An Innovative High Frequency Hyperthermia Approach against SARS-Cov-2 and Related Virus: Feasibility Analysis
Article Information
Maria Alessandra Cutolo1*, Antimo Migliaccio2, Lucia Altucci2, Antonello Cutolo3, Andrea Cusano4
1CERICT Consortium, Viale Traiano, Italy
2Department of Precision Medicine. University of Campania L. Vanvitelli, Naples, Italy
3Department of Electrical Engineering and Information Technologies, University of Naples Federico II, Naples, Italy
4Department of Engineering, Corso Garibaldi, Benevento, Italy
*Corresponding author: Maria Alessandra Cutolo, CERICT Consortium, Viale Traiano, Benevento, Italy
Received: 01 June 2021; Accepted: 10 June 2021; Published: 27 July 2021
Citation: Maria Alessandra Cutolo, Antimo Migliaccio, Lucia Altucci, Antonello Cutolo, Andrea Cusano. An Innovative High Frequency Hyperthermia Approach against SARS-Cov-2 and Related Virus: Feasibility Analysis. Archives of Clinical and Biomedical Research 5 (2021): 569-580.
View / Download Pdf Share at FacebookAbstract
Heating is a strong enemy of SARS-CoV and related virus. Starting from both this consideration and from the basic principle of the microwave ovens which are based on the strong absorption, from organic tissues, of the radiation centered around 2.15 GHz, we examine the feasibility of using this frequency range to both lower the strength of the SARS-CoV (and related virus) inside the human body and to easily sterilize objects or closed rooms. This is only a preliminary theoretical feasibility analysis, which, of course, should be experimentally tested.
Keywords
Covid; Sars-Cov-2; Hyperthermia; Microwaves
Covid articles; Sars-Cov-2 articles; Hyperthermia articles; Microwaves articles
Article Details
1. Introduction
It is well known that, in many diseases like the one caused by COVID-19, major damages arise from the antibody reaction generated to fight the virus itself. Many drugs have been demonstrated to be effective against these viruses but only at low infection levels. This means that, in the worst cases, even only a decrease of the infection level might have beneficial results against this disease. On the other hand, hyperthermia is demonstrated to work well in many cases like, for instance, some kinds of cancers [1-4]. On this line of argument, the idea of exploiting hyperthermia against COVID-19 to reduce its strength and the corresponding antibody reaction might be speculatively applied [5-12]. In passing, we note that the idea of using heat against Covid and other disease is not new [5-22]. In particular, radiofrequency electrical hyperthermia has been investigated [19-24].
A profound difference arises between cancer and the COVID-19 case. In cancer, the illness is typically localized, and this means that, in that area, temperatures higher than 50°C can be locally reached without inducing relevant damages to the healthy tissues. From this point of view, the second case is different because the infected area often coincides with the entire body. Therefore, the operational temperature should be sufficiently high to reduce the infection, but also low enough to avoid any tissue damage. In addition, after checking if this condition can be fulfilled, a very precise temperature control of the inner tissues is required in order to avoid any negative collateral effect. Taking advantage on the strong absorption from the human tissues of the microwave radiation centered around 2,15 GHz, it seems reasonable to use this radiation to induce a well-controlled hyperthermia to decrease the virus strength. We remind that this radiation has been chosen because it is strongly absorbed by water and, hence, it is the radiation used in the microwave ovens. In this paper, we analyze the feasibility of a microwave-based approach against SARS-CoV-2 virus.
Thus, after some general considerations in Sec.2, we analyze the temperature influence on SARS-CoV-2 virus in sec.3 and the temperature limits for human organs in Sec.4. Then, in Sec.5 the main outlines of the potential approach coupled to a simple sensor for an accurate temperature control inside the body are discussed. Finally, the possibility of using this approach for a cheap sterilization of closed rooms is briefly analyzed.
