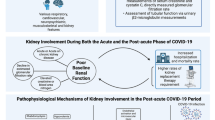
Abstract
Introduction
Kidney transplant recipients and patients on the waiting list for kidney transplant who acquire SARS-CoV-2 infection are at serious risk of developing severe COVID-19, with an increased risk of mortality for the their immunosuppressive state; other risk factors for mortality have been identified in some comorbidities such as obesity, diabetes, asthma and chronic lung disease.
Materials and Methods
The COVID-19 pandemic has led to a sharp reduction in kidney transplants in most countries, mainly due to the concern of patients on the waiting list for their potential increased susceptibility to acquire SARS-CoV-2 infection in healthcare facilities and for the difficulties of transplant centers to ensure full activity as hospitals have had to focus most of their attention on COVID-19 patients. Indeed, while the infection curve continued its exponential rise, there was a vertical decline in kidney donation/transplant activity.
Conclusion
This review article focuses on the damage induced by SARS-CoV-2 infection on kidney and on the adverse effect of this pandemic on the entire kidney transplant sector.
Similar content being viewed by others
Introduction
The Severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) has been first identified in humans in 2019. COVID-19 is the name given to the disease associated with SARS-CoV-2 infection, characterized by high morbidity and mortality. A few months after its first identification SARS-CoV-2 infection quickly spread worldwide and was declared to be a pandemic by the World Health Organization (WHO) on March 11, 2020 [1].
SARS-CoV-2 Spike proteins bind to ACE2 receptors on human cells, the first essential step for the subsequent virus entry. COVID-19 affects primarily the respiratory system with an interstitial pneumonia frequently severe and seldom life threatening, but several other organs or systems may be affected due to the widespread presence of ACE2 receptors in human body. The possible effects on some organs are summarized in Table 1.
SARS-Cov-2 trigger a systemic inflammatory syndrome which in some cases may become serious and lead to a multiorgan failure [10,11,12,13,14].
SARS-CoV-2 remain asymptomatic in 60–70% of cases, while the remaining 30–40% became symptomatic after an average incubation of 5–6 days, length differing from a subject to another mainly according to age and intensity the immune reaction. The most frequent clinical symptoms are fever (89%), cough (68%), fatigue (35%), sputum production (30%), shortness of breath (15%); headaches, weakness, sore throat, and gastrointestinal disorders (nausea, vomiting, diarrhea); pleural pain occur in 10–15% of cases [15,16,17,18,19,20,21,22]. Abnormalities at the computerized tomography (CT) scanning are observed in 86.2% of pneumonia cases, the more common pattern being frosted glass opacities and an irregular bilateral shading. Laboratory abnormalities include lymphocytopenia (83% of cases), thrombocytopenia (36%) and leukopenia (34%), increased levels of C-reactive protein, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine kinase (CK), urea nitrogen (BUN) and d-dimer [17]. Compared with younger subjects, those aging 60 or more are more prone to acquire SARS-CoV-2 infection and, once infected, more frequently show a severe course of the illness [16, 17, 22,23,24]. The primary cause of COVID-19 severity and mortality are respiratory failure (69.5%), sepsis or multiorgan failure (28%), heart failure (14.6%) and kidney failure (3.7%).
Of clinical relevance are the damage caused by SARS-CoV-2 to the brain, the cardiovascular system, and the kidney. The involvement of nervous system is proven either by the frequent occurrence of cognitive impairment and lack of attention and memory, possibly due to prolonged hypoxia, by the high frequency of anosmia, and by some cases of delirium or lethargy, Guillain Barré syndrome, trigeminal neuralgia or necrotizing hemorrhagic encephalopathy [25]. The cytokine storm has been claimed as one of the causes of the encephalic damage, but it may not be excluded that SARS-CoV-2 may overcome the blood–brain barrier, as already observed in patients with SARS.
A significant increase in inflammatory response may damage the heart and blood vessels, with an increased risk of vasculitis and myocarditis, responsible, in severe cases, of fatal cardiac arrhythmias and damage of heart tissue which may lead to myocardial infarction. The excessive inflammatory response would induce a cascade of reactions leading to blood clotting, with an increased risk of intravascular clots and pulmonary embolisms [26, 27].
This review article focusses on the involvement of kidneys in patients with SARS-CoV-2 infection, with special emphasis on patients undergoing chronic dialysis and on kidney transplant recipients.
Renal involvement in SARS-CoV-2 infection
SARS-CoV-2 Spike proteins bind to ACE2 receptors on kidney cells, which act as vectors for virus entry. The virus-induced cytokine storm causes Acute Kidney Injury (AKI) in nearly 4% of patients hospitalized for COVID-19, but up to 30% in those who died. Postmortem analysis found SARS‐CoV‐2 NP antigen in kidney tubules, complement C5b‐9 deposition on tubules and D68+ macrophage infiltration in tubulointerstitial, suggesting that the virus is a trigger for a direct cytopathic damage of kidney [28,29,30,31,32,33,34,35,36,37,38,39,40]. Like in other organs, SARS-CoV-2 infection induces micro-clots even in kidneys, which may result in filter clotting and blockage of organ function. Hematuria and proteinuria are present in most cases as markers of glomerular involvement [30, 31, 33, 35,36,37].
A kidney damage may be also induced by the drugs used to cure COVID-19. Indeed, immunosuppressive drugs administered to control the cytokine storm may favor SARS-CoV-2 replication. In addition, it cannot be excluded that some nonspecific antivirals, indiscriminately used in the hope they might act against SARS-CoV-2, are harmful to the kidney [28,29,30,31].
The degree of renal impairment goes hand in hand with the severity of the clinical manifestations of COVID-19 and is more relevant both in patients admitted to intensive care units, especially if intubated, and in elderly patients, especially if suffering from other serious comorbidities such as diabetes, hypertension, and obesity [28, 32, 34, 40,41,42].
SARS-CoV-2 infection may induce kidney damage both in transplanted and in non-transplanted patients, either with health or damaged kidneys, including those undergoing dialysis. Worthy of mention, renal replacement therapy is required in about 20% of COVID-19-related severe AKI [43,44,45].
