A Case-Control of Patients with COVID-19 to Explore the Association of Previous Hospitalisation Use of Medication on the Mortality of COVID-19 Disease: A Propensity Score Matching Analysis
Abstract
:1. Introduction
2. Results
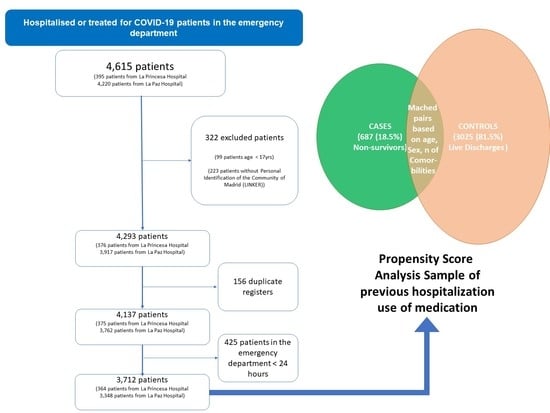
2.1. Case-Control Results
2.1.1. Characteristics of the Patients
2.1.2. Propensity Score Matching Analysis
3. Discussion
3.1. Final Substances Significantly Associated with Mortality
3.1.1. Digoxin
3.1.2. Folic Acid
3.1.3. Mirtazapine
3.1.4. Linagliptin
3.1.5. Enalapril
3.1.6. Atorvastatin
3.1.7. Allopurinol
3.1.8. Acetylsalicylic Acid
3.2. Final Substances Significantly Associated with Survival
3.2.1. Enoxaparine and Bemiparine
3.2.2. Oral Rehydration Salts
3.2.3. Azithromycin
3.2.4. Cefuroxime
3.2.5. Inhaled Glucocorticoids and Bronchodilators
3.2.6. Loratadine
3.2.7. Colchicine
3.3. Strengths and Limitations
4. Materials and Methods
4.1. Study Design and Population
4.2. Clinical Data Collection
4.3. Variables and Exposure
4.4. Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Safford, M.M. Clinical Characteristics of COVID-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Garg, S.; Kim, L.; Whitaker, M.; O’Halloran, A.; Cummings, C.; Holstein, R.; Fry, A. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, 1–30 March 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Cao, B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Peng, Z. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Cao, B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Zhong, N.S. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-Converting Enzyme 2 (ACE2) as a SARS-CoV-2 Receptor: Molecular Mechanisms and Potential Therapeutic Target. Intensive Care Med. 2020, 46, 586–590. Available online: http://link.springer.com/10.1007/s00134-020-05985-9 (accessed on 29 June 2021). [CrossRef] [Green Version]
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; Hochman, J.S. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N. Engl. J. Med. 2020, 382, 2441–2448. [Google Scholar] [CrossRef]
- Jarcho, J.A.; Ingelfinger, J.R.; Hamel, M.B.; D’Agostino, R.B., Sr.; Harrington, D.P. Inhibitors of the Renin-Angiotensin-Aldosterone System and COVID-19. N. Engl. J. Med. 2020, 382, 2462–2464. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.J.; Xie, J.; Li, H. Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality among Patients with Hypertension Hospitalized with COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef]
- Bean, D.M.; Kraljevic, Z.; Searle, T.; Bendayan, R.; Pickles, A.; Folarin, A.; Dobson, R.J. Treatment with AVE-inhibitors is associated with less severe disease with SARS-COVID-19 infection in a multi-site UK acute Hospital Trust. MedRxiv 2020. [CrossRef] [Green Version]
- Li, G.; De Clercq, E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat. Rev. Drug Discov. 2020, 19, 149–150. [Google Scholar] [CrossRef] [Green Version]
- Lu, H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci. Trends 2020, 14, 69–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Siddique, R.; Shereen, M.A.; Ali, A.; Liu, J.; Bai, Q.; Xue, M. Emergence of a Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2: Biology and Therapeutic Options. J. Clin. Microbiol. 2020, 58, e00187-20. [Google Scholar] [CrossRef] [Green Version]