2. General Remarks
2019-nCoV is a novel human coronavirus in addition to coronavirus 229E, NL63, OC43, HKU1, Middle East respiratory syndrome-related coronavirus (MERSr-CoV) and severe acute respiratory syndrome-related coronavirus (SARS-CoV). 2019-nCoV is an enveloped single-stranded plus stranded RNA virus with a diameter of 60–140 nm, spherical or elliptical in shape and pleomorphic. Zhao et al observed that angiotensin-converting enzyme 2 (ACE2) is the receptor for SARS-CoV-2 [11]. In the normal human lung, ACE2 is expressed on both type I and II alveolar epithelial cells, up to 83% the type II alveolar cells expressing ACE2. The fact that in the alveolar cells men display higher ACE2 expression levels than women might explain the worse course of SARS-Cov2 syndrome in males. The binding of SARS-CoV-2 on ACE2 causes an elevated expression of ACE2, which can create some damages on alveolar cells. In most cases it appears that the alveolar cells damage act as an engine of further reactions leading even to the cell death. Wang et al reported that the receptor-binding ability of SARS-CoV-2 through its specific SPIKE protein is 10 to 20 times stronger than that of SARS-CoV [5]. These features make 2019-nCoV a terrific threat for the whole mankind, which is fostering the efforts of many groups all around the world. Waiting for an extensive mass vaccination, many attempts are under way to look for a suitable therapy.
An increasing body of evidence suggests that a subgroup of patients with severe COVID-19 develops a so-called “cytokine storm” syndrome, which plays a central role even in the respiratory failure from acute respiratory distress syndrome (ARDS), recognized as the leading cause of mortality in patients with COVID-19. The secondary/acquired haemophagocytic lymphohistiocytosis (sHLH) is an hyperinflammatory syndrome resulting a sudden and fatal hypercytokinaemia which involves not only lungs and leads to multiorgan failure [7]. The sHLH is characterized by high and unremitting fever, cytopenias, and hyperferritinaemia. The pulmonary involvement (mainly ARDS) is observed in more than 50% of patients, so that it is difficult discriminating whether the ARDS is a primary feature of COVID-19 or it should be considered as a severe sHLH consequence. These patients present increased IL-2, IL-6 and IL-7, granulocyte CSF, interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and TNF-α. In particular, IL-6 and ferritin increase can be considered as predictors of fatality for COVID-19. Therefore, control of the hyper-inflammatory state together with reduction of viral load are issues of paramount importance for an effective therapy against COVID-19. In this regard the action of anti-inflammatory drugs deserves great consideration. The role of corticosteroids has been highly debated as during previous pandemics (SARS and MERS), were not routinely recommended and in the early protocols their use was also discouraged for COVID-19-associated lung injury. More recently, it has been reported that glucocorticoids could provide advantages in the COVID-19 treatment and their timely use might improve the early fever, promote the absorption of pneumonia and result in a better oxygenation.
Moreover, in hyperinflammation, immunesuppress-ion is likely to be beneficial. Re-analysis of data from a phase 3 randomized controlled trial of IL-1 blockade (anakinra*) in sepsis, showed significant survival benefit in patients with hyperinflammation, without increased adverse events. Similarly, although controversial, using of tocilizumab (IL-6 receptor blockade) appeared to be effective in some, even severe cases of COVID-19 pneumonia associated with elevated IL-6 in China, and its use has been approved also in Europe. As concerns antiviral drugs effective in reducing the viral load, the use of remdesivir, a drug endowed with host polymerase selectivity against the Ebola virus, has been reported in some successful cases [8] but further investigation strongly reduced interest for this drug. Also, for Favipiravir, previously known as T-705, a prodrug of a purine nucleotide, favipiravir ribofuranosyl-5′-triphosphate, encouraging results have been reported but further investigation is needed before drawing conclusions on its efficacy [9]. We envisage that a combined therapy to decrease the viral load by physical methods in support of the pharmacological therapy used to manage ARDS and sHLH might represent an option. COVID-19 (and related viruses) start to lose their virulence when the environment temperature start overpassing 38°C. Considering the literature, human organs might easily tolerate temperatures up to 44°C for up to 5 minutes. Thus, the idea of a therapy intrinsically based on a strictly controlled heating of the inner part of the human body at temperature around 42-44°C for short periods of times might represent an option. An example of localized treatment of the proposed approach is offered by the Herpes Zoster infection which is due to a virus for which any medical action is not radically efficient [10].