Kidney disease developed during SARS-CoV-2 infection is associated with an increased risk of mortality [41, 42] and patients in the end-stage renal disease (ESRD) are at an increased risk for severe COVID-19. European centers report a mortality rate of 20–30% in COVID-19 patients undergoing chronic dialysis [46,47,48,49,50,51,52,53,54,55], mostly related to the direct SARS-CoV-2 invasion of kidneys followed by a strong cytokine storm, acute respiratory distress syndrome and hypovolemia. Similar rates are reported from same centers in the USA [56, 57].
Several patients on dialytic treatment are old and suffer of concomitant diseases making them extremely fragile if affected by COVID-19 [58,59,60,61]. Therefore, great care must be taken so that renal patients in dialytic treatment do not become infected with SARS-CoV-2. The initial manifestations of the illness, frequently including nonspecific symptoms like anosmia, gastro-intestinal disorders and altered state of consciousness, should alert the healthcare personnel to suspect COVID-19, which brings clinical and logistical decisions in term of patients’ treatment and isolation and healthcare workers protection. Preventive measures to reduce the risk of infection should include providing separate rooms for dialyzing SARS-CoV-2 positive patients and assigning dedicated staff members, checking patients and healthcare personnel at their entry into the kidney unit for body temperature, any other symptoms and molecular nasopharyngeal swab to identify those eventually infected with SARS-CoV-2. Furthermore, a periodic monitoring of patients and staff with molecular nasopharyngeal swab is required. Other preventive actions consist in suspending from work the health personnel tested positive, isolate any tested positive patient, distancing the patients from each other, use of adequate protective equipment both for patients and healthcare personnel. The risk of transmission of the virus following these recommendations can certainly be reduced even if not completely abolished [62].
This review focuses on kidney damage developed during SARS-CoV-2 infection in kidney transplant recipients, considered vulnerable and at risk of serious complications due to the immunosuppressive therapy administered to prevent rejection [63,64,65,66,67,68,69,70,71,72].
COVID-19 in kidney transplant patients
National registries of some Nations and multicenter studies have provided valuable information on COVID-19 epidemiology in kidney transplant recipients. A 14/1000 incidence rate of COVID-19 has been reported by a European study conducted by the ERA-EDTA from the French and Spanish registry [73], a 17.7/1000 rate from the Spanish registry [74], and a 9.5/1000 incidence from the French registry [75]. Similar incidence was reported by the kidney registry of the Dutch-speaking Belgian Society of Nephrology (14.0/1000) [76] and by multicenter studies analyzing kidney transplant subjects from the USA, Italy, and Spain [77, 78], reporting incidences 2–5 times higher in renal transplants than in subject from the general population. Of note, higher incidences were reported by single-center analyses conducted in areas with a high incidence of SARS-CoV-2 infection [79,80,81].
Renal transplant patients with COVID-19 age nearly 60, and two-third of them are males. Comorbidities reflect patients’ age (diabetes, hypertension, cardiovascular and pulmonary diseases) and are frequently serious being transplanted subjects under immunosuppressive therapy to prevent rejection [50, 62,63,64, 82,83,84,85]; this frequently leads COVID-19 toward a severe course [85].
AKI is a common complication in transplanted patients with COVID-19, frequently serious and associated with an increased admission to intensive care unit (ICU) and mortality [43,44,45]. Aziz et al. [86] reviewed 19 articles reporting the incidence of AKI in COVID-19 patients either kidney transplanted or not (Table 2); compared to non-transplanted patients, kidney transplant recipients develop AKI more frequently (27.5 versus 13.3%) and more frequently required renal replacement therapy (RRT) (15.4% versus 3.3%). A high incidence of AKI in COVID-19 kidney transplanted patients was also reported by Marinaki et al. [87] in a systemic review on 420 patients, of whom 44% developed AKI and 23% required RRT. A similar incidence of AKI (47%) was reported in a multicenter study on 104 kidney transplant patients hospitalized for COVID-19 in Spain [71].
The management of most kidney transplant recipients with COVID-19 required reduction or withdrawal of antimetabolites and calcineurin inhibitors (CNI), and in a smaller number of cases withdrawal of mTOR inhibitors (mTORi) [71, 126, 127]. In some center, immunosuppression has been replaced with methylprednisolone [71, 126, 127].
Several studies reported a 20–30% mortality rate in kidney transplant recipients hospitalized for COVID‐19 [80, 128,129,130,131]. Accordingly, the French IMPORTANT study noted that the absolute number of deaths found in April 2020 was more than twofold higher than those observed in April 2018 and in April 2019 [132].
Of note, kidney recipients from 1-year or less may have a higher mortality rate than those transplanted from more time [132]. COVID‐19 kidney transplant recipients and Covid-19 patients from general population share the same risk factors for mortality, including an advanced age, frailty, diabetes, obesity and pre‐existing pulmonary or cardiovascular comorbidities [78, 130, 133,134,135], but a malfunctioning allograft is an additional risk factor for kidney transplant recipients [130] (Table 2).
The European Renal Association COVID-19 Database, involving 98 centers in 26 countries, mainly in Europe, enrolled 1073 patients with COVID-19 from February to May 2020, of whom 305 were kidney transplant and 768 dialysis patients, all with complete information on primary outcome and 28-day mortality [130]. The 28-day probability of death was 21.3% in kidney transplant and 25.0% in dialysis group, difference not significant after adjusting for sex, age, and frailty. Mortality was associated with an advanced age in kidney transplant patients, and with an advanced age and frailty in dialysis patients. Multivariate analysis identified other significant risk of death like a higher respiration rate, decreased kidney function and use of prednisone at presentation for kidney transplant patients, and obesity, dyspnea, high body temperature, high pulse rate and elevated liver enzymes at presentation for dialysis patients. For most kidney transplant subjects the dosage of immunosuppressive drugs were reduced or discontinued within the second day of hospitalization [133].