- Rishita, P.; Thommana, M.V.; Ruiz, M.B.; Ayna, S. Therapeutic Options for COVID-19: A Review. Cureus 2020, 12, e10480. [Google Scholar] [CrossRef]
- Reiffel, J.A. Propensity Score Matching: The ‘Devil is in the Details’ Where More May Be Hidden than You Know. Am. J. Med. 2020, 133, 178–181. [Google Scholar] [CrossRef]
- Peltzer, B.; Manocha, K.K.; Ying, X.; Kirzner, J.; Ip, J.E.; Thomas, G.; Liu, C.F.; Markowitz, S.M.; Lerman, B.B.; Safford, M.M.; et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J. Cardiovasc. Electrophysiol. 2020, 12, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Pollard, B.S.; Blanco, J.C.; Pollard, J.R. Classical Drug Digitoxin Inhibits Influenza Cytokine Storm, with Implications for COVID-19 Therapy. In Vivo 2020, 34, 3723–3730. [Google Scholar] [CrossRef]
- Cho, J.; Lee, Y.J.; Kim, J.H.; Kim, S.I.; Kim, S.S.; Choi, B.S.; Choi, J.H. Antiviral activity of digoxin and ouabain against SARS-CoV-2 infection and its implication for COVID-19. Sci. Rep. 2020, 10, 16200. [Google Scholar] [CrossRef]
- Vamos, M.; Erath, J.W.; Hohnloser, S.H. Digoxin-associated mortality: A systematic review and meta-analysis of the literature. Eur. Heart J. 2015, 36, 1831–1838. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Q.; Zhang, R.; Chen, M.T.; Wang, Q.S.; Zhang, Y.; Huang, X.H.; Wang, J.; Yan, J.H.; Li, Y.G. Digoxin Is Associated with Increased All-Cause Mortality in Patients with Atrial Fibrillation Regardless of Concomitant Heart Failure: A Meta-Analysis. J. Cardiovasc. Pharmacol. 2015, 66, 270–275. [Google Scholar] [CrossRef]
- Ouyang, A.J.; Lv, Y.N.; Zhong, H.L.; Wen, J.H.; Wei, X.H.; Peng, H.W.; Zhou, J.; Liu, L.L. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am. J. Cardiol. 2015, 115, 901–9066. [Google Scholar] [CrossRef]
- Vamos, M.; Erath, J.W.; Benz, A.P.; Lopes, R.D.; Hohnloser, S.H. Meta-Analysis of Effects of Digoxin on Survival in Patients with Atrial Fibrillation or Heart Failure: An Update. Am. J. Cardiol. 2019, 123, 69–74. [Google Scholar] [CrossRef]
- Fedele, D.; De Francesco, A.; Riso, S.; Collo, A. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: An overview. Nutrition 2021, 81, 111016. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.L.; Nguyen, L.M.; Noble, K.N.; Aronoff, D.M. COVID-19-related disease severity in pregnancy. Am. J. Reprod. Immunol. 2020, 84, e13339. [Google Scholar] [CrossRef] [PubMed]
- Spanish Agency for Medicines and Health Products. Technical Data Sheet Mirtazapina Cinfa®. Available online: https://cima.aemps.es/cima/dochtml/ft/67068/FT_67068.html (accessed on 29 June 2021).
- Spanish Agency for Medicines and Health Products. Technical Data Sheet Qudix®. Available online: https://cima.aemps.es/cima/dochtml/ft/70169/FT_70169.html (accessed on 29 June 2021).
- Coupland, C.; Dhiman, P.; Morriss, R.; Arthur, A.; Barton, G.; Hippisley-Cox, J. Antidepressant use and risk of adverse outcomes in older people: Population based cohort study. BMJ 2011, 343, d4551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seon, J.Y.; Kim, S.; Hong, M.; Lim, M.K.; Oh, I.H. Risk of COVID-19 diagnosis and death in patients with mental illness: A cohort study. Epidemiol. Psychiatr. Sci. 2021, 30, e68. [Google Scholar] [CrossRef] [PubMed]
- Spanish Agency for Medicines and Health Products. Technical Data Sheet Trajenta®. Available online: https://cima.aemps.es/cima/dochtml/ft/11707004/FT_11707004.html (accessed on 29 June 2021).