3. The Temperature Limits for SARS-Cov-2
One of the first issues of this proposal is establishing which temperature and exposure time is necessary for inactivation of coronaviruses. A quite satisfactory bundle of data exists on human (Severe Acute Respiratory Syndrome [SARS] coronavirus and Middle East Respiratory Syndrome [MERS] coronavirus) or zoonotic coronaviruses and their inactivation by different temperatures used for thermal disinfection [11]. From most of the studies with original data it can be concluded that a thermal disinfection at 60°C for 30 min, 65°C for 15 min and 80°C for 1 min is effective to strongly reduce coronavirus infectivity by at least 4 log10. The effect of heat can be due, as for other viruses, to protein denaturation induced by thermal aggregation of the SARS-CoV membrane protein [12]. It was also shown that the nucleo-capsid protein of SARS-CoV is completely denatured in 10 min at 55°C [13]. It is worth to note that all data described above were obtained with coronaviruses in suspension. Therefore, it cannot be ruled out that the results on dry surfaces might be different, although there is no reason to argue that it could be more stable. It would be interesting to assess whether the same temperature sensitivity is maintained even in the living organisms and how the temperature can affect other features, such as pathogenicity and spreading.
A recent study carried out in Wuhan [14] describes that 1 unit increase of temperature and absolute humidity were related to the decreased COVID-19 mortality at lag 3 and lag 5, respectively. Based on these findings, the temperature variation and humidity may significantly affect the COVID-19 mortality. This view is supported by several further reports [15], and this left room to the hope that COVID-19 pandemic could have been attenuated when the weather becomes warmer. Unfortunately, this hope is completely disproved gone, as a dramatic spreading of CoV-2 in warm-climate regions has been observed. More interestingly, a study in elderly patients, which are at the highest risk of fatality, showed that fever was generally more prominent in surviving patients. This was likely due to the lower baseline body temperature frequently measured in elderly subjects which results in a lower maximum temperature of fever, so that lowering the threshold temperature for fever should be avoided in surveillance [16]. These observations lead to probe the hypothesis that high temperature could be used to inhibit or weaken the virus spreading even in a living body and therefore, could represent an option for the adjuvant therapy of critical patients.
This prompt us to hypothesize using hyperthermia for therapy of several viruses including SARS-CoV-2. Two types of hyperthermia therapy could be considered. The whole-body and local hyperthermia therapy. The Whole-body hyperthermia therapy (WBHT) is the elevation of the core body temperature to 42 degrees. In vitro studies have shown that 42 degrees C is cytocidal for virally infected lymphocytes of patients with AIDS, and even more effective when heating is repeated 4 days later. In particular, the patients have been submitted to low temperature WBHT for 1 hour at 40 degrees C and repeated 96 hours later, or high temperature WBHT for 1 hour at 42 degrees C and repeated 96 hours later. In one year of follow-up after WBHT, there were positive effects of the therapy on frequency of AIDS defining events, Karnofsky score, and weight maintenance. Furthermore, two success-ive WBHT treatments were performed in four patients who were treated with protease inhibitor/ triple drug therapy but with partially unsatisfactory response. In follow-up for 6 months, plasma HIV RNA and CD4 improved after WBHT, and these patients remained clinically well suggesting that WBHT may provide advantages in patients with sub-optimal response to protease inhibitor therapy [17].
