamin D Examinations. Nutrients 2021, 13, 431. [Google Scholar] [CrossRef]
- Cavalier, E.; Huyghebaert, L.; Rousselle, O.; Bekaert, A.C.; Kovacs, S.; Vranken, L.; Peeters, S.; Le Goff, C.; Ladang, A. Simultaneous measurement of 25(OH)-vitamin D and 24, 25(OH)2-vitamin D to define cut-offs for CYP24A1 mutation and vitamin D deficiency in a population of 1200 young subjects. Clin. Chem. Lab Med. 2020, 58, 197–201. [Google Scholar] [CrossRef]
- DeLuca, H.F.; Suda, T.; Schnoes, H.K.; Tanaka, Y.; Holick, M.F. 25, 26-dihydroxycholecalciferol, a metabolite of vitamin D3 with intestinal calcium transport activity. Biochemistry 1970, 9, 4776–4780. [Google Scholar] [CrossRef]
- Coldwell, R.D.; Trafford, D.J.; Makin, H.L.; Varley, M.J. Specific mass fragmentographic assay for 25, 26-dihydroxyvitamin D in human plasma using a deuterated internal standard. J. Chromatogr. 1985, 338, 289–302. [Google Scholar] [CrossRef]
- Zelzer, S.; Meinitzer, A.; Enko, D.; Simstich, S.; Le Goff, C.; Cavalier, E.; Herrmann, M.; Goessler, W. Simultaneous determination of 24, 25- and 25, 26-dihydroxyvitamin D3 in serum samples with liquid-chromatography mass spectrometry—A useful tool for the assessment of vitamin D metabolism. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1158, 122394. [Google Scholar] [CrossRef]
- Loeffelholz, M.J.; Alland, D.; Butler-Wu, S.M.; Pandey, U.; Perno, C.F.; Nava, A.; Carroll, K.C.; Mostafa, H.; Davies, E.; McEwan, A.; et al. Multicenter Evaluation of the Cepheid Xpert Xpress SARS-CoV-2 Test. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Wolters, F.; Grünberg, M.; Huber, M.; Kessler, H.H.; Prüller, F.; Saleh, L.; Fébreau, C.; Rahamat-Langendoen, J.; Thibault, V.; Melchers, W.J. European multicenter evaluation of Xpert® Xpress SARS-CoV-2/Flu/RSV test. J. Med Virol. 2021. [Google Scholar] [CrossRef]
- Livingston, M.; Plant, A.; Dunmore, S.; Hartland, A.; Jones, S.; Laing, I.; Ramachandran, S. Detectable respiratory SARS-CoV-2 RNA is associated with low vitamin D levels and high social deprivation. Int. J. Clin. Pract. 2021. [Google Scholar] [CrossRef]
- Brenner, H. Vitamin D Supplementation to Prevent COVID-19 Infections and Deaths-Accumulating Evidence from Epidemiological and Intervention Studies Calls for Immediate Action. Nutrients 2021, 13, 411. [Google Scholar] [CrossRef]
- Szeto, B.; Zucker, J.E.; LaSota, E.D.; Rubin, M.R.; Walker, M.D.; Yin, M.T.; Cohen, A. Vitamin D Status and COVID-19 Clinical Outcomes in Hospitalized Patients. Endocr. Res. 2021, 46, 66–73. [Google Scholar] [CrossRef]
- Baktash, V.; Hosack, T.; Patel, N.; Shah, S.; Kandiah, P.; Van Den Abbeele, K.; Mandal, A.K.; Missouris, C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med. J. 2020. [Google Scholar] [CrossRef]