A 33% mortality rate in kidney transplant recipients COVID-19 was reported by an Italian study [80] and a 30% rate by the French registry [75]. In a prospective French cohort study, 1216 kidney transplant recipients were enrolled, of whom 66 (5%) were diagnosed with COVID-19. The mortality rate was 1% for all patients enrolled and 24% for the COVID-19 transplant recipients [75]. Factors independently associated with COVID-19 were non-white ethnicity, obesity, diabetes, asthma, and chronic pulmonary disease.
An Italian survey and consensus on COVID-19 and kidney transplantation examined 60 kidney transplant recipients tested positive for SARS-CoV-2, of whom 57 required hospitalizations. Of the 57, 17 were hospitalized in ICU and 11 died; notably, 18 transplant professionals acquired SARS-CoV-2 infection [136].
The TANGO international transplant consortium reported the clinical data of 144 COVID‐19 kidney transplant recipients collected in 12 centers [70]. Comorbidities were frequent in this study group: hypertension in 95% of cases, diabetes in 52%, obesity in 49%, preexisting heart diseases in 28% and preexisting lung damage in 19%. In the attempt to treat COVID-19, hydroxychloroquine, antibiotics, tocilizumab and differently each other associated antivirals were administered; immunosuppressive maintenance regimen included tacrolimus in 91% of cases, mycophenolate in 77%, mTOR inhibitor in 7.5%, and steroids in 86%. AKI occurred in 52% of patients and the mortality rate was 32% [74]. A 20.5% mortality was reported by a multicenter cohort study in the USA on 482 COVID-19 solid organ transplant recipients collected in 50 transplantation centers, of whom 318 had received kidney or kidney/pancreas transplant [137].
Other kidney transplantation centers in New York have reported a COVID-19 mortality rate ranging from 13%–30% [89, 101, 138,139,140].
Alberici et al. [141] described the clinical events of 20 kidney transplant recipients hospitalized for COVID-19 pneumonia. X-ray examination showed bilateral infiltrates in half patients, and unilateral changes or no infiltrates in the other half. Immunosuppression was withdrawn at admission in all cases, replaced by 16 mg/day methylprednisolone administered together with non-specific antivirals and hydroxychloroquine. Six patients developed AKI, one of whom required dialysis treatment. The increase in IL-6 serum value required tocilizumab treatment in six cases. Five kidney transplant recipients died within 15 days from the onset of symptom.
Researchers from the Montefiore Medical Center in New York observed 36 consecutive adult kidney transplant recipients with COVID-19. Of these 36, 94% had hypertension, 69% diabetes and 17% heart disease; viral pneumonia was detected in 27 patients, of whom 11 received mechanical ventilation. Six patients received renal replacement therapy and 10 died during a 14–28-day follow-up period [128].
Crespo et al. [78] diagnosed COVID-19 in 16 of 324 (4.9%) kidney transplant recipients aged ≥ 65 years, all but 1 with pneumonia and 33% with renal graft dysfunction. Immunosuppressive treatment was reduced or abolished. COVID-19 treatment included hydroxychloroquine and azithromycin, and tocilizumab in some cases. Eight of the 16 COVID-19 kidney transplants died within an average of 3 days after admission, most of them obese, frail, and/or with a heart disease [78].
Some clinical cases highlight particular aspects of SARS-CoV-2 infection in kidney transplant recipients. Thammathiwat et al. [142] described the case of a 58-year-old patient who developed COVID-19 2 years after kidney transplantation on maintenance immunosuppressive therapy with tacrolimus, mycophenolate mofetil (MMF), and prednisolone. COVID-19 presented with diarrhea, fever, and pneumonia with X-ray pulmonary bilateral multifocal patchy infiltrations. Darunavir/ritonavir, hydroxychloroquine, azithromycin, and favipiravir were administered since hospitalization and tacrolimus and MMF were discontinued 2 days after; tocilizumab was administered after IL-6 serum level has significantly raised. High-flow nasal cannula oxygen therapy was required. This patient significantly improved by the day 10 of hospitalization. The interaction between tacrolimus and anti-viral treatment was considered responsible of a reversible AKI developed during the hospitalization [142].
Yamada et al. [143] reported the case of a 49-year-old female who developed COVID-19 25 years after kidney transplantation. This patient presented clinical features of nephrotic syndrome. Light microscopy showed acute tubular injury in the absence of significant glomerular lesions in kidney biopsy, while electron microscopy showed a diffuse minimal podocyte injury [89].
There are two case reports documenting the household transmission of SARS-CoV-2 infection involving a kidney transplant recipient. Chen et al. reported a family cluster of Covid-19, father, mother, and son, of whom the father was a kidney transplant recipient. Initial symptoms were similar in these three patients, but the course of the disease was different. The father needed hospitalization in respiratory ICU, treatment for COVID-19 and reduction/withdrawal of immunosuppressive drugs, replaced by low-dose methylprednisolone; his wife was admitted to the respiratory isolation ward, and home assistance was assigned to the son. All family members recovered [124]. Sakulkonkij et al. described a family cluster of four COVID‐19 patients occurred in Thailand, of whom one was a kidney transplant recipient under immunosuppressive treatment [144]. The clinical presentation and the severity of COVID-19 were different in these four patients, depending on the immune status of subjects. The 38-year-old son of the female index case who underwent living-related kidney transplantation in 2014 showed allograft disfunction and a severe course of the illness with a severe pneumonia and Clostrioides difficile colitis requiring intensive treatment [144]. All patients recovered and became PCR negative for SARS-2CoV-2, even if repeated testing showed a prolonged positivity in the lower respiratory tract of the kidney transplant recipient. These family clusters of SARS-CoV-2 infection underline the importance of preventing the intra-family transmission of the virus, especially if some cohabitants are more susceptible to infection and predisposed to a severe course of COVID-19.
Of note, a recent study highlighted some differences in the clinical presentation and outcome of COVID-19 between patients on the waiting list for kidney transplant and kidney transplant recipients [145]. Despite similar demographics and comorbidity burden, patients on the waiting list required hospitalization more frequently (p = 0.03) and were at higher risk of mortality (p = 0.02). A multivariate analysis developed in the same study identified waiting list status, age, and male gender as independently associated with mortality [145].