4. The Hyperthermia and Human Organs
There are several reports indicating that SARS-CoV-2 is very fleeting at temperature around 37°C, especially in wet environments, studies on virus stability in the living cells are substantially lacking [5-17]. Dewhirst et al. [18] provided an exhaustive report of the time-temperature damage threshold of the human organs, having shown that animal tissues can tolerate temperature of 43-43.5°C for several hours without irreversible damage. Successive studies carried out in pigs [19] demonstrated that blood heating to 45 and 48°C through an extracorporeal circulation device was effective in maintaining body temperature around 42-42,5°C without major damages in organs, including brain; a severe rhabdomyolysis, which was not described by other laboratories, was, instead, observed. However, the WBHT as well as the local hyperthermia are currently used as an adjuvant therapy against many cancers, to increase the cytotoxic effect of many chemotherapeutic agents and enhance the effects of immunotherapy. A rather recent study showed that localized heating between 43 and 45°C by means of administration of magnetic nanoparticles is effective in glioblastoma without major negative effects [20]. In this scenario, since a few years many reports have shown significant advantages of modulated electro-hyperthermia over conventional hyperthermia in the treatment of several types of cancers, such as lymphomas, uterus cervix and colorectal carcer [20-23]. Modulated electro-hyperthermia (mEHT), is a loco-regional electromagnetic hyperthermia method that uses a capacitive-impedance coupled current and allow to induce temperature increase in selected tissues [22]. These findings pave the way to the strategy of breaking down the virus aggressiveness by increasing temperature of the most affected tissues. Beside these desirable thermal effects on the virus, hyperthermia can induce pleiotropic effects that might be beneficial. At fever-range of 39-42°C, the whole-body heating increases perfusion thereby lowering interstitial fluid pressure and reducing hypoxia in several tissues and tumor models. In addition, hyperthermia increases oxygenation via modulation of HIF-1 transcriptional activity. It was observed that hyperthermia activates the ERK pathway, with consequent increase of NADPH oxidase expression and activity [24]. NADPH activity increases intracellular superoxide anions, which, in turn, lead to HIF-1 stabilization. In sum, hyperthermia lowers oxygen consumption by decree-sing the mitochondrial membrane potential, thus improving overall oxygenation levels. Furthermore, hyperthermia strongly increases cytotoxic T cell killing when applied in conjunction with a recom-binant hyper-IL-6 fusion protein (H-IL-6). H-IL-6 is comprised of IL-6 and s-IL-6R [25]. Therefore, it is conceivable that hyperthermia, may contribute to reduce the release and need for circulating inter-leukins to exert cytotoxic effects on infected cells.
5. The Basic Idea of the Microwave Approach
Very recent findings have shown that SARS-CoV-2, in contrast to SARS-CoV, replicated more efficiently at lower temperatures compared with those observed in the upper respiratory tract. It appears that SARS-CoV-2 infection resultes in 10-fold higher titers released in the apical compartment between 72 and 96 hours post infection. In contrast, SARS-CoV replication was almost identical at 33°C and 37°C and showed no significant differences over the entire course of infection [26]. These results are compatible with the molecular characteristics of SARS-CoV-2 that make it a heat-susceptible pathogen [27]. It is worth of mention to notice that it has been shown that elevated temperature can inhibit SARS-CoV-2 replication throughout respiratory epithelium independent on the effects of IFN-mediated innate immune defenses [21-30]. On the other hand, it is also well known that human organs can bear up for short times temperature over 40°C. The weakest organ is the brain. Its damage temperature is 42°C where other organs can reach 45°C for a limited time even of about ten minutes [37-30]. Accordingly, heating up demarcated areas although extended of the human body with a microwave beam to temperature lower than 45°C for a period up to some tens of minutes, might turn beneficial. This approach could provide significant advantages over the whole body hyperthermia as would prevent dangerous effects on the more heat sensitive organs. Although it is presumable that this approach does not completely eliminate the virus population, a strong reduction could be hypothesized. Furthermore, in contrast with the fever, whose effects have been exhaustively described by Belon et al. [29], implying a shift of the physiological hypothalamic set-point due to massive release of pro-inflammatory cytokines, such as IL-1, IL-6 and TNFa the physical heating would also result in lowering the immune reaction, which is a major cause of death, even allowing the combined use of other therapies profiting of the low infection level. Very recent results suggest that hyperthermia-mediated HSFs/HSP70 overexpression has an inhibitory effect on NLRP3 inflammosome and cytokine storm during SARS-CoV 2 infection [30]. Of course, the exact values of both the operation temperature and the exposition time should be determined through an appropriate set of tests. The heating approach may be obtained in many different ways among which we remind electro thermal treatments based on the use of radiowaves with frequency around a few ten of MHz [21-24].
