Abstract
Introduction
The impact of the COVID-19 pandemic on routine medical care may result in altered healthcare resource use in patients with immune-mediated conditions. The aim of this study was to determine the impact of treatment interruptions in patients with and without COVID-19 infections who were treated with targeted immunomodulators (TIMs) in the USA.
Methods
Data from the IBM® MarketScan® Research Databases were analyzed in patients with immune-mediated conditions from January 1, 2018, through December 31, 2020. Healthcare resource use (HCRU) including hospitalizations, emergency department (ED) visits, in-person outpatient visits, and respiratory outcomes was assessed in a cohort of patients without COVID-19 who had uninterrupted versus interrupted TIM use. The impact of treatment interruption on HCRU and respiratory outcomes was also evaluated in a cohort of patients with COVID-19. Results from adjusted logistic regression were reported as adjusted odds ratios (aORs) with 95% confidence intervals.
Results
Approximately 25% of patients in both the COVID-19 (N = 787) and non-COVID-19 cohorts (N = 77,178) experienced interruptions in TIM therapy. In the non-COVID-19 cohort, the likelihood of being hospitalized was 20% less in patients with uninterrupted versus interrupted TIM use (aOR = 0.80, 95% CI 0.71–0.90). Patients with uninterrupted TIM use had a similar likelihood of an ED visit (aOR = 0.99, 95% CI 0.91–1.08) and respiratory outcome (aOR = 0.97, 95% CI 0.71–1.31) versus patients with interrupted TIM use. The likelihood of having an in-person outpatient visit was 87% greater in patients with uninterrupted versus interrupted TIM use (aOR = 1.87, 95% CI 1.81–1.94). Similar findings were observed in the COVID-19 cohort.
Conclusion
This analysis of real-world claims data showed that uninterrupted TIM use was not associated with an increased likelihood of hospitalizations, ED visits, or negative respiratory outcomes compared to interrupted TIM use among patients with immune-mediated conditions, regardless of COVID-19 diagnosis.
Similar content being viewed by others
Why carry out this study? |
The COVID-19 pandemic had a major impact on routine clinical practice and significant interruptions to medical care due potentially to overburdened healthcare systems and a reluctance to attend in-person healthcare visits |
Treatment interruption may be of particular relevance in the management of patients with immune-mediated conditions not only due to limited access to medical care, but also because of unclear treatment guidance and fear of severe COVID-19 infection |
It is unclear how the COVID-19 pandemic impacts treatment interruption of targeted immunomodulators and subsequently the effect on healthcare resource utilization in patients with immune-mediated conditions |
What was learned from the study? |
In patients with immune-mediated conditions, there was no increase in the likelihood of being hospitalized or having an emergency department visit or a negative respiratory outcome between patients with uninterrupted versus interrupted targeted immunomodulator use, regardless of COVID-19 diagnosis |
More frequent in-person outpatient or virtual visits were observed in both the uninterrupted and interrupted targeted immunomodulator use cohorts and may be explained by the need for routine treatment monitoring of patients with immune-mediated conditions |
Introduction
The 2019 novel coronavirus (COVID-19) pandemic continues to be a major health crisis, particularly with the emergence of COVID-19 variants more virulent than the original virus. As of June 22, 2021, > 179 million cases have been reported worldwide, resulting in > 3.8 million deaths [1]. The first case appeared in the US in January 2020 [2], and on June 22, 2021, the number of cases in the US was > 34 million and > 617,000 deaths had been reported [1]. Medical care of patients during this pandemic may have been interrupted, especially in the treatment of chronic immune-mediated conditions. Continuation of therapy is critical to the efficacy of targeted immunomodulators (TIMs) in patients with immune-mediated conditions [3]. Early in the pandemic, an estimated 41% of US adults delayed or avoided medical care including urgent or emergency care (12%) and routine care (32%) because of concerns regarding COVID-19, highlighting the immense disruption to both medical care and routine management of complex diseases [4].
