- 1Department of Emergency Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
- 2Department of Otolaryngology, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
- 3Department of Medical Research and Department of Integrated Surgery, Hsinchu Biomedical Science Park Medical Center (National Taiwan University Hospital Hsin-Chu Biomedical Park Branch), Hsinchu, Taiwan
- 4Department of Pathology, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
- 5Department of Laboratory Medicine, National Taiwan University Hospital Hsin-Chu Biomedical Park Branch, Hsinchu, Taiwan
- 6Department of Laboratory Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
- 7Center for Quality Management, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan
- 8Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
- 9Department of Internal Medicine, National Taiwan University Hospital Hsin-Chu Biomedical Park Branch, Hsinchu, Taiwan
Accurate detection of anti-SARS-CoV-2 antibodies provides a more accurate estimation of incident cases, epidemic dynamics, and risk of community transmission. We conducted a cross-sectional seroprevalence study specifically targeting different populations to examine the performance of pandemic control in Taiwan: symptomatic patients with epidemiological risk and negative qRT-PCR test (Group P), frontline healthcare workers (Group H), healthy adult citizens (Group C), and participants with prior virologically-confirmed severe acute respiratory syndrome (SARS) infection in 2003 (Group S). The presence of anti−SARS−CoV−2 total and IgG antibodies in all participants were determined by Roche Elecsys® Anti−SARS−CoV−2 test and Abbott SARS-CoV-2 IgG assay, respectively. Sera that showed positive results by the two chemiluminescent immunoassays were further tested by three anti-SARS-CoV-2 lateral flow immunoassays and line immunoassay (MIKROGEN recomLine SARS-CoV-2 IgG). Between June 29 and July 25, 2020, sera of 2,115 participates, including 499 Group P participants, 464 Group H participants, 1,142 Group C participants, and 10 Group S participants, were tested. After excluding six false-positive samples, SARS-CoV-2 seroprevalence were 0.4, 0, and 0% in Groups P, H, and C, respectively. Cross-reactivity with SARS-CoV-2 antibodies was observed in 80.0% of recovered SARS participants. Our study showed that rigorous exclusion of false-positive testing results is imperative for an accurate estimate of seroprevalence in countries with previous SARS outbreak and low COVID-19 prevalence. The overall SARS-CoV-2 seroprevalence was extremely low among populations of different exposure risk of contracting SARS-CoV-2 in Taiwan, supporting the importance of integrated countermeasures in containing the spread of SARS-CoV-2 before effective COVID-19 vaccines available.
Introduction
Coronavirus disease 2019 (COVID-19), which emerged at the end of 2019 in China and is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly evolved to a pandemic and impacts healthcare, public health, and the socioeconomic system tremendously (1, 2). The risk of a community outbreak of COVID-19 in Taiwan is especially high because of its geographic proximity and frequent person-to-person contacts with China. By virtue of its experience in coping with the severe impact of severe acute respiratory syndrome (SARS) in 2003 (3, 4), Taiwan responded to this global public health emergency promptly and has maintained a record of limited community transmission of COVID-19 (5). The first confirmed COVID-19 case in Taiwan was imported from China on January 20, 2020, and was identified at the airport entry quarantine system (6). Though cases from sporadic family clusters and one nosocomial outbreak have been reported, most of the confirmed COVID-19 cases in Taiwan were imported from aboard. As of August 28, 2020, the latest confirmed indigenous COVID-19 case reported by Taiwan authorities was on April 13, 2020. Meanwhile, only 487 confirmed cases, including 55 indigenous cases, have been reported in Taiwan (7). The number of confirmed COVID-19 cases per million Taiwan population was 20.4, ranking 204 out of 209 countries (8).
Although early success in the control of the COVID-19 pandemic was achieved, Taiwan faces an increasing risk of COVID-19 community transmission due to the rapid spread of SARS-CoV-2 globally and the rising influx of business and returning travelers. Furthermore, the risk of circulating SARS-CoV-2 in the community from untested mild or asymptomatic patients or from symptomatic patients with false-negative results by real-time reverse transcriptase-polymerase chain reaction (qRT-PCR) assay remains a serious concern (9). All these issues could seriously confound the estimation of incident cases, epidemic dynamics, and ongoing risk of COVID-19 community transmission from current viral nucleic acid testing-based reporting data (10–14).
Serological testing, i.e., detection of anti-SARS-CoV-2 antibodies in a person’s blood, has been proposed as a useful laboratory tool in the diagnosis of current or recovered COVID-19 infection, screening of recovered COVID-19 patients for convalescent plasma therapy, SARS-CoV-2 seroprevalence studies, and monitoring immune responses to COVID-19 vaccine candidates (15). Although a false-positive result has been reported, the detection sensitivity of many serological tests for COVID-19 infection is high, especially after 2–3 weeks of symptom onset (16–19). Therefore, population-based serological tests might provide a more accurate estimation of SARS-CoV-2 transmission and disease burden that comprehensively include COVID-19 patients that are asymptomatic, with false negative qRT-PCR testing results, and with qRT-PCR-confirmed infection.
In the seroprevalence study, we conducted a serological survey targeting three groups of population with two automated immunoassays simultaneously: (i) symptomatic patients with risk of SARS-CoV-2 exposure, (ii) healthcare workers (HCWs) responsible for screening or taking care of suspected or confirmed COVID-19 patients, and (iii) citizens without identifiable risk of contracting SARS-CoV-2. We hypothesized that these three groups of population specifically have different roles in the control of community transmission of SARS-CoV-2 in Taiwan (see Supplementary Table). In addition, because previous studies demonstrated a cross-reactive antibody response between SARS-CoV-2 and severe acute respiratory syndrome coronavirus (SARS-CoV) infection (20, 21), individuals with virologically confirmed SARS-CoV infection in 2003 were separately invited and analyzed to avoid overestimating the seroprevalence among study populations from cross-reactivity between the two genetically close virus (22). Our goal was first to estimate the true seroprevalence among populations with different SARS-CoV-2 exposure risks. The second goal was to understand the anti-SARS-CoV-2 antibody response among individuals with SARS in 2003.
Policies Against the COVID-19 Pandemic in Taiwan
At the beginning of the COVID-19 outbreak from December 2019, Taiwan had implemented proactive measures against this novel virus. The containment measures and policies were adjusted dynamically according to the severity and global situations of the epidemic. Strategies in Taiwan were integrated and composed of three main components: border control, healthcare system response, and public engagement (see Supplementary Table). Before April 30, travel restrictions were applied to countries with known or suspected widespread community transmission of SARS-CoV-2. The number of people entering Taiwan decreased significantly from 2,262,692 in January to 22,822 in April, with a total of 3,638,171 people between January 1st and April 30. All entry travelers received a COVID-19 Health Declaration and Home Quarantine Notice to self-report if they experienced any COVID-19-compatible symptoms before going through the immigration. Febrile or symptomatic travelers identified at the airport or during the 14-day quarantine period will undergo a SARS-CoV-2 qRT-PCR test. Confirmed COVID-19 patients with qRT-PCR test will be admitted to a hospital isolation room to prevent the spread of the virus in the community. HCWs in hospitals or nursing facilities comply with strict infection prevention and control guidelines recommended by Taiwan Centers for Disease Control. Personal protective equipment (PPE) are fully supplied for first-line HCWs, especially for the quarantine ward or intensive care unit (ICU) staffs taking care of suspected or confirmed COVID-19 patients. All citizens are encouraged to wear a mask, maintain hand hygiene and social distancing, and avoid mass gathering (23). In addition to purchasing vaccines with emergency use authorization from abroad, Taiwan government also supports SARS-CoV-2 vaccine development by local manufacturers. By April 30, a total of 62,844 individuals suspected of SARS-CoV-2 infection were reported to the Taiwan Centers for Disease Control and received at least one qRT-PCR test for the diagnosis of COVID-19. Among them, 429 COVID-19 patients were diagnosed, including 343 imported cases.
Materials and Methods
Hospital Settings, Study Design, and Participant Enrollment
Two hospitals in northern Taiwan participated in this cross-sectional seroprevalence study. The National Taiwan University Hospital (NTUH), located in Taipei, is a 2,700-bed teaching hospital that provides both primary and tertiary care. The NTUH Hsin-Chu Biomedical Park Branch (NTUH HBP Branch) is a 475-bed community hospital in Hsinchu County and is about 62 km from NTUH. The study was conducted on June 29 to July 12, 2020, in NTUH and on July 25, 2020, in NTUH HBP branch. Study approvals from the institutional review board were obtained from NTUH (202004128RINB) and NTUH HBP Branch (202007007RIPB).