Prevention of the deleterious effects of Covid-19 on kidney transplantation process
The COVID-19 pandemic has been responsible worldwide of a strong reduction in renal transplants [146], mostly due to the difficulties of transplant centers to ensure full activity since their hospitals were overrun by patient with COVID-19 and for the fear of waitlisted patients to acquire SARS-CoV-2 infection in healthcare structures. Consequently, to the exponential rise of the daily number of patients with SARS-CoV-2 infection corresponded a vertical decline in kidney. donation/transplantation. It is appropriate to consider patients waitlisted for kidney transplantation and kidney transplant recipients among the most in need of the anti-COVID-19 vaccination, as already established in many countries with three or more subsequent doses of vaccine; this will most probably lead to a consistent reduction in mortality rate.
Conclusion
The spread of the COVID-19 pandemic has radically changed the habits of people worldwide, subjected the healthcare systems of most Nations to a strong organizational pressure and caused millions of deaths. The healthcare operators had to face an extraordinary long-lasting emergency, particularly involving the intensive care units.
The kidney transplant sector has faced numerous significant hardships related to the COVID-19 pandemic. Patients on the waiting list for kidney transplantation and those already transplanted are at serious risk of contracting SARS-CoV-2 infection due to immunosuppression developed during a long-lasting chronic kidney disease, enhanced in kidney transplant recipients from immunosuppressive treatment to prevent rejection. Both clinical conditions lead to a greater sensitivity to acquire SARS-CoV-2 infection once exposed to the virus and, for those infected, to a serious clinical course, with an increased risk of mortality compared to subjects with normal immune systems. Additional risks of mortality have been identified in comorbidities which are frequent in these patients. Therefore, patients should be instructed by doctors and psychologists in care on how not to become infected with SARS-CoV-2 and encouraged to undergo anti-COVID-19 vaccination.
Thus COVID-19 has a dramatic impact on waitlisted patients, decreasing their opportunities for kidney transplantation and posing significant mortality risk. To provide them with the opportunity for kidney transplantation, transplant centers should adapt their strategies to the COVID-19 emergency, both by increasing safety within the hospital and by adopting appropriate communication systems with patients.
Fortunately, the COVID-19 pandemic is in remission in some Western countries and in China, thanks to lockdown procedures imposed on populations in the pre-vaccination era and the subsequent extensive use of effective anti-COVID-19 vaccines. Nevertheless, a third wave, smaller than the previous two, is already underway and a fourth one has been foreseen or has just begun in some countries. This portends a broad recovery in the activities of many kidneys transplant centers in Western countries, whereas the problem remains completely unsolved in developing countries, where the scarce vaccine stocks and the large number of inhabitants makes an effective resumption of renal transplantation activity unlikely in the next 2–3 years.
References
World Health Organization. Coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Acessed 27 Sept 2021.
Mohamed AA, Alawna M. Role of increasing the aerobic capacity on improving the function of immune and respiratory systems in patients with coronavirus (COVID-19): a review. Diabetes Metab Syndr. 2020;14:489–96.
Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and Liver. J Hepatol. 2020;73:1231–40.
Kopel J, Perisetti A, Gajendran M, Boregowda U, Goyal H. Clinical insights into the gastrointestinal manifestations of COVID-19. Dig Dis Sci. 2020;65:1932–9.
Bougakov D, Podell K, Goldberg E. Multiple neuroinvasive pathways in COVID-19. Mol Neurobiol. 2021;58:564–75.
Li YP, Ma Y, Wang N, Jin ZB. Eyes on coronavirus. Stem Cell Res. 2021;51:102200.
Savtale S, Hippargekar P, Bhise S, Kothule S. Prevalence of Otorhinolaryngological symptoms in Covid 19 patients. Indian J Otolaryngol Head Neck Surg. 2021. https://doi.org/10.1007/s12070-021-02410-5.
Pellicori P, Doolub G, Wong CM, et al. COVID-19 and its cardiovascular effects: a systematic review of prevalence studies. Cochrane Database Syst Rev. 2021;3: CD013879. https://doi.org/10.1002/14651858.CD013879.
Brienza N, Puntillo F, Romagnoli S, Tritapepe L. Acute kidney injury in coronavirus disease 2019 infected patients: a meta-analytic study. Blood Purif. 2021;50:35–41.
Mehta OP, Bhandari P, Raut A, Kacimi SEO, Huy NT. Coronavirus disease (COVID-19): comprehensive review of clinical presentation. Front Public Health. 2021;15: 582932. https://doi.org/10.3389/fpubh.2020.582932.
Giovanetti M, Cella E, Benedetti F, et al. SARS-CoV-2 shifting transmission dynamics and hidden reservoirs potentially limit efficacy of public health interventions in Italy. Commun Biol. 2021;4:489. https://doi.org/10.1038/s42003-021-02025-0.
Monari C, Sagnelli C, Maggi P, et al. More Severe COVID-19 in patients with active cancer: results of a multicenter cohort study. Front Oncol. 2021;11: 662746. https://doi.org/10.3389/fonc.2021.662746.
Vitiello P, Sica A, Ronchi A, Pastore F, Casale B, Argenziano G. Cutaneous lymphomas management during COVID-19 pandemic. Ital J Dermatol Venerol. 2021;156:248–9. https://doi.org/10.23736/S0392-0488.20.06717-6.
Ciotti M, Angeletti S, Minieri M, Giovannetti M, Benvenuto D, Pascarella S, Sagnelli C, Bianchi M, Bernardini S, Ciccozzi M. COVID-19 outbreak: an overview. Chemotherapy. 2020. https://doi.org/10.1159/000507423.
Heymann DL, Shindo N. COVID-19: what is next for public health? Lancet. 2020;395:542–5. https://doi.org/10.1016/S0140-6736(20)30374-3.
Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. https://doi.org/10.1038/s41586-020-2012-7.
Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. https://doi.org/10.1056/NEJMoa2002032.