In this paper we propose to get the heating effect by using microwave radiation centered around 2,15 GHz. The basic heating mechanism at this frequency is due to the strong absorption from the water thus implying that any biological tissue can be easily heated by electromagnetic radiation at this frequency. We explicitly observe that this is the radiation normally used in the microwave ovens. In turn, this means that the technology is very well assessed very chip and very easy to use. In addition, its wavelength is about one hundred times smaller than that used in the previously mentioned case. This results in a more directive beam which is easier to be controlled in order to get a specific radiation pattern especially in very small environments like those where the therapy must be applied. From a technical point of view we can that many practical configurations can be easily implemented. In the first example, a linear array of microwave sources (at 2.15 GHz) is used to make a scan of the body of the patient. The power of the beam and the scan velocity determine the temperature increase inside the body. Another configuration can be attained by using a square array of microwave sources which irradiate all the body. The power of the beam and the exposure time determine the temperature increase of the body. In both cases the cooling time after the irradiation is of the order of a few minutes. Of course, to determine the values of the involved parameters to be used an appropriate research strategy and accurate settings are required.
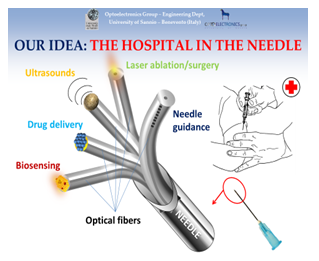
The process must be accompanied by a precise temperature control of the inner temperature of the body. This might be achieved by taking advantage on a simplified version of the so-called ‘Hospital in the Needle’. By combining nanophotonics, material science and optical fiber sensors [31-36], the Hospital in the Needle has been designed [32-39] in a configuration where a bunch of optical fibers can be integrated inside a needle. Each fiber is differently functionalized in order to work out a different medical function. In the thinnest needle (gauge 26) about ten optical fibers can be inserted, each of which is customized to perform a specific medical action. This is a strategy finalized to realize a single needle already shown to be able to perform in one shot all the following operations:
- Detection of cancer markers and mRNA
- High resolution localized ultrasound diagnostics integrated inside the needle
- Recognition of sick areas by linear and non-linear optical spectroscopy
- Ultrasound and laser surgery and ablation
- Localized optically controlled drug delivery
- System for automatic guidance of the needle
For the optical fibers that carry out laser surgery, it is possible to consider the products already available on the market. All the other sensorized optical fibers are instead innovative devices, essentially based on an emerging technology in the field of optical fiber sensors called 'Lab-on-Fiber'. This technology involves the integration of optical fibers with materials defined on a micro- and nano-metric scale, which transform the simple fiber into an effective device capable of performing the aforementioned functions. The optical fiber, thanks to its intrinsic properties (small dimensions, flexibility, low weight, biocompatibility, etc.), is well suited to be inserted inside needles and catheters for medical use. Optical fibers, in addition to ensuring the transmission of light signals, can be sensorized by integrating components and functional materials, typically around their tip. The choice of the type and size of the integrated component / material clearly depends on the physical / chemical parameter to be detected. The use of optical fibers for the development of medical probes is a common practice. However, all the devices proposed so far are designed to perform the single function. In other words, sensorized optic fibers are integrated on the single needle or catheter for the single function, thus limiting the flexibility of use. Moreover, the approaches proposed so far concerning hybrid systems, based on different technologies for the development of multi-function probes, have given rise to rather bulky devices, whose dimensions limit their use only in some particular contexts. Consequently, the miniaturized and minimally invasive devices are mono-functional, since the multifunctional ones are more invasive and not very flexible so that they can be used only in well-defined application contexts. So far this precision medicine strategy has been demonstrated suitable in the following cases: detection of thyroid cancer markers [33, 34], loco regional anesthesia [37], optically driven drug delivery [26-28, 35], localized ultrasound diagnostics [39], optical activation of “optodrugs” and temperature control [39, 40].