The seroprevalence of anti-SARS-CoV-2 antibodies was determined in three target populations: (i) Population P: patients with epidemiological risk of SARS-CoV-2 exposure (returned travelers or foreigners for international business; certain occupations such as HCWs not responsible for screening or taking care of COVID-19 patients, airport staffs, flight crew or local tour guide; having contact with known or suspected COVID-19 patient; and cluster phenomenon of infection such as presence of fever or airway symptom in a family or workplace) and compatible symptom who visited epidemic outpatient clinic or emergency department for COVID-19 screening during January 21 to April 30, 2020 (in NTUH). Detailed information about specific epidemiological risk exposure is shown in Table 1; (ii) Population H: HCWs responsible for screening or taking care of suspected or confirmed COVID-19 patients since January 21 to April 30, 2020, which included staffs in the emergency department, epidemic outpatient clinic, COVID-19 quarantine ward or ICU, and clinical laboratory (during that period, 16 qRT-PCR-confirmed COVID-19 patients, including six referred from other hospitals after diagnosis, were hospitalized in NTUH with a total of 480 patient-days in the general isolation ward and 74 patient-days in the ICU (in NTUH]); (iii) Population C: adult citizens who were eligible for a hepatitis screening program on July 25, 2020 (in NTUH HBP Branch). Among patients with the risk of SARS-CoV-2 exposure and who received COVID-19 screening at NTUH during the study period, 10 qRT-PCR-confirmed COVID-19 patients were not included because we aimed to identify undiagnosed COVID-19 patients under the quarantine and surveillance policy in Taiwan. Besides the three aforementioned populations for the seroprevalence study, we also enrolled individuals with virologically documented SARS-CoV infection in 2003 to clarify the cross-reactivity between anti-SARS-CoV and anti-SARS-CoV-2 antibody response. Eligible individuals were invited by postal mail, telephone call, and poster. Informed consent was obtained from all adult participants or from their legal representatives for participants younger than 20 years. Sex and age of all participants were recorded at the time of blood sampling. Clinical information including initial presentation, date of symptom onset, and epidemiological risk for SARS-CoV-2 exposure of screened patients was retrieved from electronic medical records of the study hospital.
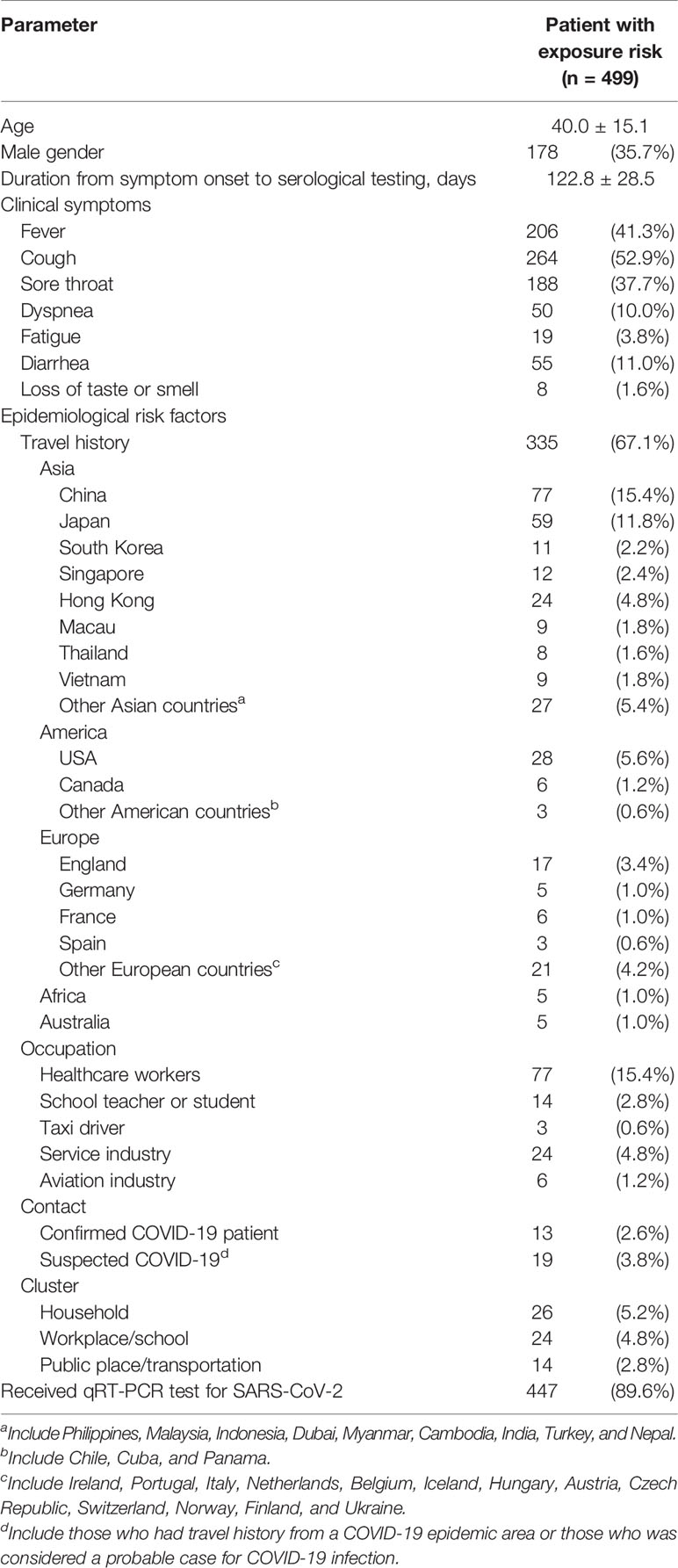
Table 1 Clinical characteristics and epidemiological risk factors of contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), of the 499 participants who visited the study hospital for coronavirus disease 2019 (COVID-19) screening.
Sample Collection and Anti-SARS-CoV-2 Antibody Testing
After obtaining the informed consent, 3 ml of peripheral venous blood from each participant was collected. The serum of the collected blood samples was stored at −20°C before testing. We used two automated chemiluminescent immunoassays to detect anti−SARS−CoV−2 antibodies. The Roche Elecsys® Anti−SARS−CoV−2 assay is an electrochemiluminescence immunoassay using the recombinant nucleocapsid protein (N protein) for the detection of total antibodies (including IgG) against SARS−CoV−2 with Cobas e immunoassay analyzers (Roche Diagnostics Basel, Switzerland) (Roche test) (18, 24). The Abbott SARS-CoV-2 IgG assay is a chemiluminescent microparticle immunoassay that qualitatively detects IgG antibodies on the SARS-CoV-2 N protein in human serum and plasma using the ARCHITECT i System (Abbott Laboratories, IL, USA) (Abbott test) (19, 25). Sample with a reported cutoff index (COI) greater than 1.00 in Roche test and index sample/calibrator (index S/C) greater than 1.40 in Abbott test was considered as a positive result of the test, respectively.
The serum of all participants with known SARS infection history and the serum of participants in the seroprevalence study with a positive result for anti-SARS-CoV-2 antibodies by either the Roche test or Abbott test were further tested with three qualitative lateral flow immunoassays (LFIA) for anti-SARS-CoV-2 antibodies: (i) Wondfo SARS-CoV-2 Antibody Test (Guangzhou Wondfo Biotech Co., Ltd., China) (Wondfo test), (ii) ASK COVID-19 IgG/IgM Rapid Test (TONYAR Biotech Inc., Taiwan) (ASK test), and (iii) Dynamiker 2019-nCoV IgG/IgM Rapid Test (Dynamiker Biotechnology [Tianjin] Co., Ltd., China) (Dynamiker test) (17). The ASK test and Dynamiker test detect either anti-SARS-CoV-2 IgG and IgM antibodies separately, and the Wondfo test detects total antibodies against SARS-CoV-2 within 5–15 min. The viral protein labeled was the SARS-CoV-2 spike protein (S protein) in the Wondfo and ASK tests and SARS-CoV-2 N protein in the Dynamiker test. A weakly positive result (any shade of color in the test lines) of an antibody rapid testing was considered positive according to the manufacturers’ instructions. In our previous studies, the three serological tests of lateral flow immunoassay method showed comparable performance in detecting anti-SARS-CoV-2 antibodies but no cross-reactivity with autoantibodies or antibodies against other coronavirus or non-coronavirus than serological tests of chemiluminescent immunoassay method (17, 26). In addition, a serological test for cytomegalovirus (CMV) IgM/IgG antibodies was performed to detect possible cross-reactivity between anti-SARS-CoV-2 and anti-CMV antibodies to increase diagnostic specificity. Finally, we used recomLine SARS-CoV-2 IgG (MIKROGEN Diagnostik GmbH, Neuried, Germany), a line immunoassay specifically identifying antibodies against the individual antigens of the coronaviruses (nucleocapsid protein [NP], receptor binding domain [RBD], and S1 from SARS-CoV-2 as well as NP for human coronavirus 229E, NL63, OC43, and HKU1), as a confirmatory test to clarify the initial results of the Roche and Abbott tests among participants in the seroprevalence study (27).