- Kilis-Pstrusinska, K.; Akutko, K.; Braksator, J.; Dancewicz, A.; Grosman-Dziewiszek, P.; Jamer, T.; Juszczyńska, K.; Konikowska, K.; Koruba, M.; Pupek, M.; et al. Kidney Dysfunction and Its Progression in Patients Hospitalized Duo to COVID-19: Contribution to the Clinical Course and Outcomes. J. Clin. Med. 2021, 10, 5522. [Google Scholar] [CrossRef]
- de Abajo, F.J.; Rodríguez-Martín, S.; Lerma, V.; Mejía-Abril, G.; Aguilar, M.; García-Luque, A.; Laredo, L.; Laosa, O.; Centeno-Soto, G.A.; Ángeles Gálvez, M.; et al. MED-ACE2-COVID-19 study group. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: A case-population study. Lancet 2020, 395, 1705–1714. [Google Scholar] [CrossRef]
- Mackey, K.; King, V.J.; Gurley, S.; Kiefer, M.; Liederbauer, E.; Vela, K.; Sonnen, P.; Kansagara, D. Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults: A Living Systematic Review. Ann. Intern. Med. 2020, 173, 195–203. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. Br. J. Pharmacol. 2020, 177, 4825–4844. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Gari, A.; Elshony, N.; Shaheen, H.M.; Abubakar, M.B.; Adeyemi, S.B.; Al-Kuraishy, H.M. Hypertension and its management in COVID-19 patients: The assorted view. Int. J. Cardiol. Cardiovasc. Risk Prev. 2021, 11, 200121. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Anawalt, B.; Boyce, A.; Chrousos, G.; de Herder, W.W.; Dhatariya, K.; Dungan, K.; Grossman, A.; Hershman, J.M.; Hofland, J.; et al. Lipid and Lipoprotein Levels in Patients with COVID-19 Infections. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Sammalkorpi, K.; Valtonen, V.; Kerttula, Y.; Nikkilä, E.; Taskinen, M.R. Changes in serum lipoprotein pattern induced by acute infections. Metabolism 1988, 37, 859–865. [Google Scholar] [CrossRef]
- Spanish Agency for Medicines and Health Products. Technical Data Sheet Veklury®. Available online: https://cima.aemps.es/cima/dochtml/ft/1201459002/FT_1201459002.html (accessed on 29 June 2021).
- Spanish Agency for Medicines and Health Products. Technical Data Sheet Kaletra®. Available online: https://cima.aemps.es/cima/dochtml/ft/01172006/FT_01172006.html (accessed on 29 June 2021).
- Spanish Agency for Medicines and Health Products. Technical Data Sheet Roactemra®. Available online: https://cima.aemps.es/cima/dochtml/ft/108492007/FT_108492007.html#4-datos-cl-nicos (accessed on 29 June 2021).
- Singh, J.A.; Edwards, N.L. Gout management and outcomes during the COVID-19 pandemic: A cross-sectional internet survey. Ther. Adv. Musculoskelet Dis. 2020, 12, 1759720X20966124. [Google Scholar] [CrossRef]
- Madan, A.; Siglin, J.; Khan, A. Comprehensive review of implications of COVID-19 on clinical outcomes of cancer patients and management of solid tumors during the pandemic. Cancer Med. 2020, 9, 9205–9218. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Mehta, J.L. Meta-Analysis of the Effect of Aspirin on Mortality in COVID-19. Am. J. Cardiol. 2021, 142, 158–159. [Google Scholar] [CrossRef]
- Paar, V.; Wernly, B.; Zhou, Z.; Motloch, L.J.; Hoppe, U.C.; Egle, A.; Lichtenauer, M. Anti-coagulation for COVID-19 treatment: Both anti-thrombotic and anti-inflammatory? J. Thromb. Thrombolysis 2020, 51, 226–231. [Google Scholar] [CrossRef]
- Echeverría-Esnal, D.; Martin-Ontiyuelo, C.; Navarrete-Rouco, M.E.; Cuscó, M.D.-A.; Ferrández, O.; Horcajada, J.P.; Grau, S. Azithromycin in the treatment of COVID-19: A review. Expert Rev. Anti Infect. Ther. 2021, 19, 147–163. [Google Scholar] [CrossRef]