Macera M, De Angelis G, Sagnelli C, Coppola N, Vanvitelli Covid-Group. Clinical presentation of COVID-19: case series and review of the literature. Int J Environ Res Public Health. 2020;17:5062. https://doi.org/10.3390/ijerph17145062.
Sica A, Casale D, Rossi G, et al. The impact of the SARS-CoV-2 infection, with special reference to the haematological setting. J Med Virol. 2021;93:223–33. https://doi.org/10.1002/jmv.26197.
Sica A, Sagnelli C, Casale B, et al. How fear of COVID-19 can affect treatment choices for anaplastic large cell lymphomas ALK+ therapy: a case report. Healthcare (Basel). 2021;9:135. https://doi.org/10.3390/healthcare9020135.
Creta M, Sagnelli C, Celentano G, et al. SARS-CoV-2 infection affects the lower urinary tract and male genital system: a systematic review. J Med Virol. 2021;93:3133–42. https://doi.org/10.1002/jmv.26883.
Haitao T, Vermunt JV, Abeykoon J, et al. COVID-19 and sex differences: mechanisms and biomarkers. Mayo Clin Proc. 2020;95:2189–203. https://doi.org/10.1016/j.mayocp.2020.07.024.
Sagnelli C, Gentile V, Tirri R, et al. Chronic conventional disease-modifying anti-rheumatic drugs masking severe SARS-CoV-2 manifestations in an elderly rheumatic patient. J Infect. 2020;81:979–97. https://doi.org/10.1016/j.jinf.2020.05.043.
Peluso G, Campanile S, Scotti A, et al. COVID-19 and living donor kidney transplantation in Naples during the pandemic. Biomed Res Int. 2020. https://doi.org/10.1155/2020/5703963.
Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med. 2021;384:481–3.
Dhakal BP, Sweitzer NK, Indik JH, et al. SARS-CoV-2 infection and cardiovascular disease: COVID-19 heart. Heart Lung Circ. 2020;29:973–87. https://doi.org/10.1016/j.hlc.2020.05.101.
Sagnelli C, Celia B, Monari C, Cirillo S, De Angelis G, Bianco A, Coppola N. Management of SARS-CoV-2 pneumonia. J Med Virol. 2021;93:1276–87. https://doi.org/10.1002/jmv.26470.
Bo Diao CW, Wang R, Feng Z, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Nat Commun. 2021;12:2506. https://doi.org/10.1038/s41467-021-22781-1.
Farkash EA, Wilson AM, Jentzen JM. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol. 2020. https://doi.org/10.1681/ASN.2020040432.
Hassanein M, Radhakrishnan Y, Sedor J, et al. COVID-19 and the kidney. Cleve Clin J Med. 2020;87:619–31. https://doi.org/10.3949/ccjm.87a.20072.
Farouk SS, Fiaccadori E, Cravedi P, Campbell KN. COVID-19 and the kidney: what we think we know so far and what we don’t. J Nephrol. 2020;33:1213–8. https://doi.org/10.1007/s40620-020-00789-y.
Gagliardi I, Patella G, Michael A, Serra R, Provenzano M, Andreucci M. COVID-19 and the kidney: from epidemiology to clinical practice. J Clin Med. 2020;9:2506. https://doi.org/10.3390/jcm9082506.
Meena P, Bhargava V, Rana DS, Bhalla AK, Gupta A. COVID-19 and the kidney: a matter of concern. Curr Med Res Pract. 2020;10:165–8. https://doi.org/10.1016/j.cmrp.2020.07.003.
Adapa S, Chenna A, Balla M, et al. COVID-19 pandemic causing acute kidney injury and impact on patients with chronic kidney disease and renal transplantation. J Clin Med Res. 2020;12:352–61. https://doi.org/10.14740/jocmr4200.
Ahmadian E, Hosseiniyan Khatibi SM, Razi Soofiyani S, et al. Covid-19 and kidney injury: pathophysiology and molecular mechanisms. Rev Med Virol. 2021;31: e2176. https://doi.org/10.1002/rmv.2176.
Alvarez-Belon L, Sarnowski A, Forni LG. COVID-19 infection and the kidney. Br J Hosp Med (Lond). 2020;81:1–8. https://doi.org/10.12968/hmed.2020.0574.
Ng JH, Bijol V, Sparks MA, Sise ME, Izzedine H, Jhaveri KD. Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis. 2020;27:365–76. https://doi.org/10.1053/j.ackd.2020.09.003.
Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in patients with liver and kidney diseases: an early systematic review and meta-analysis. Trop Med Infect Dis. 2020;5:80.
Taverna G, Di Francesco S, Borroni EM, et al. The kidney, COVID-19, and the chemokine network: an intriguing trio. Int Urol Nephrol. 2021;53:97–104. https://doi.org/10.1007/s11255-020-02579-8.
Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1339–48. https://doi.org/10.1007/s00134-020-06153-9.
Gansevoort RT, Hilbrands LB. CKD is a key risk factor for COVID-19 mortality. Nat Rev Nephrol. 2020;16:705–6. https://doi.org/10.1038/s41581-020-00349-4.
Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–6.
Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31:1157–65.
Yang X, Tian S, Guo H. Acute kidney injury and renal replacement therapy in COVID-19 patients: a systematic review and meta-analysis. Int Immunopharmacol. 2021;90: 107159. https://doi.org/10.1016/j.intimp.2020.107159.
Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;8:209–18.
Novick TK, Rizzolo K. Cervantes L COVID-19 and kidney disease disparities in the United States. Adv Chronic Kidney Dis. 2020;27:427–33. https://doi.org/10.1053/j.ackd.2020.06.005.
Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76:428–55. https://doi.org/10.1111/all.14657.
Prasad N, Agrawal SK, COVID-19 Working Group of Indian Society of Nephrology. COVID 19 and acute kidney injury. Indian J Nephrol. 2020;30:161–5. https://doi.org/10.4103/ijn.IJN_120_20.
Benedetti C, Waldman M, Zaza G, Riella LV, Cravedi P. COVID-19 and the kidneys: an update. Front Med (Lausanne). 2020;7:423. https://doi.org/10.3389/fmed.2020.00423.