Statistical Analysis and Case Number Estimate
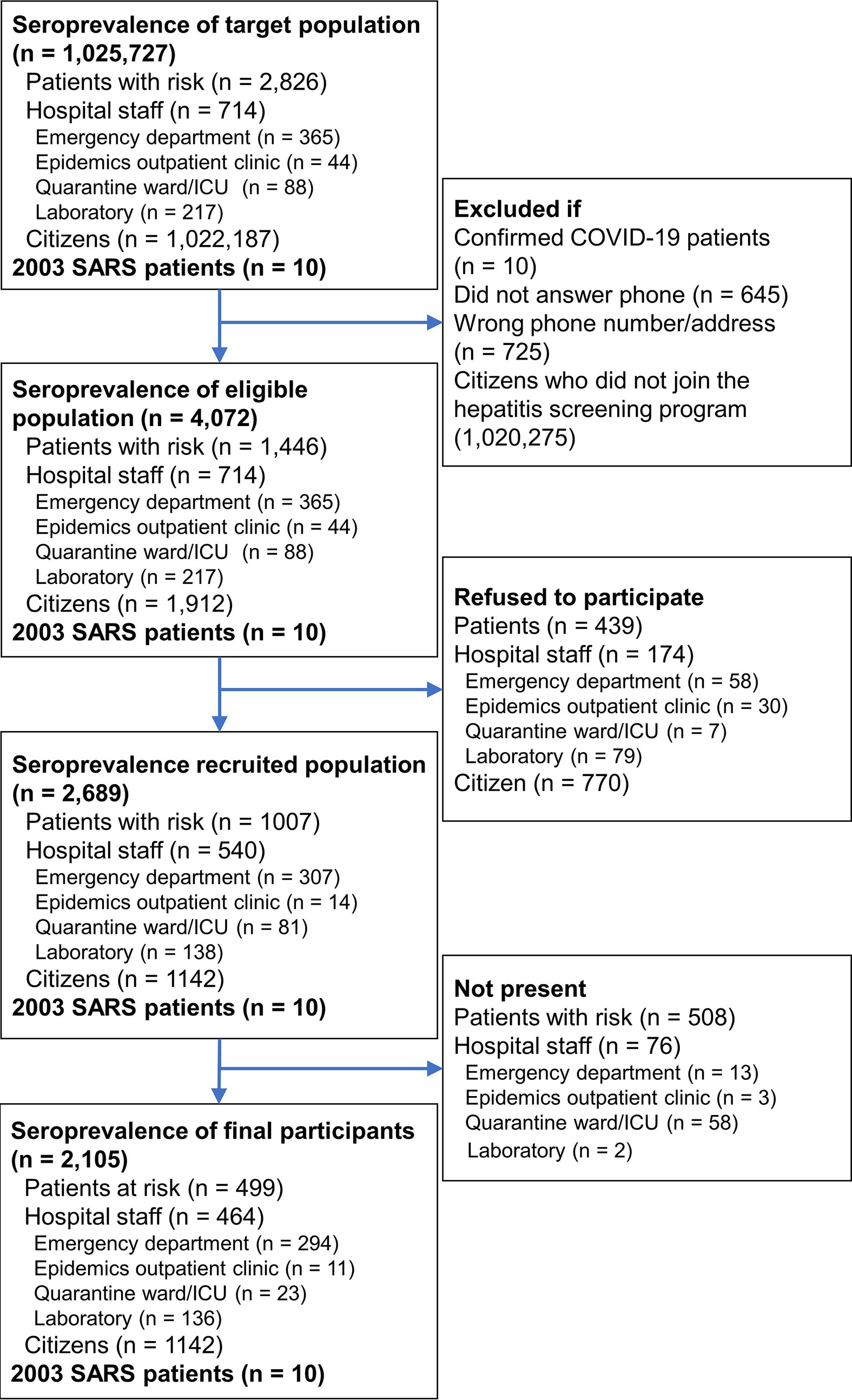
We calculated means and standard deviations (SDs) for age variables and percentages for categorical variables. Binomial proportion confidence interval (CI) was used to estimate the range of seroprevalence. The required sample size was estimated using a single proportion population formula (28) with a 95% confidence level and 1% margin of error. The population size for Population P and Population H were retrieved from electronic medical records and personnel registry of the study hospital (NTUH) and were 2,826 and 714, respectively (Figure 1). The population size of 1,022,187 for Population C was the total population of HsinChu City and HsinChu County at the end of 2020 according to government statistics (29, 30). Expected seroprevalence for Population P, Population H, and Population C were estimated to be 3, 2 and 1% according to the seroprevalence reports at the time of study (31–33). The minimum number of participants for Population P, Population H, and Population C were estimated at 802, 367, and 381, respectively. Data were analyzed using SPSS for Windows (IBM SPSS Statistics v26).
Results
In the seroprevalence study, 5,452 individuals were initially invited. Individuals who had previously confirmed SARS-CoV-2 infection, failed to be contacted, refused to participate, or did not show up during the study period were excluded. Finally, a total of 2,105 individuals, including 499 patients with risk for COVID-19 infection (Group P), 464 hospital staffs (Group H), and 1,142 citizens (Group C), provided consent and were tested for anti-SARS-CoV-2 antibodies (Figure 1). Of the 499 Group P participants, 308 (61.7%) had travel history from China (15.4%), other Asian countries (26.5%), European countries (10.4%), American countries (7.4%), African countries (1.0%), and Australia (1.0%) within 2 weeks of symptom onset. qRT-PCR was performed in 447 (89.6%) of the Group P participants and all were negative for SARS-CoV-2. Age and male sex percentage were 40.0 ± 15.1 years and 35.7%, respectively. Duration from symptom onset to serological testing in Group P participants was 122.8 ± 28.5 days (Table 1). Age and male sex percentage were 37.4 ± 10.8 years and 35.0% for Group H participants, 49.5 ± 12.6 years and 50.2% for Group C participants, and 50.6 ± 10.7 years and 40.0% for Group S participants, respectively.
In the seroprevalence study including 2105 Groups P, H and C participants, a total of eight participants were positive for anti-SARS-CoV-2 antibodies in either the Roche test (six participants) or Abbott test (four participants) (Table 2). The sera of eight participants were further tested with three LFIAs, line immunoassay, and anti-CMV IgG/IgM antibodies. Among them, only two participants were further confirmed as true positive with the line immunoassay. Both of them were returning travelers from the United States on February 29 and March 29, respectively (Figure 2). They had fever and airway symptom but negative results on multiple screening qRT-PCR assay during the quarantine period. Another six participants were judged stringently as false positive due to a negative result for anti-SARS-CoV-2 S1, RBD, or N-protein antibodies in line immunoassay. LFIAs for the six participants’ sera were all negative for anti-SARS-CoV-2 antibody response and were compatible with the results in the line immunoassay. Of the six sera interpreted as false positive result for anti-SARS-CoV-2 antibodies, cross-reactivity with seasonal human coronavirus and possibly with anti-CMV IgM/IgG antibody was observed in five participants.
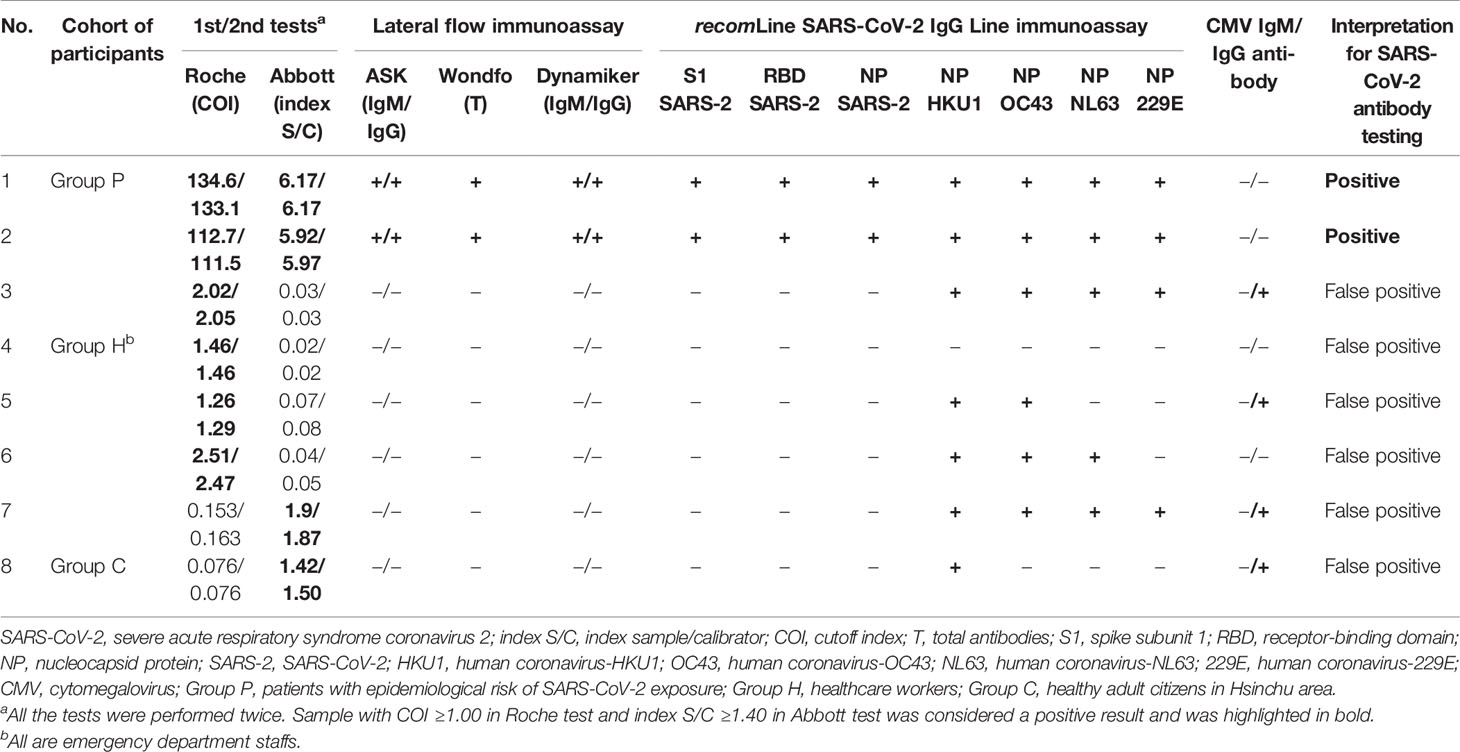
Table 2 Summary of eight participants with positive results from either Roche Elecsys® Anti−SARS−CoV−2 test (Roche) or Abbott SARS-CoV-2 IgG assays (Abbott) in a seroprevalence survey (n = 2105) conducted from June 25 to July 29, 2020.