- Durojaiye, A.B.; Clarke, J.-R.D.; Stamatiades, G.A.; Wang, C. Repurposing cefuroxime for treatment of COVID-19: A scoping review of in silico studies. J. Biomol. Struct. Dyn. 2020, 39, 4547–4554. [Google Scholar] [CrossRef]
- Elfiky, A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: An in silico perspective. J. Biomol. Struct. Dyn. 2021, 39, 3204–3212. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, K.; Al-Duhaidahawi, D.; Taskin Tok, T. Using integrated computational approaches to identify safe and rapid treatment for SARS-CoV-2. J. Biomol. Struct. Dyn. 2021, 39, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, D.V.; Bafadhel, M. Inhaled corticosteroids in virus pandemics: A treatment for COVID-19? Lancet Respir. Med. 2020, 8, 846–847. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Nicolau, D.V., Jr.; Langford, B.; Mahdi, M.; Jeffers, H.; Mwasuku, C.; Krassowska, K.; Fox, R.; Binnian, I.; Glover, V.; et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): A phase 2, open-label, randomised controlled trial. Lancet Respir. Med. 2021, 9, 763–772. [Google Scholar] [CrossRef]
- Hou, Y.; Ge, S.; Li, X.; Wang, C.; He, H.; He, L. Testing of the inhibitory effects of loratadine and desloratadine on SARS-CoV-2 spike pseudotyped virus viropexis. Chem. Interact. 2021, 338, 109420. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Halim, D.A.; Jodhinata, C.; Yanto, T.A.; Kurniawan, A. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Clin. Exp. Pharmacol. Physiol. 2021, 48, 823–830. [Google Scholar] [CrossRef]
- Royal Decree 1090/2015, of 4 December, Regulating Clinical Trials with Medicinal Products, Ethics Committees for Investigation with Medicinal Products and the Spanish Clinical Studies Registry. Available online: https://www.aemps.gob.es/legislacion/espana/investigacionClinica/docs/Royal-Decree-1090-2015_4-December.pdf (accessed on 13 December 2021).
- Organic Law 3/2018 of 5 December 2018, on the Protection of Personal Data and Guarantee of Digital Rights. 2018. Available online: https://noticias.juridicas.com/base_datos/Laboral/632849-lo-3-2018-de-5-dic-proteccion-de-datos-personales-y-garantia-de-los-derechos.html (accessed on 20 December 2021).
- Novel Coronaviris (nCoV) Acute Respiratory Infection Clinical Characterisation Data Tools. nCoV Case Records form Version 1.3. Adapted from Sprint Sari Case Report Form by ISARIC. 24 February 2020. Available online: https://media.tghn.org/medialibrary/2020/03/ISARIC_COVID-19_CRF_V1.3_24Feb2020.pdf (accessed on 16 April 2020).
- Borobia, A.M.; Carcas, A.J.; Arnalich, F.; Álvarez-Sala, R.; Monserrat-Villatoro, J.; Quintana, M. A Cohort of Patients with COVID-19 in a Major Teaching Hospital in Europe. J. Clin. Med. 2020, 9, 1733. [Google Scholar] [CrossRef]
- ATC/DDD Index 2020 Updated 16 December 2019. Available online: https://www.whocc.no/atc_ddd_index/> (accessed on 4 September 2020).
- Drugbank Pharmaceutical Knowledge Base that is Enabling Major Advances across the Data-Driven Medicine Industry. Available online: https://www.drugbank.ca/drugs (accessed on 4 September 2020).
- R Foundation. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 29 June 2021).
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio, PBC: Boston, MA, USA, 2021. Available online: http://www.rstudio.com/ (accessed on 29 June 2021).