As TIM use in the treatment of immune-mediated conditions can result in a reduced immune response, there is concern that treated patients may have an increased risk of COVID-19 infections and/or a more severe course of COVID-19 illness including hospitalizations, complications, and mortality [5, 6]. There is limited guidance on the continued use of TIMs during the COVID-19 pandemic. At the beginning of the pandemic, several professional medical organizations such as National Psoriasis Foundation, American Academy of Dermatology, American College of Rheumatology, Medical Dermatology Society, and American College of Gastroenterology recommended discontinuation or postponement of some TIMs [7,8,9,10,11]. As a consequence of unclear guidance, patients have forgone treatment, as shown in a recent study [12] of patients with rheumatic conditions without diagnosis of COVID-19 or respiratory disease where 14.9% of patients stopped taking a disease-modifying antirheumatic drug (DMARD) and 16.5% stopped taking a biologic or Janus kinase inhibitor because of concerns over COVID-19 infection; most of these interruptions were not recommended by a physician. Furthermore, results from this study on healthcare-related patient behavior [12] revealed that 56.6%, 42.3%, and 36.0% of patients avoided an office visit, laboratory testing, and other testing, respectively [12]. Thus, more research is needed to understand the consequences of treatment interruption in patients with immune-mediated conditions who may be at greater risk for COVID-19. To address this gap, we sought to evaluate the impact of treatment interruptions on healthcare resource utilization among patients with immune-mediated conditions with and without COVID-19 who were treated with TIMs in the US.
Methods
Study Design
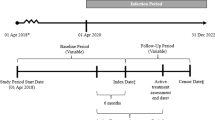
This study sought to determine the impact of treatment interruptions on patients with immune-mediated conditions, with and without COVID-19, who were treated with TIMs in the US. We conducted two separate analyses in patients with immune-mediated conditions. The first analysis evaluated patients who did not have COVID-19 and compared healthcare resource utilization and respiratory outcomes in patients with uninterrupted versus interrupted TIM use. The second analysis evaluated COVID-19 patients and compared healthcare resource utilization and respiratory outcomes in those with uninterrupted versus interrupted TIM use.
Data Source
This was a retrospective observational study using de-identified data from the IBM® MarketScan® Research Databases. The MarketScan databases are a private sector health data resource reflecting healthcare experience for > 41.1 million covered individuals, including employees and dependents and Medicare-eligible retirees with employer-provided Medicare supplemental plans. The MarketScan databases represent one of the largest collections of de-identified patient data available for healthcare research in the US.
Patient-level data were retrieved from the MarketScan databases from January 1, 2018, through December 31, 2020. This study used anonymized data; therefore, Ethics Committee approval was not required. Data were de-identified following the statistical de-identification rules of the Health Insurance Portability and Accountability Act (HIPPA) and managed according to customer data use agreements.
Identification of Study Population
Non-COVID-19 Patients
For non-COVID-19 patients, a random date between March 13, 2020, and August 3, 2020, was assigned to each patient. Non-COVID-19 patients who were treated with TIMs were selected using the following criteria: patients had to have ≥ 2 claims with diagnosis codes (Supplementary Table 1) for immune-mediated conditions (ankylosing spondylitis, atopic dermatitis, Crohn’s disease, hidradenitis suppurativa, psoriasis, psoriatic arthritis, rheumatoid arthritis, or ulcerative colitis) during the 24 months before the assigned random date. Patients were also required to have ≥ 2 claims (pharmacy or medical) for TIM use within 12 months before the assigned random date with ≥ 1 claim for a TIM within 6 months before the assigned random date based on codes from the Healthcare Common Procedure Coding System (Supplementary Table 2). Non-COVID-19 patients also had to have continuous enrollment ≥ 12 months before and ≥ 150 days after the assigned random date with no diagnosis of COVID-19.
COVID-19 Patients
COVID-19 patients were selected if they had ≥ 1 claim with International Classification of Disease, 10th revision, Clinical Modification (ICD-10-CM) for COVID-19 between March 13, 2020 (the date a national emergency was declared in the US) and August 3, 2020 (see Supplementary Table 1 for ICD-10-CM codes). TIM-treated patients with immune-mediated conditions were identified using the following criteria: patients had to have ≥ 2 claims with diagnosis codes for immune-mediated conditions (ankylosing spondylitis, atopic dermatitis, Crohn’s disease, hidradenitis suppurativa, psoriasis, psoriatic arthritis, rheumatoid arthritis, or ulcerative colitis) during the 24 months before the initial COVID-19 diagnosis (see Supplementary Table 1 for ICD-10-CM codes). Patients also had to have ≥ 2 claims (pharmacy or medical) for a TIM use within 12 months before the COVID-19 diagnosis with ≥ 1 claim for a TIM within 6 months before the COVID-19 diagnosis based on codes from the Healthcare Common Procedure Coding System. TIM use included tumor necrosis factor inhibitors, CD20 inhibitors, interleukin (IL)-6 inhibitors, T-cell inhibitors, IL-12/23 inhibitors, IL-23 inhibitors, IL-17 inhibitors, IL-4/13 inhibitors, Janus kinase inhibitors, phosphodiesterase 4 inhibitors, and an integrin inhibitor. A complete list of therapies and healthcare procedure codes is provided in Supplementary Table 2. Lastly, patients had to have continuous enrollment ≥ 12 months before and ≥ 150 days after the COVID-19 diagnosis.