Dadson P, Tetteh CD, Rebelos E, Badeau RM, Moczulski D. Underlying kidney diseases and complications for COVID-19: a review. Front Med (Lausanne). 2020;7: 600144. https://doi.org/10.3389/fmed.2020.600144.
Lin L, Wang X, Ren J, et al. Risk factors and prognosis for COVID-19-induced acute kidney injury: a meta-analysis. BMJ Open. 2020;10: e042573. https://doi.org/10.1136/bmjopen-2020-042573.
Longo N, Calogero A, Creta M, et al. Outcomes of renal stone surgery performed either as predonation or ex vivo bench procedure in renal grafts from living donors: a systematic review. Biomed Res Int. 2020;2020:6625882. https://doi.org/10.1155/2020/6625882.
Viscardi G, Zanaletti N, Ferrara MG, et al. Atypical haemolytic-uraemic syndrome in patient with metastatic colorectal cancer treated with fluorouracil and oxaliplatin: a case report and a review of literature. ESMO Open. 2019;4: e000551. https://doi.org/10.1136/esmoopen-2019-000551.
Calogero A, Gallo M, Sica A, et al. Gastroenterological complications in kidney transplant patients. Open Med (Wars). 2020;15:623–34. https://doi.org/10.1515/med-2020-0130.
Calogero A, Sagnelli C, Peluso G, et al. Physical activity in elderly kidney transplant patients with multiple renal arteries. Minerva Med. 2020. https://doi.org/10.23736/S0026-4806.20.06573-8.
Fisher M, Yunes M, Mokrzycki MH. Chronic hemodialysis patients hospitalized with COVID-19: short-term outcomes in Bronx, New York. Kidney 360. 2020;1:755–62. https://doi.org/10.34067/KID.0003672020.
Valeri AM, Robbins-Juarez SY, Stevens JS. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31:1409–15.
Sharma P, Ng JH, Bijol V, Jhaveri KD, Wanchoo R. Pathology of COVID-19-associated acute kidney injury. Clin Kidney J. 2021;14:i30–9. https://doi.org/10.1093/ckj/sfab003.
Chen YT, Shao SC, Hsu CK, et al. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24:346. https://doi.org/10.1186/s13054-020-03009-y.
Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY). 2020;12:12493–503. https://doi.org/10.18632/aging.103579.
Yang Q, Yang X. Incidence and risk factors of kidney impairment on patients with COVID-19: a meta-analysis of 10180 patients. PLoS ONE. 2020;15: e0241953. https://doi.org/10.1371/journal.pone.0241953.
Ng JH, Hirsch JS, Hazzan A, et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2021;77:204-215.e1. https://doi.org/10.1053/j.ajkd.2020.09.002.
Kant S, Menez SP, Hanouneh M, et al. The COVID-19 nephrology compendium: AKI, CKD, ESKD and transplantation. BMC Nephrol. 2020;21:449. https://doi.org/10.1186/s12882-020-02112-0.
Jangamani R, Thirumal C, Sundar S. Coronavirus disease 2019 and the kidney. APIK J Internal Med. 2020;8:172. https://doi.org/10.4103/ajim.ajim_33_20.
Izzedine H, Jhaveri KD. Acute kidney injury in patients with COVID-19: an update on the athophysiology. Nephrol Dial Transplant. 2021;36:224–6.
Alberici F, Delbarba E, Manenti C, et al. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep. 2020;5:580–5. https://doi.org/10.1016/j.ekir.2020.04.001.
Johnson KM, Belfer JJ, Peterson GR, Boelkins MR, Dumkow LE. Managing COVID-19 in renal transplant recipients: a review of recent literature and case supporting corticosteroid-sparing immunosuppression. Pharmacotherapy. 2020;40:517–24. https://doi.org/10.1002/phar.2410.
Marinaki S, Tsiakas S, Korogiannou M, et al. A systematic review of COVID-19 infection in kidney transplant recipients: a universal effort to preserve patients’ lives and allografts. J Clin Med. 2020;9:2986. https://doi.org/10.3390/jcm9092986.
Kumar RN, Tanna SD, Shetty AA, Stosor V. COVID-19 in an HIV-positive kidney transplant recipient. Transpl Infect Dis. 2020;22: e13338. https://doi.org/10.1111/tid.13338.
Imam A, Abukhalaf SA, Imam R, et al. Kidney transplantation in the times of COVID-19—a literature review. Ann Transplant. 2020;25: e925755. https://doi.org/10.12659/AOT.925755.
Raja MA, Mendoza MA, Villavicencio A, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando). 2021;35: 100588. https://doi.org/10.1016/j.trre.2020.10058.
Moris D, Kesseli SJ, Barbas AS. Kidney transplant recipients infected by COVID-19: review of the initial published experience. Transpl Infect Dis. 2020;22: e13426. https://doi.org/10.1111/tid.13426.
Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–8.
Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. Covid-19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21:1825–37. https://doi.org/10.1111/ajt.16369.
Caillard S, Anglicheau D, Matignon M, et al. An initial report from the French SOT COVID registry suggests high mortality due to Covid-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–58.
De Meester J, De Bacquer D, Naesens M, et al. Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: a regionwide registry study. J Am Soc Nephrol. 2020;32:385–96.
Cravedi P, Suraj SM, Azzi Y, et al. COVID-19 and kidney transplantation: results from the TANGO international transplant consortium. Am J Transplant. 2020;20:3140–8.
Favà A, Cucchiari D, Montero N, et al. Clinical characteristics and risk factors for severe COVID-19 in hospitalized kidney transplant recipients: a multicentric cohort study. Am J Transplant. 2020;20:3030–41.
Elias M, Pievani D, Randoux C, et al. COVID-19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J Am Soc Nephrol. 2020;31:2413–23.
Bossini N, Alberici F, Delbarba E, et al. Kidney transplant patients with SARS-CoV-2 infection: the Brescia Renal COVID task force experience. Am J Transplant. 2020;20:3019–29.
Crespo M, Pérez-Sáez MJ, Redondo-Pachón D, et al. COVID-19 in elderly kidney transplant recipients. Am J Transplant. 2020;20:2883–9.