| [ALL] n = 3712 n (%) | Case (Deceased) n = 687 n (%) | Control (Live Discharges) n = 3025 n (%) | OR | p.ratio | p.overall | |
|---|---|---|---|---|---|---|
| Sex | <0.001 | |||||
| Man | 1777 (47.9%) | 419 (61.0%) | 1358 (44.9%) | Ref. | Ref. | |
| Woman | 1930 (52.0%) | 268 (39.0%) | 1662 (54.9%) | 0.52 [0.44;0.62] | <0.001 | |
| ‘Missing’ | 5 (0.13%) | 0 (0.00%) | 5 (0.17%) | 0.00 [0.00;3.55] | 0.261 | |
| Age, years (mean (SD)) | 62.0 [48.0;78.0] | 83.0 [75.0;88.0] | 57.0 [44.0;72.0] | 1.10 [1.09;1.11] | <0.001 | <0.001 |
| Arterial hypertension | <0.001 | |||||
| No | 2190 (59.0%) | 223 (32.5%) | 1967 (65.0%) | Ref. | Ref. | |
| Yes | 1511 (40.7%) | 462 (67.2%) | 1049 (34.7%) | 3.88 [3.25;4.66] | <0.001 | |
| ‘Missing’ | 11 (0.30%) | 2 (0.29%) | 9 (0.30%) | 1.96 [0.20;9.55] | 0.406 | |
| Diabetes mellitus | <0.001 | |||||
| No | 3043 (82.0%) | 459 (66.8%) | 2584 (85.4%) | Ref. | Ref. | |
| Yes | 656 (17.7%) | 225 (32.8%) | 431 (14.2%) | 2.94 [2.42;3.56] | <0.001 | |
| ‘Missing’ | 13 (0.35%) | 3 (0.44%) | 10 (0.33%) | 1.69 [0.30;6.59] | 0.433 | |
| Non-complicated diabetes mellitus | <0.001 | |||||
| No | 3225 (86.9%) | 536 (78.0%) | 2689 (88.9%) | Ref. | Ref. | |
| Yes | 421 (11.3%) | 134 (19.5%) | 287 (9.49%) | 2.34 [1.85;2.95] | <0.001 | |
| ‘Missing’ | 66 (1.78%) | 17 (2.47%) | 49 (1.62%) | 1.74 [0.93;3.10] | 0.062 | |
| Complicated diabetes mellitus | <0.001 | |||||
| No | 3545 (95.5%) | 623 (90.7%) | 2922 (96.6%) | Ref. | Ref. | |
| Yes | 100 (2.69%) | 45 (6.55%) | 55 (1.82%) | 3.84 [2.50;5.85] | <0.001 | |
| ‘Missing’ | 67 (1.80%) | 19 (2.77%) | 48 (1.59%) | 1.86 [1.02;3.24] | 0.031 | |
| Dislipemia | <0.001 | |||||
| No | 2526 (68.0%) | 334 (48.6%) | 2192 (72.5%) | Ref. | Ref. | |
| Yes | 1168 (31.5%) | 350 (50.9%) | 818 (27.0%) | 2.81 [2.36;3.34] | <0.001 | |
| ‘Missing’ | 18 (0.48%) | 3 (0.44%) | 15 (0.50%) | 1.31 [0.24;4.67] | 0.641 | |
| Obesity | 0.001 | |||||
| No | 3190 (85.9%) | 564 (82.1%) | 2626 (86.8%) | Ref. | Ref. | |
| Yes | 459 (12.4%) | 102 (14.8%) | 357 (11.8%) | 1.33 [1.04;1.70] | 0.021 | |
| ‘Missing’ | 63 (1.70%) | 21 (3.06%) | 42 (1.39%) | 2.33 [1.30;4.06] | 0.003 | |
| Chronic heart disease | <0.001 | |||||
| No | 3007 (81.0%) | 399 (58.1%) | 2608 (86.2%) | Ref. | Ref. | |
| Yes | 686 (18.5%) | 283 (41.2%) | 403 (13.3%) | 4.59 [3.80;5.54] | <0.001 | |
| ‘Missing’ | 19 (0.51%) | 5 (0.73%) | 14 (0.46%) | 2.33 [0.65;6.90] | 0.130 | |
| Chronic lung disease | <0.001 | |||||
| No | 3475 (93.6%) | 619 (90.1%) | 2856 (94.4%) | Ref. | Ref. | |
| Yes | 216 (5.82%) | 60 (8.73%) | 156 (5.16%) | 1.77 [1.28;2.44] | <0.001 | |
| ‘Missing’ | 21 (0.57%) | 8 (1.16%) | 13 (0.43%) | 2.84 [1.02;7.42] | 0.030 | |