- Hernández, J.L.; Nan, D.; Fernandez-Ayala, M.; García-Unzueta, M.; Hernández-Hernández, M.A.; López-Hoyos, M.; Muñoz-Cacho, P.; Olmos, J.M.; Gutiérrez-Cuadra, M.; Ruiz-Cubillán, J.J.; et al. Vitamin D Status in Hospitalized Patients with SARS-CoV-2 Infection. J. Clin. Endocrinol. Metab. 2021, 106, e1343–e1353. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Amer, O.E.; Alotaibi, N.H.; Aldisi, D.A.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Alshingetti, N.; Alomar, S.Y.; Alfawaz, H.; et al. Vitamin D status of Arab Gulf residents screened for SARS-CoV-2 and its association with COVID-19 infection: A multi-centre case-control study. J. Transl. Med. 2021, 19, 166. [Google Scholar] [CrossRef]
- Im, J.H.; Je, Y.S.; Baek, J.; Chung, M.H.; Kwon, H.Y.; Lee, J.S. Nutritional status of patients with COVID-19. Int. J. Infect. Dis. 2020, 100, 390–393. [Google Scholar] [CrossRef]
- Katz, J.; Yue, S.; Xue, W. Increased risk for COVID-19 in patients with vitamin D deficiency. Nutrition 2021, 84, 111106. [Google Scholar] [CrossRef]
- Luo, X.; Liao, Q.; Shen, Y.; Li, H.; Cheng, L. Vitamin D Deficiency Is Associated with COVID-19 Incidence and Disease Severity in Chinese People [corrected]. J. Nutr. 2021, 151, 98–103. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Ebrahimi, M.; Pazoki, M.; Kafan, S.; Tabriz, H.M.; Hadadi, A.; Montazeri, M.; Nasiri, M.; Shirvani, A.; et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE 2020, 15, e0239799. [Google Scholar] [CrossRef]
- Abrishami, A.; Dalili, N.; Torbati, P.M.; Asgari, R.; Arab-Ahmadi, M.; Behnam, B.; Sanei-Taheri, M. Possible association of vitamin D status with lung involvement and outcome in patients with COVID-19: A retrospective study. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Akbar, M.R.; Wibowo, A.; Pranata, R.; Setiabudiawan, B. Low Serum 25-hydroxyvitamin D (Vitamin D) Level Is Associated With Susceptibility to COVID-19, Severity, and Mortality: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 660420. [Google Scholar] [CrossRef]
- Rahme, M.; Al-Shaar, L.; Singh, R.; Baddoura, R.; Halaby, G.; Arabi, A.; Habib, R.H.; Daher, R.; Bassil, D.; El-Ferkh, K.; et al. Limitations of platform assays to measure serum 25OHD level impact on guidelines and practice decision making. Metabolism 2018, 89, 1–7. [Google Scholar] [CrossRef]
- Buonpane, E. Therapeutic drug monitoring of cyclosporine (CSA). Conn. Med. 1990, 54, 17–19. [Google Scholar]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Zelzer, S.; Hofer, E.; Meinitzer, A.; Fritz-Petrin, E.; Simstich, S.; Goessler, W.; Schmidt, R.; Herrmann, M. Association of vitamin D metabolites with cognitive function and brain atrophy in elderly individuals—The Austrian stroke prevention study. Aging 2021, 13, 9455–9467. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Montano-Loza, A.J. Perspective: Improving vitamin D status in the management of COVID-19. Eur. J. Clin. Nutr. 2020, 74, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.; Silva, C.B.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.E.; Ginde, A.A. Vitamin D3 to Treat COVID-19: Different Disease, Same Answer. JAMA 2021, 325, 1047–1048. [Google Scholar] [CrossRef]