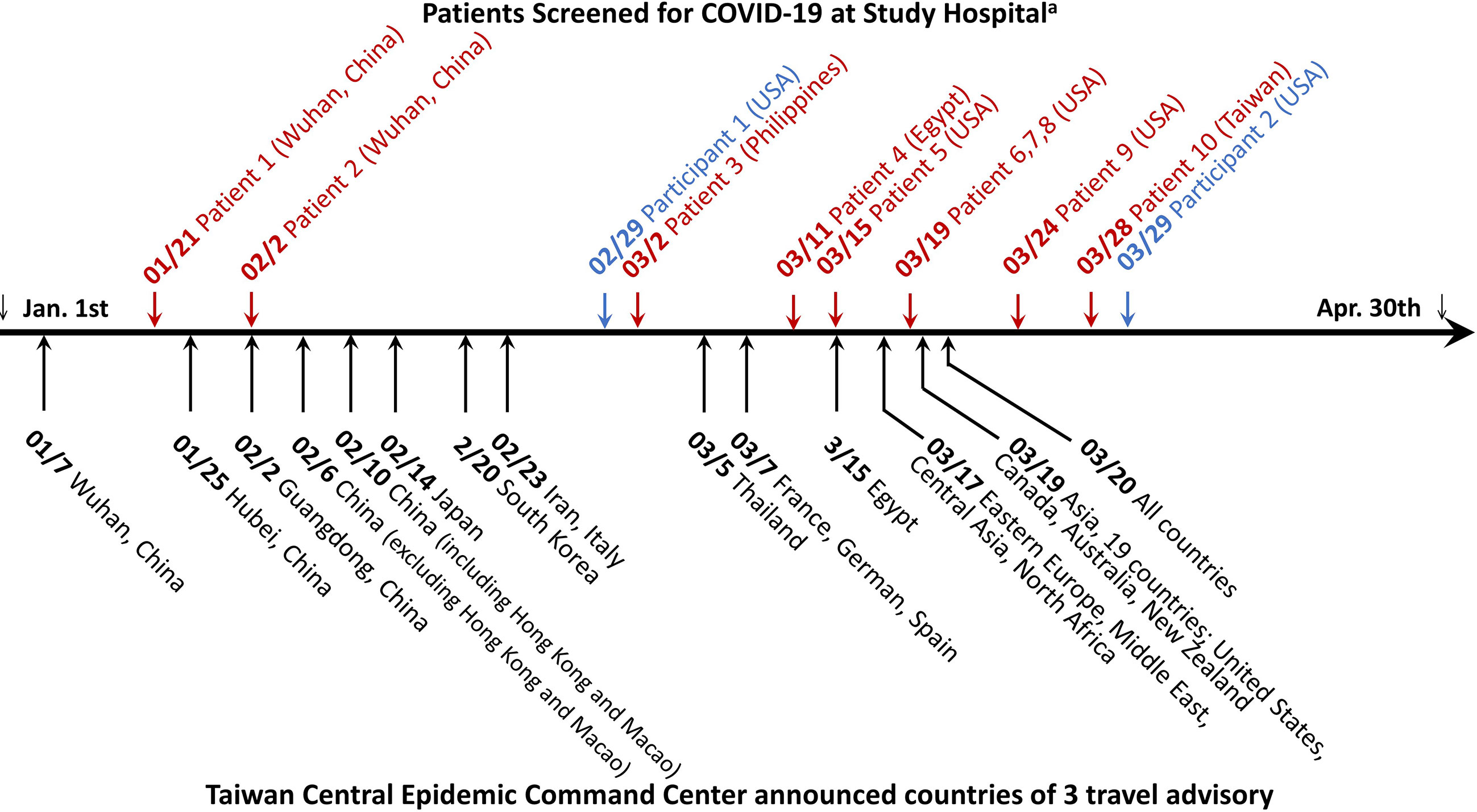
Figure 2 Timeline of COVID-19 patient diagnosed at National Taiwan University Hospital and travel advisory announced by Taiwan Central Epidemic Command Center between January and April 2020. The diagnosis of COVID-19 was achieved either by a positive qRT-PCR testing result at initial screening visit in the emergency department or epidemic outpatient clinic (letters in red color) or retrospectively by serological test in this study (letters in blue color). The number-slash-number before COVID-19 patient denotes the latest date of leaving an area or country reporting COVID-19 outbreak for the 11 imported cases and the date of COVID-19 diagnosis for the one indigenous case. The city or country name indicates the travel location of the 11 imported cases, except one indigenous case who did not have travel history abroad.
Ten individuals with previous SARS infection history participated in this study to help evaluate the cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections (Group S). All of the 10 Group S participants were incumbent or retired HCWs. None of them took care of or screened patients with confirmed or suspected COVID-19 infection before testing. They also had no known epidemiological risk of contracting SARS-CoV-2 in the past half year before study enrollment. Among the 10 Group S participants, eight (80.0%) were positive for anti-SARS-CoV-2 antibodies in the Roche test, but none were positive in the Abbott test and the three LFIA tests. Five Group S participants were positive for anti-SASR-CoV-2 S1 and RBD antibodies in the line immunoassay. One Group S participant who showed high cutoff index (COI) value (71.97 and 73.22, respectively; COI ≥1.0 considered as reactive) was positive for anti-SASR-CoV-2 S1, RBD, and NP antibodies in the line immunoassay (Table 3). Figure 3 demonstrates the results of the recomLine SARS-CoV-2 IgG line immunoassay for two qRT-PCR-confirmed, hospitalized COVID-19 patients and eight participants in this study.
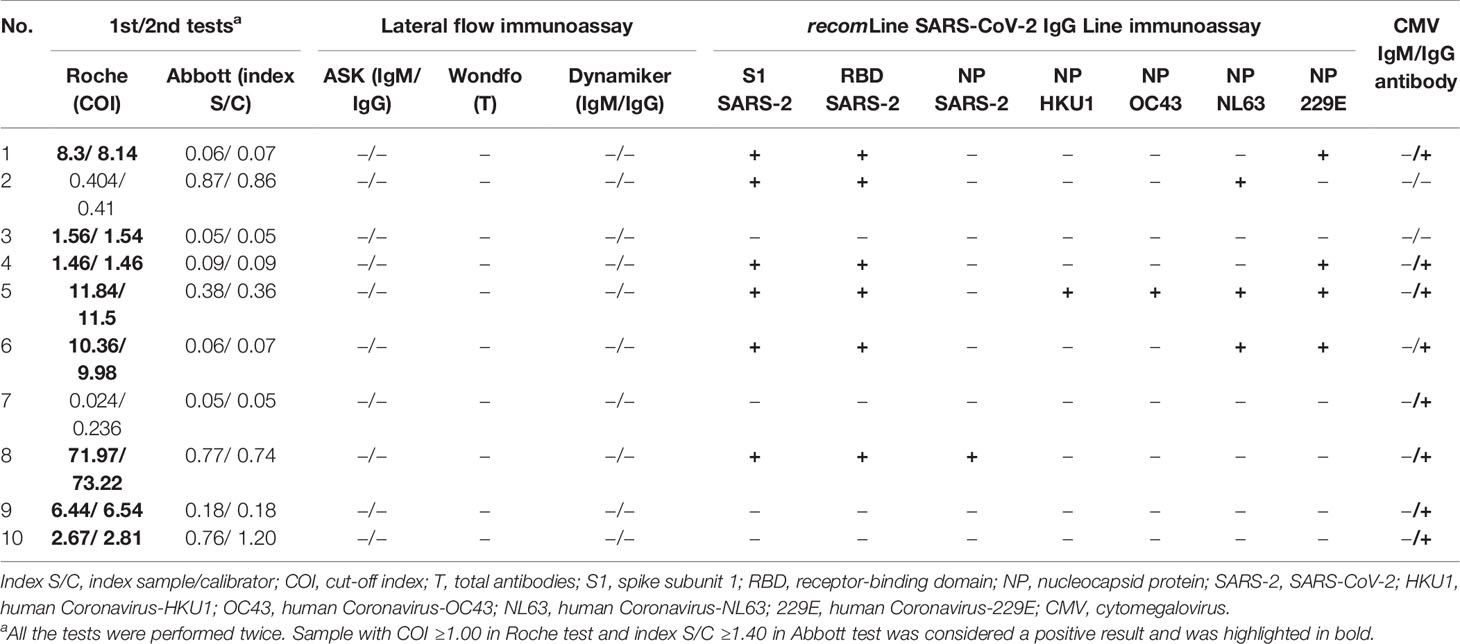
Table 3 Summary of results of different antibody assays from 10 healthcare workers with virologically-documented SARS-CoV infection in 2003.