Bitencourt L, Pedrosa AL, de Brito SBCS, Fróes ACF, de Carvalho ST, Fonseca GG, Ferreira GC, Fradico PF, Simões E Silva AC. Curr Drug Targets. 2021;22:52–67. https://doi.org/10.2174/1389450121999201013151300.
Alfishawy M, Elbendary A, Mohamed M, Nassar M. COVID-19 mortality in transplant recipients. Int J Organ Transplant Med. 2020;11:145–62.
Stasi A, Castellano G, Ranieri E, et al. SARS-CoV-2 and viral sepsis: immune dysfunction and implications in kidney failure. J Clin Med. 2020;9:4057. https://doi.org/10.3390/jcm9124057.
Toapanta N, Torres IB, Sellarés J, et al. Kidney transplantation and COVID-19 renal and patient prognosis. Clin Kidney J. 2021;14:i21–9. https://doi.org/10.1093/ckj/sfab030.
Aziz F, Mandelbrot D, Singh T, et al. Early report on published outcomes in kidney transplant recipients compared to nontransplant patients infected with coronavirus disease 2019. Transplant Proc. 2020;52:2659–62.
Marinaki S, Tsiakas S, Korogiannou M, et al. A systematic review of COVID-19 infection in kidney transplant recipients: a universal effort to preserve patients’ lives and allografts. J Clin Med. 2020;9:2986. https://doi.org/10.3390/jcm9092986.
Sánchez-Álvarez JE, Pérez Fontán M, Jiménez Martín C, et al. SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN). Nefrologia. 2020;40:272–8.
Lubetzky M, Aull MJ, Craig-Schapiro R. Kidney allograft recipients, immunosuppression, and coronavirus disease-2019: a report of consecutive cases from a New York City transplant center. Nephrol Dial Transplant. 2020;35:1250–61.
Ali T, Al-Ali A, Fajji L, et al. Coronavirus disease-19: Disease severity and outcomes of solid organ transplant recipients: different spectrum of disease in different populations? Transplantation. 2021;105:121–7.
Mehta SA, Leonard J, Labella P, et al. Outpatient management of kidney transplant recipients with suspected COVID-19-Single-center experience during the New York City surge. Transpl Infect Dis. 2020;22: e13383. https://doi.org/10.1111/tid.13383.
Demir E, Uyar M, Parmaksiz E, et al. COVID-19 in kidney transplant recipients: a multicenter experience in Istanbul. Transpl Infect Dis. 2020;22: e13371. https://doi.org/10.1111/tid.13371.
Benotmane I, Gautier-Vargas G, Wendling MJ, et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am J Transplant. 2020;20:3162–72.
Akalin E, Azzi Y, Bartash R, et al. COVID-19 and kidney transplantation PT. N Engl J Med. 2020;382:2475–7. https://doi.org/10.1056/NEJMc2011117.
Montagud-Marrah E, Cofan F, Torregrosa JV, et al. Preliminary data on outcomes of SARS-CoV-2 infection in a Spanish single centre cohort of kidney recipients. Am J Transplant. 2020;20:2958–9. https://doi.org/10.1111/ajt.15970.
Felldin M, Softeland JM, Magnusson J, et al. Initial report from a Swedish high-volume transplant center after the first wave of the COVID-19 pandemic. Transplantation. 2020;105:108–14. https://doi.org/10.1097/TP.0000000000003436.
Hartzell S, Bin S, Benedetti C, et al. Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients. Am J Transplant. 2020;20:3149–61. https://doi.org/10.1111/ajt.16261.
Malekhosseini SA, Nikoupour H, Gholami S, et al. A report of 85 cases of COVID-19 and abdominal transplantation from a single center: what are the associated factors with death among organ transplantation patients. Transplantation. 2021;105:90–9.
Columbia University Kidney Transplant P. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. JASN. 2020;31:1150–6.
Abrishami A, Samavat S, Behnam B, et al. Clinical course, imaging features, and outcomes of COVID-19 in kidney transplant recipients. Eur Urol. 2020;78:281–6. https://doi.org/10.1016/j.eururo.2020.04.064.
Nair V, Jandovitz N, Hirsch JS. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1819–25.
Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77:748–54.
Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID- 19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20:1849–58. https://doi.org/10.1111/ajt.15929.
Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020. https://doi.org/10.1016/j.eururo.2020.03.030.
Banerjee D, Popoola J, Shah S, et al. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;S0085–2538:30361–6. https://doi.org/10.1016/jkint.2020.03.018.
Gandolfini I, Delsante M, Fiaccadori E, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1941–3. https://doi.org/10.1111/ajt.15891.
Kim Y, Kwon O, Paek JH, et al. Two distinct cases with COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:2269–75. https://doi.org/10.1111/ajt.15947.
Bartiromo M, Borchi B, Botta A. Threatening drug-drug interaction in a kidney transplant patient with Coronavirus Disease 2019 (COVID-19). Transpl Infect Dis. 2020;22: e13286. https://doi.org/10.1111/tid.13286.
Arpali E, Akyollu B, Yelken B, et al. Case report: a kidney transplant patient with mild COVID-19. Transpl Infect Dis. 2020;22: e13296. https://doi.org/10.1111/tid.13296.
Billah M, Santeusanio A, Delaney V, et al. A catabolic state in a kidney transplant recipient with COVID-19. Transpl Int. 2020;33:1140–1. https://doi.org/10.1111/tri.13635.
Bussalino E, De Maria A, Russo R, Paoletti E. Immunosuppressive therapy maintenance in a kidney transplant recipient with SARS-CoV-2 pneumonia: a case report. Am J Transplant. 2020;20:1922–4. https://doi.org/10.1111/ajt.15920.
Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20:1875–8. https://doi.org/10.1111/ajt.15874.
Meziyerh S, Zwart TC, van Etten RW, et al. Severe COVID-19 in a renal transplant recipient: a focus on pharmacokinetics. Am J Transplant. 2020;20:1896–901. https://doi.org/10.1111/ajt.15943.