| Median, Q1–Q3 | Exitus | Recovery | p-Value * | ||
| age | n = 34 | 80 (68–88) | n = 114 | 59 (45–78) | <0.001 |
| peak IL-6 pg/mL during observation | n = 22 | 147.5 (89.2–279.0) | n = 55 | 47.1 (15.3–128) | 0.001 |
| peak CRP mg/L during observation | n = 32 | 103.2 (54.0–195.8) | n = 107 | 40.7 (6.5–107.6) | <0.001 |
| Total Number (%) | Exitus | Recovery | p-Value + | ||
| female | 15 (44.1) | 56 (49.1) | 0.608 | ||
| male | 19 (55.9) | 58 (50.9) | |||
| ICU admission | 12 (35.3) | 23 (20.2) | 0.069 | ||
| normal ward | 22 (64.7) | 91 (79.8) | |||
| renal disease no | 19 (55.9) | 94 (82.5) | 0.001 | ||
| renal disease yes | 15 (44.1) | 20 (17.5) | |||
| CAD no | 19 (55.9) | 86 (75.4) | 0.028 | ||
| CAD yes | 15 (44.1) | 28 (24.6) | |||
| ambulant | 0 (0) | 35 (30.7) | <0.001 | ||
| resident | 34 (100) | 79 (69.3) | |||
| preexisting disease no | 4 (11.8) | 48 (42.1) | 0.001 | ||
| preexisting disease yes | 30 (88.2) | 66 (57.9) | |||
| cancer no | 24 (70.6) | 103 (90.4) | 0.015 | ||
| cancer yes | 9 (26.5) | 10 (8.8) | |||
| hypertension | 10 (29.4) | 64 (56.1) | 0.006 | ||
| normotension | 24 (70.6) | 50 (43.9) | |||
| oxygen therapy no | 4 (11.8) | 50 (44.2) | 0.001 | ||
| oxygen therapy yes | 18 (52.9) | 44 (38.9) | |||
| CPAP | 3 (8.8) | 10 (8.8) | |||
| mechanical ventilation | 9 (26.5) | 9 (8.0) | |||
| pulmonary disease no | 28 (82.4) | 98 (86.0) | 0.701 | ||
| pulmonary disease yes | 6 (17.6) | 15 (13.2) | |||
| diabetes mellitus no | 23 (67.6) | 77 (86.5) | 0.016 | ||
| diabetes mellitus yes | 11 (32.4) | 12 (13.5) | |||
| Vitamin D deficiency < 30 nmol/L yes | 10 (29.4) | 17 (14.9) | 0.075 | ||
| Vitamin D deficiency no | 24 (70.6) | 97 (85.1) | |||
| Total Cohort n = 148 (Analyzed) | Exitus n = 34 | Recovery n = 114 | p-Values * Exitus Versus Recovery | |
|---|---|---|---|---|
| Median (Q1–Q3) | Median (Q1–Q3) | Median (Q1–Q3) | ||
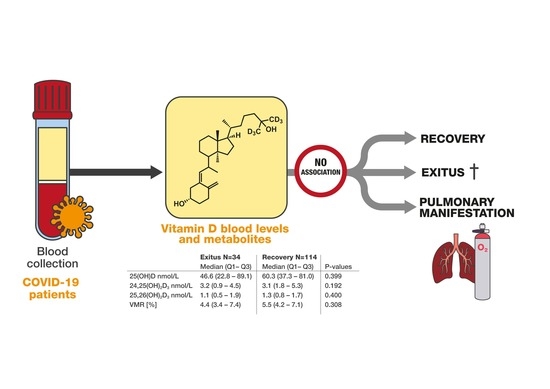
| 25(OH)D nmol/L | 57.6 (34.3–81.5) | 46.6 (22.8–89.1) | 60.3 (37.3–81.0) | 0.399 * |
| 24,25(OH)2D3 nmol/L | 3.1 (1.4–5.2) | 3.2 (0.9–4.5) | 3.1 (1.8–5.3) | 0.192 * |
| 25,26(OH)2D3 nmol/L | 1.2 (0.7–1.7) | 1.1 (0.5–1.9) | 1.3 (0.8–1.7) | 0.400 * |
| VMR [%] | 5.5 (3.9–7.2) | 4.4 (3.4–7.4) | 5.5 (4.2–7.1) | 0.308 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelzer, S.; Prüller, F.; Curcic, P.; Sloup, Z.; Holter, M.; Herrmann, M.; Mangge, H. Vitamin D Metabolites and Clinical Outcome in Hospitalized COVID-19 Patients. Nutrients 2021, 13, 2129. https://doi.org/10.3390/nu13072129
Zelzer S, Prüller F, Curcic P, Sloup Z, Holter M, Herrmann M, Mangge H. Vitamin D Metabolites and Clinical Outcome in Hospitalized COVID-19 Patients. Nutrients. 2021; 13(7):2129. https://doi.org/10.3390/nu13072129
Chicago/Turabian StyleZelzer, Sieglinde, Florian Prüller, Pero Curcic, Zdenka Sloup, Magdalena Holter, Markus Herrmann, and Harald Mangge. 2021. "Vitamin D Metabolites and Clinical Outcome in Hospitalized COVID-19 Patients" Nutrients 13, no. 7: 2129. https://doi.org/10.3390/nu13072129