Accordingly, an optical fiber can be hosted inside a needle (a 26 gauge or even thinner needle is good enough) and it is possible to measure in this way the inner temperature of the body with an accuracy better than 0,1°C. This can be easily made by inserting a temperature optical fiber sensor inside the needle. This solution allows to control the inner temperature of a body during the proposed treatment with a precision better than 0,1°C and with a very low invasiveness. In this way, a very thin needle (with a diameter smaller than one millimeter) can be used as a precise temperature sensor in order to keep under control the inner temperature of the body during the treatment thus avoiding any dangerous collateral effect. More precisely the sensor is made by an optical fiber with its terminal part realized to be sensitive to the temperature. The termination of the fiber is provided by a temperature sensitive film or by a fiber bragg grating [31-34]. We remind that fiber grating gratings are a periodic perturbation of the refractive index inside an optical fiber. The resulting device is normally used to filter the radiation transmitted inside an optical fiber. Alternatively, the same device is also used as an accurate temperature and strain senor as well. Both the solutions have been showed suitable in a large variety of practical cases [39, 40].
Although the heating source can be be almost uniform we must notice that it is very hard to foresee the temperature distribution inside the body. This is mainly due to the different content of water and to the different chemical composition of different tissues. In addition, both the health state and the age of the patient can play a relevant role in the actual temperature distribution inside each tissue. On this line of argument, we have thought to use a temperature sensor integrated inside a high gauge needle. This amounts to saying that it is possible to control the actual temperature inside a tissue with a great accuracy and with a very poor invasiveness. We underline that the hospital in the needle and hence a sensorized needle are able to measure the temperature in a single point even if with a very high precision. This means that a few needles must be used to make an accurate temperature control of the tissues while our process is under way. The optimized number of the needles is far beyond the objectives of this paper and it will be the determined inside an incoming research project.
As a final remark, this approach can also be used for a fast, cheap and easy sterilization process. Nowadays sterilization involves either ultraviolet radiation or the use of a liquid disinfectants (e. g. Alcoholic solutions, quaternary ammonium derivatives). To sterilize a closed room, a simple nebulizer which injects in the room a mixture of simple water with sterilizing liquid might be proposed. Then, a small microwave source working at 2.15 Ghz (the frequency of microwave ovens) might be applied. A room with a surface of 30 m2 and a height of a 3 m has a volume of 90’ m3. In order to sterilize an environment like this about one liter of water would be needed. In SI units, cs = 1.005 + 1.82H where 1.005 kJ/kg°C is the heat capacity of dry air, 1.82 kJ/kg°C the heat capacity of water vapor, and H is the specific humidity in kg water vapor per kg dry air in the mixture. To bring this water at a temperature of about 60°C energy is needed. If the environment temperature is of about 20°C an energy of about 40.000 cal equivalent to about 160.000 joules should be provided. If we assume a microwave source of about 500 watts (typical sources for a small microwave oven), we see that we need about 5 minutes to sterilize a room like this. The sterilization is safe because the microwave radiation is fully absorbed by the vapor and the operator is on the back of the source in a totally safe position.
A this point it is important to clarify the different behavior of the microwave radiation in the two case described in the first part of this paper. When used for a room sterilization process, the microwave radiation is, in a very minor part, absorbed by the surface of the objects present in the room. The major part of the radiation is absorbed by the air thus inducing its heating. On the other side, when the microwave radiation is used to heat the inner tissues of the patient, most of the radiation is absorbed by the patient and, therefore, no other radiation is present in the room.
6. Conclusions and Future Trends
In this review we have critically approached the basis of a radically new approach against Sars-CoV2 and related virus. The next step should be a set of in vitro tests to check the validity of our approach, before trying to extend its validation on in vivo models.
Ethics approval and consent to participate
This point does not apply to our case as we do not perform any experiment on either alnimals or human beings.
Consent for publication
All the authors give their own consent for publication of this paper.
Competing interests
There is not competing interest between the content
of this paper and anyone of the authors.
Funding
There is no specific funding on this research. It has been developed outside of any of the research projects on which anyone of the authors is actually involved.
Authors' contributions
Antonello Cutolo and Andrea Cusano had the the basic idea discussed in the paper. Maria Alessandra Cutolo worked out the feasibility analysis. Antimo Migliacco and Lucia Altucci have performed all the
medical analysis.
Acknowledgements
Non acknowledgement is present.
Availability of data and material
All the materiali contained in this papere cannot be made available before publication.