Marx D, Moulin B, Fafi-Kremer S, et al. First case of COVID-19 in a kidney transplant recipient treated with belatacept. Am J Transplant. 2020;20:1944–6. https://doi.org/10.1111/ajt.15919.
Ning L, Liu L, Li W, et al. Novel coronavirus (SARSCoV-2) infection in a renal transplant recipient: case report. Am J Transplant. 2020;20:1864–8. https://doi.org/10.1111/ajt.15897.
Hsu JJ, Gaynor P, Kamath M, et al. COVID-19 in a high-risk dual heart and kidney transplant recipient. Am J Transplant. 2020;20:1911–5. https://doi.org/10.1111/ajt.15936.
Seminari E, Colaneri M, Sambo M, et al. SARS Cov-2 infection in a renal-transplanted patients. A case report. Am J Transplant. 2020;20:1882–4. https://doi.org/10.1111/ajt.15902.
Huang J, Lin H, Wu Y, et al. COVID-19 in posttransplant patients—report of 2 cases. Am J Transplant. 2020;20:1879–81. https://doi.org/10.1111/ajt.15896.
Kates OS, Fisher CE, Stankiewicz-Karita HC, et al. Earliest cases of coronavirus disease 2019 (COVID-19) identified in solid organ transplant recipients in the United States. Am J Transplant. 2020;20:1885–90. https://doi.org/10.1111/ajt.15944.
Zhong Z, Zhang Q, Xia H, et al. Clinical characteristics and immunosuppressant management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20:1916–21. https://doi.org/10.1111/ajt.1592.
Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20:1859–63. https://doi.org/10.1111/ajt.15869.
Wang J, Li X, Cao G, et al. COVID-19 in a kidney transplant patient. Eur Urol. 2020;77:769–70. https://doi.org/10.1016/j.eururo.2020.03.036.
de Barros Machado DJ, Ianhez LE. COVID-19 pneumonia in kidney transplant recipients—where we are? Transpl Infect Dis. 2020. https://doi.org/10.1111/tid.13306.
Chen S, Yin Q, Shi H, et al. A familial cluster, including a kidney transplant recipient, of Coronavirus Disease 2019 (COVID-19) in Wuhan, China. Am J Transplant. 2020;20:1869–74.
Fontana F, Alfano G, Mori G, et al. COVID-19 pneumonia in a kidney transplant recipient successfully treated with tocilizumab and hydroxychloroquine. Am J Transplant. 2020;20:1902–6. https://doi.org/10.1111/ajt.15935.
Banerjee D, Popoola J, Shah S, et al. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97:1076–82.
Coates PT, Wong G, Drueke T, Rovin B, Ronco P. Early experience with COVID-19 in kidney transplantation. Kidney Int. 2020;97:1074–5. https://doi.org/10.1016/j.kint.2020.04.001.
Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–7. https://doi.org/10.1056/NEJMc2011117.
Haarhaus M, Santos C, Haase M, et al. Risk prediction of COVID-19 incidence and mortality in a large multi-national hemodialysis cohort: implications for management of the pandemic in outpatient hemodialysis settings. Clin Kidney J. 2021;4:805–13.
Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–83.
Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20:1849–58.
Thaunat O, Legeai C, Anglicheau D, et al. IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT). Kidney Int. 2020;98:1568–77. https://doi.org/10.1016/j.kint.2020.10.008.
Pérez-Sáez MJ, Blasco M, Redondo-Pachón D, et al. Use of tocilizumab in kidney transplant recipients with COVID-19. Am J Transplant. 2020;20:3182–90. https://doi.org/10.1111/ajt.16192.
Aziz H, Lashkari N, Yoon YC, et al. Effects of coronavirus disease 2019 on solid organ transplantation. Transplant Proc. 2020;52:2642–53. https://doi.org/10.1016/j.transproceed.2020.09.006.
Azzi Y, Parides M, Alani O, et al. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020;98:1559–67.
Vistoli F, Furian L, Maggiore U, et al. ID-19 and kidney transplantation: an Italian Survey and Consensus. J Nephrol. 2020;33:667–80. https://doi.org/10.1007/s40620-020-00755-8.
Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa1097.
Flythe JE, Assimon MM, Tugman MJ, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis. 2021;77:190–203.
Pereira MR, Mohan S, Cohen DJ. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–8.
Mehta SA, Leonard J, Labella P, et al. Outpatient management of kidney transplant recipients with suspected COVID-19—single-center experience during the New York City surge. Transpl Infect Dis. 2020;22: e13383. https://doi.org/10.1111/tid.13383.
Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–8. https://doi.org/10.1016/j.kint.2020.04.002.
Thammathiwat T, Tungsanga S, Tiankanon K, et al. A case of successful treatment of severe COVID-19 pneumonia with favipiravir and tocilizumab in post-kidney transplant recipient. Transplant Infect Dis. 2021;23:e13388.
Yamada M, Rastogi P, Ince D, et al. Minimal change disease with nephrotic syndrome associated with coronavirus disease 2019 after apolipoprotein L1 risk variant kidney transplant: a case report. Transplant Proc. 2020;52:2693–7. https://doi.org/10.1016/j.transproceed.2020.08.012.
Sakulkonkij P, Bruminhent J, Pankongngam C, Chalermphunchai N. A family cluster of diagnosed coronavirus disease 2019 (COVID-19) kidney transplant recipient in Thailand. Immun inflamm Dis. 2020;8:534–43.
Craig-Schapiro R, Salinas T, Lubetzky M, et al. COVID-19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients. Am J Transplant. 2021;21:1576–85. https://doi.org/10.1111/ajt.16351.
Aubert O, Yoo D, Zielinski D, Cozzi E, et al. COVID-19 pandemic and worldwide organ transplantation: a population-based study. Lancet Public Health. 2021. https://doi.org/10.1016/S2468-2667(21)00200-0.
Funding
Not to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Sagnelli, C., Sica, A., Gallo, M. et al. Renal involvement in COVID-19: focus on kidney transplant sector. Infection 49, 1265–1275 (2021). https://doi.org/10.1007/s15010-021-01706-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-021-01706-6