TIM Use Continuation Status
A distribution approach was taken to assess TIM continuation status by identifying the time window when the majority of TIM prescription refills occurred after COVID-19 diagnosis for COVID-19 patients or after the assigned random date for non-COVID-19 patients. For both cohorts, > 95% of TIM prescription refills occurred within 60 days; therefore, a time window of 60 days was used to assess TIM continuation status. TIM continuation or uninterrupted TIM use was defined as ≥ 1 prescription for any TIM use during this 60-day window, and interruption of TIM use was defined as no prescription for TIM use during the 60-day period.
Outcomes Analyzed
The date corresponding to 60 days after the COVID-19 diagnosis was the index date for COVID-19 patients, and the date corresponding to 60 days after the assigned random date was the index date for non-COVID-19 patients. For both cohorts, outcomes were assessed for 90 days after the index date. Healthcare resource utilization included the percentage of patients who were hospitalized, had an emergency department visit, had an in-person outpatient visit, or had a virtual visit (includes virtual check-in visits and telehealth visits). Respiratory outcomes based on ICD-10-CM code, included viral pneumonia (J12.89), bronchitis (J20.8 or J40), lower respiratory tract infection (J98.8 or J22), or acute respiratory distress syndrome (J80). The percentage of patients with a respiratory outcome was determined if they had a diagnosis code for any of the four respiratory outcomes. The likelihood of being hospitalized; having emergency department, in-person outpatient, or virtual visits; or a respiratory outcome was determined.
Statistical Analysis
For baseline characteristics, frequencies and percentages were reported for categorical variables and were compared using chi-square tests or Fisher’s exact tests. Means and standard deviations were reported for continuous variables and were compared using the Wilcoxon rank-sum test.
For COVID-19 and non-COVID-19 patients, results from logistic regression models for binary outcomes were reported as adjusted odds ratios (aOR) with corresponding 95% confidence intervals. The reference category for the aORs was the interrupted TIM cohort. Models were adjusted for the following baseline covariates: age, sex, region, index month, immune-mediated conditions, and Charlson Comorbidity Index (CCI). Comorbidities included in the CCI are listed in Supplementary Table 3; rheumatic conditions were excluded. Logistic regression was not performed for outcomes with < 50 events [13].
For non-COVID-19 patients, results were stratified among patients who had rheumatic (ankylosing spondylitis, psoriatic arthritis, or rheumatoid arthritis), dermatologic (atopic dermatitis, hidradenitis suppurativa, psoriasis, or psoriatic arthritis), and gastrointestinal conditions (Crohn’s disease or ulcerative colitis), separately. Since patients could have multiple conditions, the stratified groups were not mutually exclusive. Odds ratios for respiratory outcomes in stratified analyses were not reported in non-COVID-19 patients because of the small number of events (i.e., < 50). For COVID-19 patients, comparisons were not stratified by therapeutic area because of the small sample size.
Results
Baseline Characteristics
Non-COVID-19 Patients
There were 77,178 non-COVID-19 patients who met the inclusion criteria, including 59,432 (77.0%) and 17,746 (23.0%) in the uninterrupted and interrupted TIM use cohorts, respectively (Fig. 1). Patients in the uninterrupted TIM use cohort were younger than those in the interrupted use cohort (46.6 ± 14.6 versus 48.6 ± 14.6 years, P < 0.0001; Table 1). A smaller proportion of patients in the uninterrupted TIM use cohort was female than in the interrupted use cohort (56.6% versus 57.5%, P = 0.03). Patients in the uninterrupted TIM use cohort included smaller proportions of patients with psoriasis, rheumatoid arthritis, and hidradenitis suppurativa and larger proportions of patients with Crohn’s disease, ulcerative colitis, and atopic dermatitis than those in the interrupted use cohort. Patients in the uninterrupted versus interrupted TIM use cohort had a lower CCI (CCI: 0.44 ± 0.96 versus 0.52 ± 1.09, P < 0.0001), and more than half of the patients in each cohort were being treated with a tumor necrosis factor inhibitor (60.2% versus 54.1%; P < 0.0001), respectively.