Conflict of Interest Declaration
By the present, we declare that no author has any conflict of interest between his scientific and institutional activities and the results discussed in the enclosed paper An innovative high frequency hyperthermia approach against SARS-CoV-2 and related virus: feasibility analysis by Maria Alessandra Cutolo, Antimo Migliaccio, Lucia Altucci, Antonello Cutolo, Andrea Cusano, which is submitted for publication on Translation Medicine Commun-ications. Napoli December 9th 2020, The corresponding author: Maria Alessandra Cutolo.
References
- CSSR Kumar, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery- Advanced drug delivery reviews 63 (2011): 789-808.
- Johannsen M, Thiesen B, Wust P, Jordan A. Magnetic nanoparticle hyperthermia for prostate cancer, International Journal of Hyperthermia 8 (2010): 790-795.
- Hurwitz MD, Hansen JL, Prokopios-Davos S, Manola J, Wang Q, Bornstein BA, et al. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer. Cancer 117 (2011): 510-516.
- Mahmood J, Shukla HD, Soman S, Samanta S, Singh P, Kamlapurkar S, et al. Immunotherapy, Radiotherapy, and Hyperthermia: A Combined Therapeutic Approach in Pancreatic Cancer Treatment. Cancers 10 (2018): 469.
- Wang Y, Wang Y, Luo W, Huang L, Xiao J, Li F, et al. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int J Med Sci 17 (2020): 1522-1531.
- Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. L.Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2 (2020): e325-e331.
- Dolin R, Hirsch MS. Remdesivir – An Important First Step. N. Engl. J. of Medicine (2020).
- Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering (2020).
- Alonso K, Pontiggia P, Sabato A, Calvi G, Curto FC, de Bartolomei E, et al. Systemic hyperthermia in the treatment of HIV-related disseminated Kaposi's sarcoma. Long-term follow-up of patients treated with low-flow extracorporeal perfusion hyperthermia. Am J Clin Oncol 17 (1994): 353-359.
- Paggiaro AO, Carvalho VF, Gemperli R. Effect of different human tissue processing techniques on SARS-CoV-2 inactivation-review. Cell Tissue Bank 22 (2021): 1-10.
- Wang Y, Wu X, Wang Y, Li B, Zhou H, Yuan G, et al. Low stability of nucleocapsid protein in SARS virus. Biochemistry 43 (2004): 11103e8
- Ma Y, Zhao Y, Liu J, He X, Wang B, Fu S, et al. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China, Science of The Total Environment 724 (2020): 138226.
- Briz-Redón A, Serrano-Aroca A. A spatio-temporal analysis for exploring the effect of temperature on COVID-19 early evolution in Spain, Science of The Total Environment 728 (2020): 138811.
- Leung C. Risk factors for predicting mortality in elderly patients with COVID-19: A review of clinical data in China. Mechanisms of Ageing and Development 188 (2020): 111255.
- Ash Stephen R, Steinhart C R, Curfman M F, Gingrich C H, Sapir D A, Ash E L, et al. Extracorporeal whole-body hyperthermia treatments for HIV infection and AIDS. ASAIO Journal: September-October (1997): M8389.
- Dewhirst M W, Viglianti B L, Lora-Michiels M, Hanson M, Hoopes P J. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia, International Journal of Hyperthermia 3 (2003): 267-294.
- Lassche G, Frenzel T, Mignot MH, Jonkel MA , van der Hoeven JG, van Herpen CML, et al. Thermal distribution, physiological effects and toxicities of extracorporeally induced whole-body hyperthermia in a pig, Physiological Reports 8 (2020): e143E.
- Mahmoudi B, Bouras A, Bozec D, Ivkov R, Hadjipanayis Magnetic hyperthermia therapy for the treatment of glioblastoma: a review of the therapy’s history, efficacy and application in humans, International Journal of Hyperthermia 8 (2018): 1316-1328.
- Lee SY, Lee NR, Cho DH, Kim JS. Treatment outcome analysis of chemotherapy combined with modulated electro-hyperthermia compared with chemotherapy alone for recurrent cervical cancer, following irradiation. Oncol Lett 14 (2017): 73-78.
- Andocs G, Rehman M U, Zhao Q-L, Tabuchi Y, Kanamori M, Kondo T. Comparison of biological effects of modulated electro-hyperthermia and conventional heat treatment in human lymphoma U937 cells. Cell Death Discovery 2 (2016): 16039.