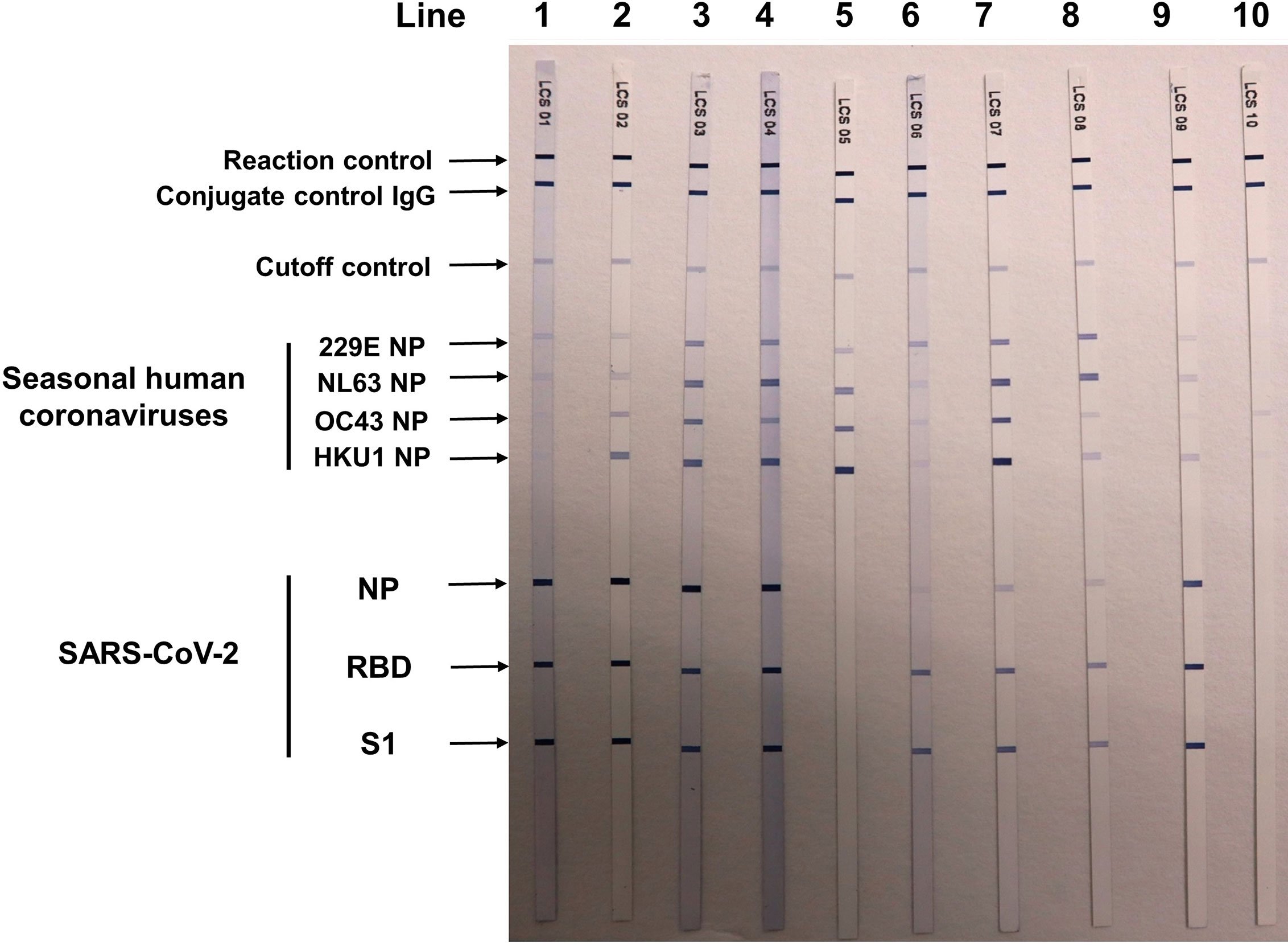
Figure 3 Results of recomLine SARS-CoV-2 IgG [Avidität] line immunoassay (MIKROGEN Diagnostik GmbH, Neuried, Germany) for two qRT-PCR-confirmed COVID-19 patients and eight participants in the seroprevalence survey. Lines 1 and 2: two patients with qRT-PCR-confirmed COVID-19 patients treated at National Taiwan University Hospital (NTUH). Lines 3–5: participant nos. 1 and 2 and 6 in Table 2. Lines 6–9: four healthcare workers (nos. 1, 5, 6, and 8 in Table 3) with SARS-CoV infection in 2003. Line 10: a patient visited NTUH with acute respiratory infection, and triplicate qRT-PCR testings for SARS-CoV-2 from respiratory samples were negative. NP, nucleocapsid protein; RBD, receptor-binding domain; S1, spike subunit 1.
Results of serological testing for anti-SARS-CoV-2 antibodies are summarize in Table 4. The crude seroprevalence among the three study groups were 0.6% (95% CI, 0.12–1.75%) in Group P participants, 0.86% (95% CI, 0.24–2.19%) in Group H participants, and 0.09% (95% CI, 0–0.49%) in Group C participants. If the six participants interpreted as having false positive testing result were exclude, the stringently judged seroprevalence among the three study groups, therefore, was 0.4% (95% CI, 0.05–1.44%) in Group P participants, 0% (95% CI, 0–0.79%) in Group H participants, and 0% (95% CI, 0–0.32%) in Group C participants. Finally, data about the distribution of the Roche and Abbott test results of the 2,105 seroprevalence study participants, stratified by stringently judged two positive and 2,103 negative results, is shown in Figure 4.
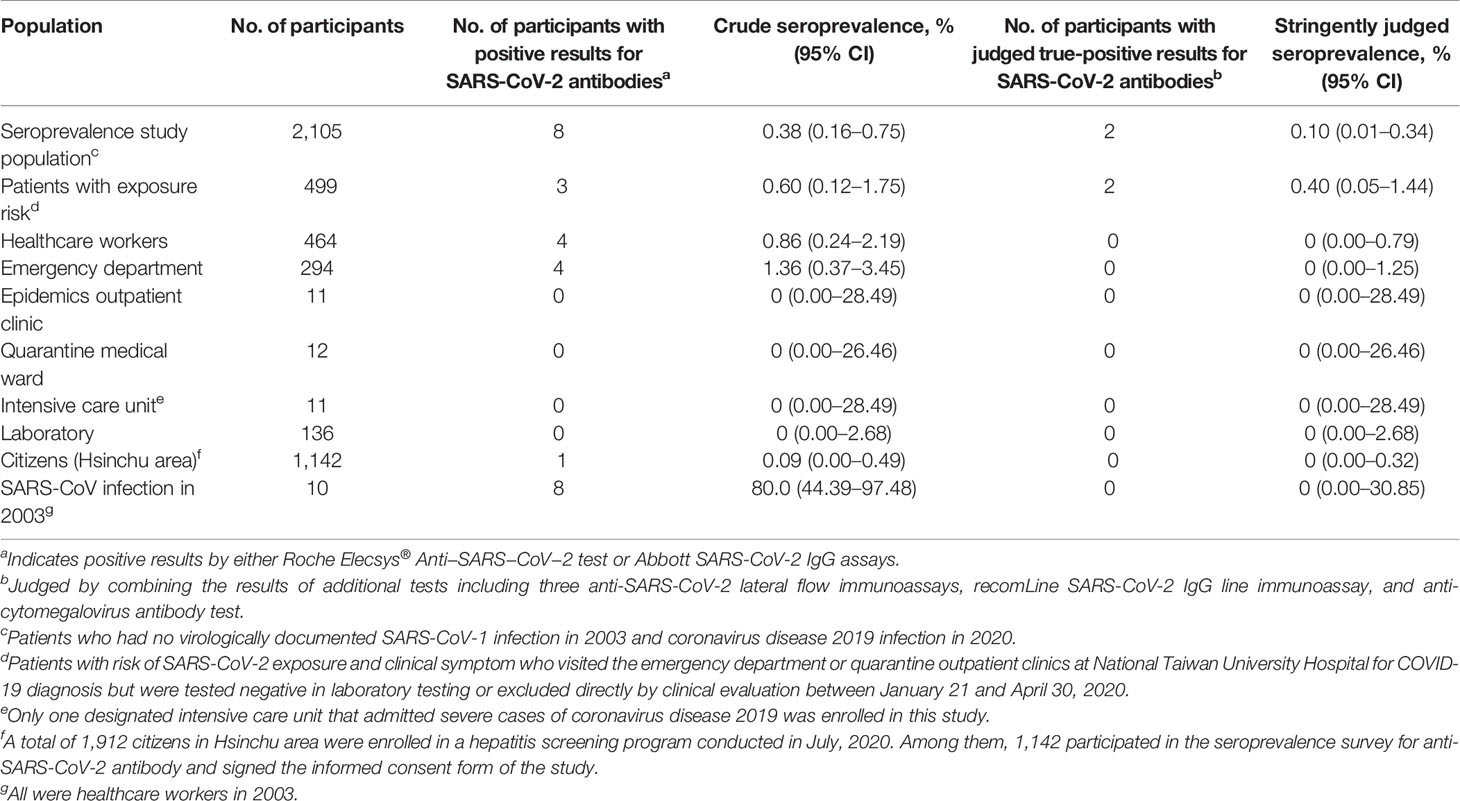
Table 4 Summary of sources of participants and corresponding seroprevalence for coronavirus disease 2019 using Roche Elecsys® Anti−SARS−CoV−2 test and Abbott SARS-CoV-2 IgG assay.