COVID-19 Patients
A total of 787 COVID-19 patients met the inclusion criteria, including 564 (71.7%) in the uninterrupted TIM use cohort and 223 (28.3%) in the interrupted TIM use cohort (Fig. 1). Patients in the uninterrupted TIM use cohort were younger than those in the interrupted TIM use cohort (45.8 ± 13.3 versus 48.4 ± 12.6 years, P = 0.03). In COVID-19 patients, there were no other significant differences in demographics between patients in the uninterrupted versus interrupted TIM use cohorts (Table 1). More patients in the uninterrupted versus interrupted TIM use cohort had Crohn’s disease (23.8% versus 14.3%, P < 0.01) while fewer patients had rheumatoid arthritis (27.5% versus 38.1%, P < 0.01). The distribution of other immune-mediated conditions was similar between the cohorts. There was no significant difference in CCI between patients in the uninterrupted versus those with interrupted TIM use cohorts [mean ± (SD): CCI 0.68 ± 1.30 versus 0.72 ± 1.27; P = 0.36], and more than half of the patients in each cohort were being treated with a tumor necrosis factor inhibitor (61.3% versus 55.2%; P = 0.13), respectively.
Outcomes
Non-COVID-19 Patients
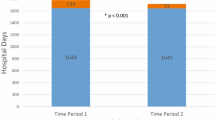
During the 90-day study period, significantly fewer non-COVID-19 patients with uninterrupted TIM use were hospitalized compared to those with interrupted TIM use (1.7% versus 2.2%, P < 0.0001; Fig. 2A). The percentages of patients with an ED visit or a respiratory outcome were similar in the uninterrupted versus interrupted TIM use cohorts. Significantly more patients had an in-person outpatient visit (67.7% versus 52.7%, P < 0.0001) or virtual visit (17.3% versus 14.0%, P < 0.0001) among patients in the uninterrupted versus interrupted TIM use cohort.
In non-COVID-19 patients, the likelihood of being hospitalized, having an ED visit, or a respiratory outcome was 20% less (aOR = 0.80, 95% CI 0.71–0.90; P < 0.001), 1% less (aOR = 0.99, 95% CI 0.91–1.08; P = 0.89), or 3% less (aOR = 0.97, 95% CI 0.71–1.31; P = 0.82), respectively, in the uninterrupted versus interrupted TIM use cohort (Fig. 3A). The likelihood of having an in-person outpatient visit or a virtual visit was 87% greater (aOR = 1.87, 95% CI 1.81–1.94; P < 0.0001) or 29% greater (aOR = 1.29, 95% CI 1.23–1.35; P < 0.0001), respectively, in the uninterrupted versus interrupted TIM use cohort.
COVID-19 Patients
During the 90-day study period, significantly fewer patients in the uninterrupted versus interrupted TIM use cohort were hospitalized (1.8% versus 4.9%, P = 0.02; Fig. 2B). The percentage of patients with an ED (6.9% versus 7.6%, P = 0.85) or virtual visit (22.9% versus 26.5%, P = 0.33) was less in the uninterrupted versus interrupted TIM use cohort, but the differences were not statistically significant. The percentage of patients with an in-person outpatient visit was greater in the uninterrupted TIM use cohort than in the interrupted TIM use cohort (84.6% versus 79.4%; P = 0.10). The percentage of patients with a respiratory outcome was less in the uninterrupted versus interrupted TIM use cohort (1.2% versus 2.2%, P = 0.34), but the difference was not statistically significant.
In COVID-19 patients, the likelihood of being hospitalized, having an ED visit, or having a respiratory outcome was 69% less (aOR = 0.31, 95% CI 0.12–0.80; P = 0.02), 5% less (aOR = 0.95, 95% CI 0.51–1.76; P = 0.87), or 31% less (aOR = 0.69, 95% CI 0.20–2.37; P = 0.56), respectively, in the uninterrupted versus interrupted TIM use cohort (Fig. 3B). The likelihood of having an in-person outpatient visit was 56% greater (aOR = 1.56, 95% CI 1.01–2.40; P = 0.045) in patients in the uninterrupted versus interrupted TIM use cohort, and the likelihood of having a virtual visit was 15% less in patients in the uninterrupted versus interrupted TIM use cohort (aOR = 0.85, 95% CI 0.59–1.24; P = 0.40).