- Vancsik T, Kovago C, Kiss E, Papp E, Forika G, Benyo Z, et al. Modulated electro-hyperthermia induced loco-regional and systemic tumor destruction in colorectal cancer allografts. J Cancer 9 (2018): 41-53.
- Jung Moon P, Sonveaux PE, Porporato P, Danhier B, Gallez I, Batinic-Haberle, et al. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc. Natl. Acad. Sci. USA 107 (2010): 20477-20482.
- Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin. Invest 12 (2011).
- Philip V’kovski, Mitra Gultom, Jenna N Kelly, Silvio Steiner, Julie Russeil, Bastien Mangeat, et al. Disparate temperature-dependent virus – host dynamics for SARS-CoV-2 and SARS-CoV in the human respiratory epithelium. BiorXiv (2021).
- Rébé C, Ghiringhelli F, Garrido C. Can the hyperthermia-mediated heat shock factor/heat shock protein 70 pathway dampen the cytokine storm during SARS-CoV-2 infection?. Br J Pharmacol (2021): 1-7.
- Herder V, Dee K, Wojtus JK, Goldfarb D, Quan Gu CR, Jarrett RF, et al. Elevated temperature inhibits SARS-CoV-2 replication in respiratory epithelium independently of the induction of IFN-mediated innate immune defences. bioRxiv (2020).
- Belon L, Skidmore P, Rohan M, Walter E. Effect of a fever in viral infections — the ‘Goldilocks’ phenomenon? World J Clin Cases 9 (2021): 296-307.
- Mancilla-Galindo J, Galindo-Sevilla N. Exploring the rational for thermotherapy in COVID-19, International Journal of Hyperthermia 1 (2021): 202-212.
- Cutolo A, Mignani AG, Tajani A. Photonics for safety and security. World Scientific Publishing Company, Singapore (2014).
- Righini G, Tajani A, Cutolo A. An Introduction to optoelectronic sensors. World Scientific Publishing Company, Singapore (2009).
- Cusano A, Arregui F, Giordano M, Cutolo A. Optochemical Nanosensors. Taylor & Francis November (2012).
- Quero G, Consales M, Severino R, Vaiano P, Boniello A, Sandomenico A, et al. High sensitive long period fiber grating biosensor for cancer biomarker detection, (2016), HEALTHINF 2016 - 9th International Conference on Health Informatics, Proceedings; Part of 9th International Joint Conference on Biomedical Engineering Systems and Technologies. BIOSTEC (2016): 561-569.
- Quero G, Severino R, Vaiano P, Consales M, Ruvo M, Sandomenico A, et al. High sensitive reflection type long period fiber grating biosensor for real time detection of Thyroglobulin, a differentiated thyroid cancer biomarker: The "Smart Health" Project. In Proceedings of SPIE - The International Society for Optical Engineering 9634 (2015): 96342G.
- Cutolo A, Cusano A, Consales M, Pisco M, Consales M, Aliberti A, et al. Ago o catetere provvvisto di uan pluralità di fibre ottiche, Italian Patent N.102019000005362, filed (2019).
- Carotenuto B, Micco A, Ricciardi A, Amorizzo E, Mercieri M, Cutolo A, et al. Optical Guidance Systems for Epidural Space Identification, IEEE Journal of Selected Topics in Quantum Electronics 2 (2017): 1-9.
- Carotenuto B, Ricciardi A, Micco A, Amorizzo E, Mercieri M, Cutolo A, et al. Optical Fiber Technology enables Smart Needles for Epidurals: in-vivo swine study. Biomed. Opt. Express 10 (2019): 1351-1364.
- Giaquinto M, Ricciardi A, Cutolo A, Cusano A. Lab on Fiber Plasmonic Probes for Ultrasound detection: A comparative study, IEEE J. Lightwave Technology 22 (2016): 5189.
- Principe S, Giaquinto M, Micco A, Cutolo MA, Riccio M, Breglio G, et al. Thermo-plasmonic lab-on-fiber optrodes, Optics & Laser Technology 132 (2020): 106502.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 