| Chronic obstructive pulmonary disease | <0.001 | |||||
| No | 3434 (92.5%) | 588 (85.6%) | 2846 (94.1%) | Ref. | Ref. | |
| Yes | 260 (7.00%) | 95 (13.8%) | 165 (5.45%) | 2.79 [2.11;3.67] | <0.001 | |
| ‘Missing’ | 18 (0.48%) | 4 (0.58%) | 14 (0.46%) | 1.38 [0.33;4.42] | 0.556 | |
| Asthma | 0.040 | |||||
| No | 3489 (94.0%) | 656 (95.5%) | 2833 (93.7%) | Ref. | Ref. | |
| Yes | 205 (5.52%) | 26 (3.78%) | 179 (5.92%) | 0.63 [0.40;0.96] | 0.024 | |
| ‘Missing’ | 18 (0.48%) | 5 (0.73%) | 13 (0.43%) | 1.66 [0.46;4.99] | 0.347 | |
| Neurological chronic disease | <0.001 | |||||
| No | 3331 (89.7%) | 543 (79.0%) | 2788 (92.2%) | Ref. | Ref. | |
| Yes | 363 (9.78%) | 139 (20.2%) | 224 (7.40%) | 3.18 [2.51;4.03] | <0.001 | |
| ‘Missing’ | 18 (0.48%) | 5 (0.73%) | 13 (0.43%) | 1.97 [0.55;5.93] | 0.218 | |
| Mild liver disease | 0.342 | |||||
| No | 3601 (97.0%) | 663 (96.5%) | 2938 (97.1%) | Ref. | Ref. | |
| Yes | 95 (2.56%) | 19 (2.77%) | 76 (2.51%) | 1.11 [0.63;1.87] | 0.680 | |
| ‘Missing’ | 16 (0.43%) | 5 (0.73%) | 11 (0.36%) | 2.01 [0.55;6.31] | 0.216 | |
| Moderate or severe liver disease | 0.043 | |||||
| No | 3662 (98.7%) | 671 (97.7%) | 2991 (98.9%) | Ref. | Ref. | |
| Yes | 35 (0.94%) | 11 (1.60%) | 24 (0.79%) | 2.04 [0.90;4.36] | 0.063 | |
| ‘Missing’ | 15 (0.40%) | 5 (0.73%) | 10 (0.33%) | 2.23 [0.60;7.18] | 0.167 | |
| Chronic kidney disease | <0.001 | |||||
| No | 3435 (92.5%) | 549 (79.9%) | 2886 (95.4%) | Ref. | Ref. | |
| Yes | 260 (7.00%) | 134 (19.5%) | 126 (4.17%) | 5.59 [4.27;7.31] | <0.001 | |
| ‘Missing’ | 17 (0.46%) | 4 (0.58%) | 13 (0.43%) | 1.62 [0.38;5.26] | 0.407 | |
| GF < 30: | <0.001 | |||||
| No | 137 (3.69%) | 65 (9.46%) | 72 (2.38%) | Ref. | Ref. | |
| Yes | 104 (2.80%) | 57 (8.30%) | 47 (1.55%) | 1.34 [0.78;2.31] | 0.261 | |
| ‘Missing’ | 3471 (93.5%) | 565 (82.2%) | 2906 (96.1%) | 0.22 [0.15;0.31] | <0.001 | |
| Solid malignant disease | <0.001 | |||||
| No | 3304 (89.0%) | 546 (79.5%) | 2758 (91.2%) | Ref. | Ref. | |
| Yes | 387 (10.4%) | 135 (19.7%) | 252 (8.33%) | 2.71 [2.14;3.42] | <0.001 | |
| ‘Missing’ | 21 (0.57%) | 6 (0.87%) | 15 (0.50%) | 2.02 [0.64;5.54] | 0.167 | |
| Haematological chronic disease | <0.001 | |||||
| No | 3481 (93.8%) | 615 (89.5%) | 2866 (94.7%) | Ref. | Ref. | |
| Yes | 211 (5.68%) | 67 (9.75%) | 144 (4.76%) | 2.17 [1.58;2.96] | <0.001 | |
| ‘Missing’ | 20 (0.54%) | 5 (0.73%) | 15 (0.50%) | 1.55 [0.44;4.52] | 0.400 | |
| Rheumatological disease | <0.001 | |||||
| No | 3284 (88.5%) | 569 (82.8%) | 2715 (89.8%) | Ref. | Ref. | |
| Yes | 412 (11.1%) | 113 (16.4%) | 299 (9.88%) | 1.80 [1.41;2.29] | <0.001 | |
| ‘Missing’ | 16 (0.43%) | 5 (0.73%) | 11 (0.36%) | 2.17 [0.59;6.80] | 0.175 | |