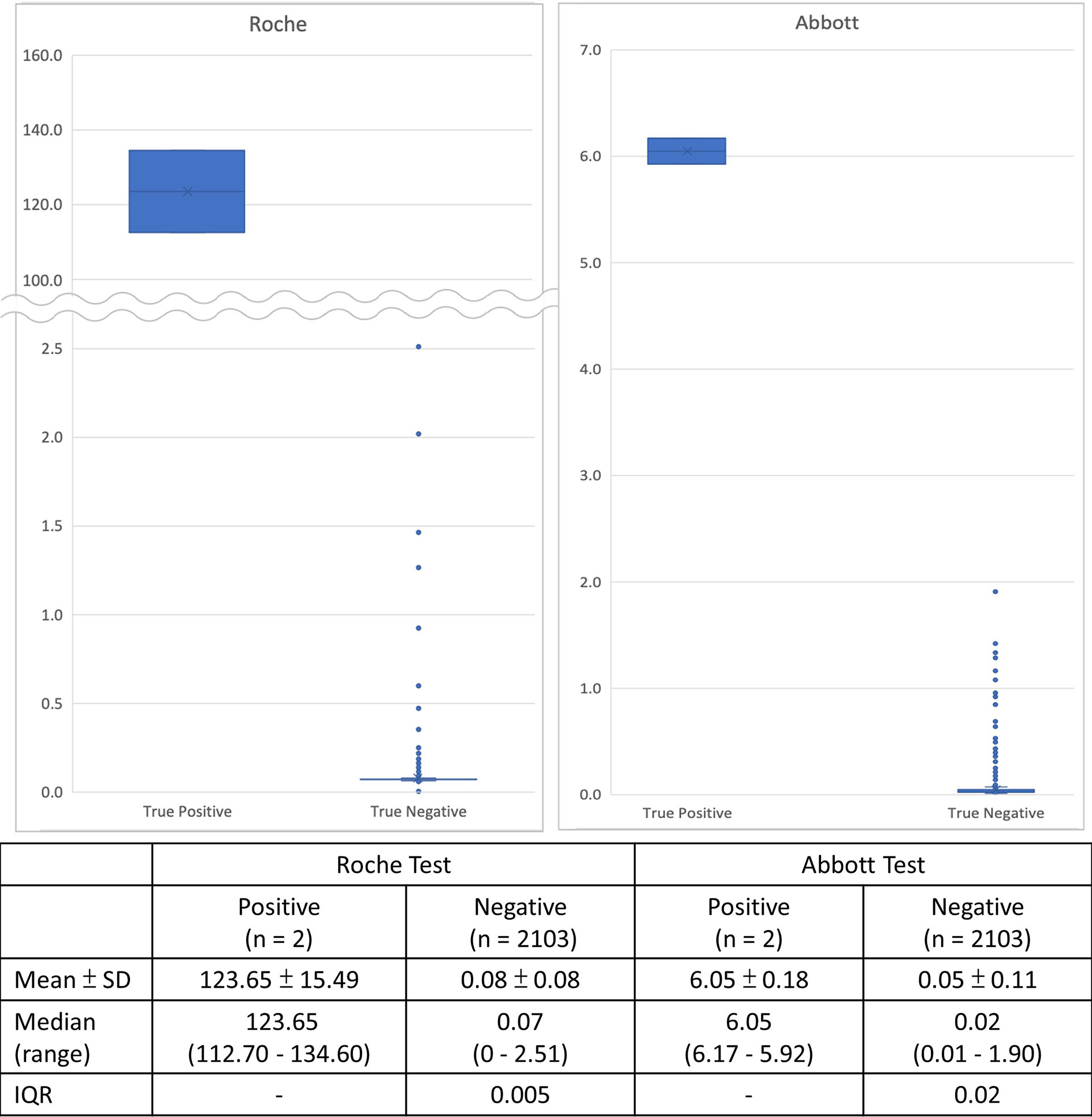
Figure 4 Distribution of serological test results of the 2,105 seroprevalence study participants of the two automated chemiluminescent immunoassays, stratified by final positive and negative interpretation from combining the results of the recomLine SARS-CoV-2 IgG line immunoassay and the three lateral flow immunoassays.
Discussion
Our study simultaneously evaluated the seroprevalence of the antibody response to SARS-CoV-2 in three different populations in northern Taiwan, with the sample collection period from late June through mid-July 2020. After strictly excluding false-positive results including those from participants with prior SARS infection in 2003, we found that the seroprevalence was 0.4% among participants who had epidemiological risk of SARS-CoV-2 exposure but were negative for COVID-19 diagnosis in hospital screening during the early stage of the COVID-19 outbreak in Taiwan. None of the HCWs and citizen participants had positive anti-SARS-CoV-2 antibody testing result in this study. The risk-stratified seroprevalence estimates are the first report from Taiwan that have successfully staved off the COVID-19 pandemic with timely implementation of effective preventive measures against the invasion of SARS-CoV-2.
Immediately after the outbreak of COVID-19 in China, Taiwan authority implemented strict public health interventions and stepwise announced international travel restrictions to prevent the spread of SARS-CoV-2 into the community. Many hospitals set up outdoor or specialized screening clinics in outpatient clinics or emergency departments to prevent nosocomial outbreak of COVID-19 in the hospital (34). From January 21 through April 30, a total of 2,826 patients with epidemiological risk for COVID-19 infection received clinical or laboratory screening in NTUH; among them 10 were diagnosed with COVID-19 infection by positive qRT-PCR test. Two COVID-19 cases out of the 499 Group P patients were additionally diagnosed by serological testing in this study. If the remaining 2,317 patients who did not participate in this study had the same 0.4% seroprevalence as of the 499 Group P participants, additional nine COVID-19 patients could be retrospectively identified by serological testing, suggesting that the exact COVID-19 patient number among the 2,826 screened patients between January and April 2020 would be 21 rather than 10, corresponding to 0.74% (21/2,826) COVID-19 disease prevalence among symptomatic patients with epidemiological risk. The detection sensitivity of the current screening strategy combining clinical evaluation and laboratory qRT-PCR testing therefore might only be 47.6% (10/21). The projected COVID-19 prevalence was much higher than the prevalence based on clinical and viral nucleic acid assay screening. These undiagnosed COVID-19 infections were likely due to the low viral load below the detection limit of the qRT-PCR testing, or with atypical or mild symptoms in which COVID-19 infection was not suspected by the visiting physician at the time of screening.
The outbreak of SARS-CoV-2 infection and consequently the emergence of a tremendous number of COVID-19 patients led to medical service system failure and staff burnout (35, 36). A high seroprevalence of up to 5–23% has been reported in many countries, especially in countries or areas experiencing severe community outbreaks of COVID-19 (14, 37–40). The HCWs responsible for screening or taking care of COVID-19 patients were even at a higher risk of contracting SARS-CoV-2, especially in areas with a short supply of PPE (41, 42). In contrast, without community transmission and only a limited number of COVID-19 cases, Taiwan attracts worldwide attention with respect to the amazing performance of containing the COVID-19 pandemic. Although an undetected circulation of SARS-CoV-2 in the community has been suspected, our study confirmed the extremely low seroprevalence of SARS-CoV-2 in Taiwan under the current epidemic controlling strategies. However, clinical and viral nucleic acid-based laboratory screening systems might diagnose less than one-half of symptomatic COVID-19 patients, as demonstrated in a previous study (43), and in the current study. It is plausible that 429 confirmed COVID-19 patients in Taiwan by April 30 indicate that additional 465 undiagnosed symptomatic COVID-19 patients escaped from the current airport and hospital screening system deduced from the data analyses in this study. Those undiagnosed symptomatic COVID-19 patients as well as unrecognized asymptomatic COVID-19 patients inevitably spread the SARS-CoV-2 into the healthcare system and community. The low seroprevalence in hospital staffs supports the effectiveness of implementing strict infection control measures and providing adequate PPE for first-line HCWs in the prevention of nosocomial transmission of the SARS-CoV-2. The low seroprevalence in our citizens demonstrates the importance of establishing physical (mask-wearing, social distancing, avoiding mass gathering) and chemical barriers (handwashing, alcohol scrub) in the interruption of SARS-CoV-2 transmission in communities.
In this study, 61.7% of patients who visited NTUH for SARS-CoV-2 infection screening had travel history 2 weeks prior to symptom onset from countries reporting confirmed COVID-19 cases. The low SARS-CoV-2 seroprevalence among these patients implies the low influx pressure of the virus from abroad. It reasonably reflects the extent and severity of the COVID-19 pandemic in the early emerging period of this novel virus. It is considered a warning, however, to the current success of COVID-19 control in Taiwan. As the severity of the COVID-19 pandemic rises globally, the risk of SARS-CoV-2 invasion and spread into Taiwan with returning travelers or international business travelers is inevitably increasing, especially from those with asymptomatic infection with prolonged communicability period and virus shedding (44–47). The risk will be even higher after Taiwan opens the border to revitalize international business to boost the economy. Additional strategies, such as a screening policy after 14 days of quarantine, should be considered to prevent the SARS-CoV-2 invasion in Taiwan.
The potential cross-reactive antibody response between SARS-CoV-2 and SARS-CoV among recovered SARS patients long after an infection was an important finding in this study. Previous studies showed that the anti-SARS-CoV IgG and neutralizing antibodies decreased markedly 2 years after the infection and were detected in only 8.7% of patients 6 years after recovery from SARS infection (48, 49). SARS-CoV-specific peripheral memory B cell response was undetectable, but memory T cell responses could be identified in 60.9% of recovered SARS patients 6 years after infection (49). One possible explanation is the increased detection sensitivity of current serological tests compared to that of ELISA kits used in previous studies (49–51). Nonetheless, our study suggested the possibility of the prolonged existence of anti-SARS-CoV antibodies after 17 years of infection causing a false-positive testing result from the cross-reactive antibody response between the two viruses with close genetic composition (52, 53). Therefore, in the seroprevalence study for COVID-19 infection in the areas with prior SARS outbreak, false-positive testing results should be considered among participants with virologically confirmed SARS infection.
This study has some limitations. First, this was a two-center study conducted in northern Taiwan. Thus, a nationwide seroprevalence study to confirm our study findings is imperative. Second, the inadequate case number of a seroprevalence study in areas with low disease prevalence might have failed to detect sporadic COVID-19 infection in the community and healthcare system. It was especially important in the estimation of the seroprevalence among patients with epidemiological risk of SARS-CoV-2 exposure in this study. Third, although both the Roche and Abbott tests are based on antibodies against SARS-CoV-2 N protein, the relative higher false-positive rate in Roche test was not further investigated in this study. Fourth, we did not test the anti-SARS-CoV antibodies in patients with virologically-documented SARS-CoV infection in 2003. It is not known whether the cross-reactivity was caused by anti-SARS-CoV antibody after 17 years since SARS-CoV infection. Fifth, returning or foreign travelers without symptoms were not included in this study. It was impossible to detect asymptomatic infection, and therefore, the threat from influx pressure of SARS-CoV-2 was underestimated. Finally, asymptomatic infection with transient or absent antibody response to SARS-CoV-2 infection remains theoretically plausible. Therefore, extent of SARS-CoV-2 transmission in the community might be further underestimated (54).
Conclusion
In conclusion, rigorous exclusion of false-positive testing results is imperative for an accurate estimate of seroprevalence and disease burden in countries with low COVID-19 disease prevalence and the existence of outbreaks of SARS in 2003. The overall SARS-CoV-2 seroprevalence was extremely low in Taiwan, reflecting the early success of nationwide containment strategies. However, with the increasing severity of the global COVID-19 pandemic and virus burden, additional countermeasures based on current strategies such as the establishment of adequate herd immunity by vaccination to prevent the invasion and spread of SARS-CoV-2 in Taiwan should be considered.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The study was conducted on June 29 to July 12, 2020, in NTUH and on July 25, 2020, in NTUH HBP branch. Study approvals from the institutional review board were obtained from NTUH (202004128RINB) and NTUH HBP Branch (202007007RIPB). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
W-PT, J-LW, C-CW, and P-RH designed the research. K-TK, C-HL, M-YC, Y-FL, C-JY, C-HH, and S-YC performed the research and analyzed the data. W-PT, J-LW, and S-YC wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by the National Taiwan University Hospital (grant numbers: NTUH 109-P12).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.626609/full#supplementary-material
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus From Patients With Pneumonia in China, 2019. N Engl J Med (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. World Health Organization. Situation Reports: Weekly Epidemiological Update – Coronavirus Disease 2019 (COVID-19) (2020). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (Accessed 28 August 2020).
3. Chen SY, Ma MH, Su CP, Chiang WC, Ko PC, Lai TI, et al. Facing an Outbreak of Highly Transmissible Disease: Problems in Emergency Department Response. Ann Emerg Med (2004) 44:93–5. doi: 10.1016/j.annemergmed.2004.01.028
4. Hsueh PR, Kao CL, Lee CN, Chen LK, Ho MS, Sia C, et al. SARS Antibody Test for Serosurveillance. Emerg Infect Dis (2004) 10:1558–62. doi: 10.3201/eid1009.040101
5. Wang CJ, Ng CY, Brook RH. Response to COVID-19 in Taiwan: Big Data Analytics, New Technology, and Proactive Testing. JAMA (2020) 323:1341–2. doi: 10.1001/jama.2020.3151
6. Taiwan Centers for Disease Control. Available at: https://www.cdc.gov.tw/Bulletin/Detail/6oHuoqzW9e_onW0AaMEemg?typeid=9 (Accessed 28 August 2020). Press Release.
7. Taiwan Centers for Disease Control. Available at: https://sites.google.com/cdc.gov.tw/2019ncov/taiwan (Accessed 28 August 2020). Press Release.
8. Our World in Data. Total COVID-19 Tests, Confirmed Cases and Deaths Per Million People. Available at: https://ourworldindata.org/grapher/covid-tests-cases-deaths-per-million (Accessed 28 August 2020).
9. Lai CC, Wang CY, Ko WC, Hsueh PR. In Vitro Diagnostics of Coronavirus Disease 2019: Technologies and Application. J Microbiol Immunol Infect (2021) 54(2):164–74. doi: 10.1016/j.jmii.2020.05.016
10. Byambasuren O, Cardona M, Bell K, Clark J, McLaws ML, Glasziou P. Estimating the Extent of Asymptomatic COVID-19 and its Potential for Community Transmission: Systematic Review and Meta-Analysis. JAMMI (2020) 5.4:223–34. doi: 10.3138/jammi-2020-0030
11. Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the Asymptomatic Proportion of Coronavirus Disease 2019 (COVID-19) Cases on Board the Diamond Princess Cruise Ship, Yokohama, Japan, 2020. Euro Surveill (2020) 25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180
12. Zhou X, Li Y, Li T, Zhang W. Follow-Up of Asymptomatic Patients With SARS-CoV-2 Infection. Clin Microbiol Infect (2020) 26:957–9. doi: 10.1016/j.cmi.2020.03.024
13. Kimball A, Hatfield KM, Arons M, James A, Taylor J, Spicer K, et al. Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep (2020) 69:377–81. doi: 10.15585/mmwr.mm6913e1
14. Havers FP, Reed C, Lim T, Montgomery JM, Klena JD, Hall AJ, et al. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern Med (2020) 10.1001/jamainternmed.2020.4130. doi: 10.1001/jamainternmed.2020.4130
15. Theel ES, Slev P, Wheeler S, Couturier MR, Wong SJ, Kadkhoda K. The Role of Antibody Testing for SARS-CoV-2: Is There One? J Clin Microbiol (2020) 58:e00797–20. doi: 10.1128/JCM.00797-20
16. Ko JH, Joo EJ, Kim SH, Kim YJ, Huh K, Cho SY, et al. Clinical Application of Rapid Diagnostic Test Kit for SARS-CoV-2 Antibodies Into the Field of Patient Care. J Microbiol Immunol Infect (2020) 54(1):97–100. doi: 10.1016/j.jmii.2020.07.003 S1684-1182(20)30160-2.
17. Wu JL, Tseng WP, Lin CH, Lee TF, Chung MY, Huang CH, et al. Four Point-of-Care Lateral Flow Immunoassays for Diagnosis of COVID-19 and for Assessing Dynamics of Antibody Responses to SARS-Cov-2. J Infect (2020) 81:435–42. doi: 10.1016/j.jinf.2020.06.023
18. Muench P, Jochum S, Wenderoth V, Ofenloch-Haehnle B, Hombach M, Strobl M, et al. Development and Validation of the Elecsys Anti-SARS-CoV-2 Immunoassay as a Highly Specific Tool for Determining Past Exposure to SARS-Cov-2. J Clin Microbiol (2020) 58:e01694–20. doi: 10.1128/JCM.01694-20
19. Bryan A, Pepper G, Wener MH, Fink SL, Morishima C, Chaudhary A, et al. Performance Characteristics of the Abbott Architect SARS-CoV-2 Igg Assay and Seroprevalence in Boise, Idaho. J Clin Microbiol (2020) 58:e00941–20. doi: 10.1128/JCM.00941-20
20. Lv H, Wu NC, Tsang OT, Yuan M, Perera RAPM, Leung WS, et al. Cross-Reactive Antibody Response Between SARS-CoV-2 and SARS-CoV Infections. Cell Rep (2020) 31:107725. doi: 10.1016/j.celrep.2020.107725
21. Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg Infect Dis (2020) 26:1478–88. doi: 10.3201/eid2607.200841
22. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
23. Taiwan Centers for Disease Control. Coronavirus Disease 2019 (COVID-19). Available at: https://www.cdc.gov.tw/en/Disease/SubIndex/ (Accessed 12 August 2020).