| HIV infection | 0.668 | |||||
| No | 3671 (98.9%) | 678 (98.7%) | 2993 (98.9%) | Ref. | Ref. | |
| Yes | 21 (0.57%) | 4 (0.58%) | 17 (0.56%) | 1.04 [0.25;3.20] | 0.905 | |
| ‘Missing’ | 20 (0.54%) | 5 (0.73%) | 15 (0.50%) | 1.47 [0.42;4.28] | 0.455 | |
| Malnutrition | 0.002 | |||||
| No | 3670 (98.9%) | 671 (97.7%) | 2999 (99.1%) | Ref. | Ref. | |
| Yes | 15 (0.40%) | 8 (1.16%) | 7 (0.23%) | 5.10 [1.61;16.6] | 0.003 | |
| ‘Missing’ | 27 (0.73%) | 8 (1.16%) | 19 (0.63%) | 1.88 [0.71;4.52] | 0.152 | |
| Dementia | <0.001 | |||||
| No | 3482 (93.8%) | 565 (82.2%) | 2917 (96.4%) | Ref. | Ref. | |
| Yes | 212 (5.71%) | 118 (17.2%) | 94 (3.11%) | 6.48 [4.82;8.72] | <0.001 | |
| ‘Missing’ | 18 (0.48%) | 4 (0.58%) | 14 (0.46%) | 1.47 [0.35;4.72] | 0.489 | |
| Mental illness | <0.001 | |||||
| No | 3327 (89.6%) | 587 (85.4%) | 2740 (90.6%) | Ref. | Ref. | |
| Yes | 365 (9.83%) | 96 (14.0%) | 269 (8.89%) | 1.67 [1.28;2.15] | <0.001 | |
| ‘Missing’ | 20 (0.54%) | 4 (0.58%) | 16 (0.53%) | 1.17 [0.28;3.63] | 0.752 | |
| Non-severe mental illness, type | 0.001 | |||||
| 1 | 224 (6.03%) | 62 (9.02%) | 162 (5.36%) | Ref. | Ref. | |
| 2 | 110 (2.96%) | 23 (3.35%) | 87 (2.88%) | 0.69 [0.38;1.22] | 0.184 | |
| 3 | 25 (0.67%) | 8 (1.16%) | 17 (0.56%) | 1.23 [0.44;3.19] | 0.643 | |
| ‘Missing’ | 3353 (90.3%) | 594 (86.5%) | 2759 (91.2%) | 0.56 [0.41;0.78] | <0.001 | |
| Severe mental illness | 0.288 | |||||
| No | 3561 (95.9%) | 663 (96.5%) | 2898 (95.8%) | Ref. | Ref. | |
| Yes | 132 (3.56%) | 19 (2.77%) | 113 (3.74%) | 0.74 [0.42;1.21] | 0.218 | |
| ‘Missing’ | 19 (0.51%) | 5 (0.73%) | 14 (0.46%) | 1.56 [0.44;4.61] | 0.399 | |
| Severe mental illness, type | 0.017 | |||||
| 1 | 74 (1.99%) | 5 (0.73%) | 69 (2.28%) | Ref. | Ref. | |
| 2 | 22 (0.59%) | 4 (0.58%) | 18 (0.60%) | 3.02 [0.54;15.7] | 0.146 | |
| 3 | 36 (0.97%) | 10 (1.46%) | 26 (0.86%) | 5.21 [1.46;21.4] | 0.005 | |
| ‘Missing’ | 3580 (96.4%) | 668 (97.2%) | 2912 (96.3%) | 3.17 [1.29;10.1] | 0.005 | |
| Charlson Comorbidity Index | 2.00 [0.00;5.00] | 5.00 [4.00;7.00] | 2.00 [0.00;4.00] | 1.57 [1.51;1.63] | <0.001 | <0.001 |
| Final Drug | ATC Code | Drug p-Value | OR | Lower Limit 95% CI | Upper Limit 95% CI | Power |
|---|---|---|---|---|---|---|
| ENOXAPARINE | B01AB05 | <0.001 | 0.11 | 0.06 | 0.21 | <0.001 |
| BEMIPARINE | B01AB12 | <0.001 | 0.18 | 0.08 | 0.37 | 0.585 |
| ORAL REHYDRATION SALTS (GLUCOSE, POTASSIUM CHLORIDE, SODIUM CHLORIDE, TRISODIUM CITRATE) | A07CA91 | <0.001 | 0.15 | 0.03 | 0.53 | 0.591 |
| AZITHROMYCIN | J01FA10 | 0.002 | 0.46 | 0.26 | 0.78 | 0.517 |
| CEFUROXIME | J01DC02 | 0.011 | 0.26 | 0.06 | 0.83 | 0.553 |
| IPRATROPIUM BROMIDE | R03BB01 | 0.006 | 0.48 | 0.27 | 0.84 | 0.516 |
| MEPYRAMINE THEOPHYLLINE ACETATE | R03DA12 | 0.015 | 0.00 | 0.00 | 0.85 | 0.853 |
| BUDESONIDE, FORMOTEROL FUMARATE | R03AK07 | 0.013 | 0.51 | 0.28 | 0.90 | 0.514 |
| LORATADINE | R06AX13 | 0.022 | 0.20 | 0.02 | 0.93 | 0.576 |
| COLCHICINE | M04AC01 | 0.022 | 0.20 | 0.02 | 0.93 | 0.576 |
| SALBUTAMOL SULPHATE | R03AC02 | 0.039 | 0.62 | 0.37 | 0.99 | 0.508 |
| Final Drug | ATC Code | Drug p-Value | OR | Lower Limit 95% CI | Upper Limit 95% CI | Power | Interactions p < 0.05 |
|---|---|---|---|---|---|---|---|
| DIGOXIN | C01AA05 | 0.011 | 3.81 | 1.20 | 15.84 | 0.553 | Chronic heart disease |
| FOLIC ACID | B03BB01 | 0.001 | 2.32 | 1.36 | 4.08 | 0.520 | Malnutrition Pregnancy |
| MIRTAZAPINE | N06AX11 | 0.001 | 2.17 | 1.32 | 3.65 | 0.517 | Mental illness |
| LINAGLIPTIN | A10BH05 | 0.025 | 2.12 | 1.05 | 4.52 | 0.519 | Chronic kidney disease |
| ENALAPRIL | C09BA02 | 0.012 | 1.93 | 1.12 | 3.39 | 0.513 | Chronic kidney disease |
| ATORVASTATIN | C10AA05 | 0.002 | 1.52 | 1.16 | 2.01 | 0.505 | Dislipemia |
| ALLOPURINOL | M04AA01 | 0.030 | 1.42 | 1.02 | 1.99 | 0.504 | Solid malignant disease |
| ACETYLSALICYLIC ACID | B01AC06 | 0.038 | 1.31 | 1.01 | 1.71 | 0.502 | Chronic heart disease Diabetes, dislipemia, obesity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monserrat Villatoro, J.; Mejía-Abril, G.; Díaz García, L.; Zubiaur, P.; Jiménez González, M.; Fernandez Jimenez, G.; Cancio, I.; Arribas, J.R.; Suarez Fernández, C.; Mingorance, J.; et al. A Case-Control of Patients with COVID-19 to Explore the Association of Previous Hospitalisation Use of Medication on the Mortality of COVID-19 Disease: A Propensity Score Matching Analysis. Pharmaceuticals 2022, 15, 78. https://doi.org/10.3390/ph15010078
Monserrat Villatoro J, Mejía-Abril G, Díaz García L, Zubiaur P, Jiménez González M, Fernandez Jimenez G, Cancio I, Arribas JR, Suarez Fernández C, Mingorance J, et al. A Case-Control of Patients with COVID-19 to Explore the Association of Previous Hospitalisation Use of Medication on the Mortality of COVID-19 Disease: A Propensity Score Matching Analysis. Pharmaceuticals. 2022; 15(1):78. https://doi.org/10.3390/ph15010078
Chicago/Turabian StyleMonserrat Villatoro, Jaime, Gina Mejía-Abril, Lucía Díaz García, Pablo Zubiaur, María Jiménez González, Guillermo Fernandez Jimenez, Inés Cancio, José Ramón Arribas, Carmen Suarez Fernández, Jesús Mingorance, and et al. 2022. "A Case-Control of Patients with COVID-19 to Explore the Association of Previous Hospitalisation Use of Medication on the Mortality of COVID-19 Disease: A Propensity Score Matching Analysis" Pharmaceuticals 15, no. 1: 78. https://doi.org/10.3390/ph15010078