24. Elecsys Anti-SARS-Cov-2. FDA. Available at: https://www.fda.gov/media/137605/download.
25. Sars-CoV-2 Igg. FDA. Available at: https://www.fda.gov/media/137383/download.
26. Chen SY, Lee YL, Lin YC, Lee NY, Liao CH, Hung YP, et al. Multicenter Evaluation of Two Chemiluminescence and Three Lateral Flow Immunoassays for the Diagnosis of COVID-19 and Assessment of Antibody Dynamic Responses to SARS-CoV-2 in Taiwan. Emerg Microbes Infect (2020) 9:2157–68. doi: 10.1080/22221751.2020.1825016
27. Recomline SARS-CoV-2 Igg. Available at: https://www.mikrogen.de/produkte/produktuebersicht/testsystem/sars-cov-2-igg-aviditaet-ruo.html.
28. Kirkwood B, Sterne J. Essentials of Medical Statistics. 2nd Edition. Malden, Mass: Wiley-Blackwell (2001). p. 512.
29. Available at: https://dep-civil.hccg.gov.tw/ch/home.jsp?id=55&parentpath=0,4,46 (Accessed 30 March 2021).
30. Available at: https://w3.hsinchu.gov.tw/house/print/city.aspx?y=109&m=12 (Accessed on 30 March 2021).
31. Xu X, Sun J, Nie S, Li H, Kong Y, Liang M, et al. Seroprevalence of Immunoglobulin M and G Antibodies Against SARS-CoV-2 in China. Nat Med (2020) 26:1193–5. doi: 10.1038/s41591-020-0949-6
32. Pan SC, Huang YS, Hsieh SM, Chen CY, Chang SY, Chang SC. A Cross-Sectional Seroprevalence for COVID-19 Among Healthcare Workers in a Tertially Care Hospital in Taiwan. J Formos Med Assoc (2021) S0929-6646(21)00019-X. doi: 10.1016/j.jfma.2021.01.002
33. Ho HL, Wang FY, Lee HR, Huang YL, Lai CL, Jen WC, et al. Seroprevalence of COVID-19 in Taiwan Revealed by Testing anti-SARS-CoV-2 Serological Antibodies on 14,765 Hospital Patients. Lancet Regional Health - Western Pac (2020) 3:100041. doi: 10.1016/j.lanwpc.2020.100041
34. Lin CH, Tseng WP, Wu JL, Tay J, Cheng MT, Ong HN, et al. A Double Triage and Telemedicine Protocol to Optimize Infection Control in an Emergency Department in Taiwan During the COVID-19 Pandemic: Retrospective Feasibility Study. J Med Internet Res (2020) 22:e20586. doi: 10.2196/20586
35. Zhang Z, Liu S, Xiang M, Li S, Zhao D, Huang C, et al. Protecting Healthcare Personnel From 2019-nCoV Infection Risks: Lessons and Suggestions. Front Med (2020) 14:229–31. doi: 10.1007/s11684-020-0765-x
36. Dewey C, Hingle S, Goelz E, Linzer M. Supporting Clinicians During the COVID-19 Pandemic. Ann Intern Med (2020) 172:752–3. doi: 10.7326/M20-1033
37. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (Ene-COVID): A Nationwide, Population-Based Seroepidemiological Study. Lancet (2020) 396:535–44. doi: 10.1016/S0140-6736(20)31483-5
38. Rosenberg ES, Tesoriero JM, Rosenthal EM, Chung R, Barranco MA, Styer LM, et al. Cumulative Incidence and Diagnosis of SARS-CoV-2 Infection in New York. Ann Epidemiol (2020) 48:23–9.e4. doi: 10.1016/j.annepidem.2020.06.004
39. Percivalle E, Cambiè G, Cassaniti I, Nepita EV, Maserati R, Ferrari A, et al. Prevalence of SARS-CoV-2 Specific Neutralising Antibodies in Blood Donors From the Lodi Red Zone in Lombardy, Italy, as At 06 April 2020. Euro Surveill (2020) 25:2001031. doi: 10.2807/1560-7917.ES.2020.25.24.2001031
40. Sood N, Simon P, Ebner P, Eichner D, Reynolds J, Bendavid E, et al. Seroprevalence of SARS-CoV-2-specific Antibodies Among Adults in Los Angeles County, California, on April 10-11, 2020. JAMA (2020) 323:2425–7. doi: 10.1001/jama.2020.8279
41. Chen Y, Tong X, Wang J, Huang W, Yin S, Huang R, et al. High SARS-CoV-2 Antibody Prevalence Among Healthcare Workers Exposed to COVID-19 Patients. J Infect (2020) 81:420–6. doi: 10.1016/j.jinf.2020.05.067
42. Torres JP, Piñera C, De La Maza V, Lagomarcino AJ, Simian D, Torres B, et al. Sars-CoV-2 Antibody Prevalence in Blood in a Large School Community Subject to a Covid-19 Outbreak: A Cross-Sectional Study. Clin Infect Dis (2020) ciaa955. doi: 10.1093/cid/ciaa955
43. Li Y, Yao L, Li J, Chen L, Song Y, Cai Z, et al. Stability Issues of RT-PCR Testing of SARS-CoV-2 for Hospitalized Patients Clinically Diagnosed With COVID-19. J Med Virol (2020) 92:903–8. doi: 10.1002/jmv.25786
44. Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. Sars-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med (2020) 382:1177–9. doi: 10.1056/NEJMc2001737
45. Huang L, Zhang X, Zhang X, Wei Z, Zhang L, Xu J, et al. Rapid Asymptomatic Transmission of COVID-19 During the Incubation Period Demonstrating Strong Infectivity in a Cluster of Youngsters Aged 16-23 Years Outside Wuhan and Characteristics of Young Patients With COVID-19: A Prospective Contact-Tracing Study. J Infect (2020) 80:e1–13. doi: 10.1016/j.jinf.2020.03.006
46. Kim GU, Kim MJ, Ra SH, Lee J, Bae S, Jung J, et al. Clinical Characteristics of Asymptomatic and Symptomatic Patients With Mild COVID-19. Clin Microbiol Infect (2020) 26:948.e1–3. doi: 10.1016/j.cmi.2020.04.040
47. Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, et al. Asymptomatic Carrier State, Acute Respiratory Disease, and Pneumonia Due to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-Cov-2): Facts and Myths. J Microbiol Immunol Infect (2020) 53:404–12. doi: 10.1016/j.jmii.2020.02.012
48. Lin Q, Zhu L, Ni Z, Meng H, You L. Duration of Serum Neutralizing Antibodies for SARS-CoV-2: Lessons From SARS-CoV Infection. J Microbiol Immunol Infect (2020) 53:821–2. doi: 10.1016/j.jmii.2020.03.015J
49. Tang F, Quan Y, Xin ZT, Wrammert J, Ma MJ, Lv H, et al. Lack of Peripheral Memory B Cell Responses in Recovered Patients With Severe Acute Respiratory Syndrome: A Six-Year Follow-Up Study. J Immunol (2011) 186:7264–8. doi: 10.4049/jimmunol.0903490
50. Liu W, Fontanet A, Zhang PH, Zhan L, Xin ZT, Baril L, et al. Two-Year Prospective Study of the Humoral Immune Response of Patients With Severe Acute Respiratory Syndrome. J Infect Dis (2006) 193:792–5. doi: 10.1086/500469
51. Wu LP, Wang NC, Chang YH, Tian XY, Na DY, Zhang LY, et al. Duration of Antibody Responses After Severe Acute Respiratory Syndrome. Emerg Infect Dis (2007) 13:1562–4. doi: 10.3201/eid1310.070576
52. Jarjour NN, Masopust D, Jameson SC. T Cell Memory: Understanding Covid-19. Immunity (2021) 54:14–8. doi: 10.1016/j.immuni.2020.12.009
53. Cox RJ, Brokstad KA. Not Just Antibodies: B Cells and T Cells Mediate Immunity to COVID-19. Nat Rev Immunol (2020) 20:581–2. doi: 10.1038/s41577-020-00436-4
Keywords: SARS-CoV-2, COVID-19, seroprevalence, cross-reactivity, SARS
Citation: Tseng W-P, Wu J-L, Wu C-C, Kuo K-T, Lin C-H, Chung M-Y, Lee Y-F, Yang B-J, Huang C-H, Chen S-Y, Yu C-J, Chen S-C and Hsueh P-R (2021) Seroprevalence Surveys for Anti-SARS-CoV-2 Antibody in Different Populations in Taiwan With Low Incidence of COVID-19 in 2020 and Severe Outbreaks of SARS in 2003. Front. Immunol. 12:626609. doi: 10.3389/fimmu.2021.626609
Received: 06 November 2020; Accepted: 21 April 2021;
Published: 18 May 2021.
Edited by:
Onur Boyman, University of Zurich, SwitzerlandReviewed by:
Carlo Cervia, University Hospital Zürich, SwitzerlandRodney Rosalia, Zan Mitrev Clinic, North Macedonia
Grégory Bouchaud, INRA Centre Angers-Nantes Pays de la Loire, France
Copyright © 2021 Tseng, Wu, Wu, Kuo, Lin, Chung, Lee, Yang, Huang, Chen, Yu, Chen and Hsueh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Po-Ren Hsueh, hsporen@ntu.edu.tw; Shey-Ying Chen, erdrcsy@gmail.com; Chong-Jen Yu, jefferyyu@ntu.edu.tw
 Wen-Pin Tseng
Wen-Pin Tseng Jhong-Lin Wu
Jhong-Lin Wu Chen-Chi Wu2,3
Chen-Chi Wu2,3 Po-Ren Hsueh
Po-Ren Hsueh