Outcomes Stratified by Therapeutic Area
Rheumatic Conditions
A total of 36,411 patients had a rheumatic condition, including 27,302 (75.0%) with uninterrupted TIM use and 9109 (25.0%) with interrupted TIM use during the study period (Supplementary Table 4). In patients with a rheumatic condition, those in the uninterrupted TIM use cohort were younger than those in the interrupted TIM use cohort (51.8 ± 11.7 years 53.1 ± 12.5; P < 0.0001). A smaller proportion of patients with uninterrupted TIM use was female (64.6% versus 66.5%; P < 0.01) compared to those with interrupted TIM use. Rheumatoid arthritis (56.0% versus 62.1% in the uninterrupted TIM use versus interrupted TIM use cohort, respectively, P < 0.0001) was the most prevalent rheumatic condition followed by psoriatic arthritis (38.2% versus 34.2%, respectively P < 0.0001) and ankylosing spondylitis (11.3% versus 9.7%, respectively, P < 0.0001). Patients with uninterrupted TIM use had a lower comorbidity burden as assessed by CCI than those with interrupted TIM use (CCI: 0.50 ± 1.03 versus 0.60 ± 1.15; P < 0.0001), and more than half of the patients in each cohort were being treated with a tumor necrosis factor inhibitor (71.5% versus 64.3%; P < 0.0001, respectively).
During the 90-day study period, significantly fewer patients with a rheumatic condition in the uninterrupted versus interrupted TIM use cohort were hospitalized (1.7% versus 2.3%, P < 0.001; Supplemental Fig. 1A). Although not statistically significant, fewer patients with a rheumatic condition had an ED visit in the uninterrupted versus interrupted TIM use cohorts (4.2% versus 4.6%, respectively, P = 0.11). Significantly more patients with a rheumatic condition had an in-person outpatient visit (70.4% versus 56.8%, P < 0.0001) or virtual visit (20.0% versus 17.0%, P < 0.0001) in the uninterrupted versus interrupted TIM use cohort. The percentages of patients with a respiratory outcome were similar among patients in the both cohorts.
For patients with a rheumatic condition, the likelihood of being hospitalized or having an ED visit was 17% less (aOR = 0.83, 95% CI 0.70–0.98; P = 0.03) or 7% less (aOR = 0.93, 95% CI 0.83–1.05; P = 0.25; Supplemental Fig. 2A), respectively, in patients with uninterrupted versus interrupted TIM use. In contrast, the likelihood of having an in-person outpatient visit or a virtual visit was 81% greater (aOR = 1.81, 95% CI 1.72–1.90; P < 0.0001) or 21% greater (aOR = 1.21, 95% CI 1.14–1.29; P < 0.0001), respectively, in patients with uninterrupted versus interrupted TIM use.
Dermatologic Conditions
A total of 36,548 patients had a dermatologic condition, including 27,684 (75.7%) with uninterrupted TIM use and 8864 (24.3%) with interrupted during the study period (Supplemental Table 5). There was no significant difference in age (47.4 ± 13.9 versus 47.8 ± 13.9 years; P = 0.20) or gender (51.9% females versus 51.4% each, P = 0.42) between the cohorts with uninterrupted versus interrupted TIM use. Psoriasis (65.9% versus 69.5% in the uninterrupted TIM use versus interrupted TIM use cohort, respectively, P < 0.0001) was the most prevalent dermatologic condition followed by psoriatic arthritis (39.4% versus 37.0%, respectively, P < 0.0001), atopic dermatitis (16.1% versus 11.4%, respectively P < 0.0001), and hidradenitis suppurativa (3.9% versus 4.2%, respectively P = 0.33). The comorbidity burden as determined by CCI was similar between patients with a dermatologic condition in the uninterrupted versus interrupted TIM use cohorts (CCI: 0.45 ± 0.98 versus 0.46 ± 1.01; P = 0.92). More than one-third of patients with a dermatologic condition in the uninterrupted versus interrupted TIM use cohorts was being treated with tumor necrosis factor inhibitors (38.6% versus 41.5%; P < 0.0001).
During the 90-day study period, significantly fewer patients with a dermatologic condition in the uninterrupted TIM use cohort were hospitalized (1.2% versus 1.6%, P < 0.01; Supplemental Fig. 1B) compared with those in the interrupted TIM use cohort. The percentages of patients with a respiratory outcome or an ED visit were similar regardless of whether TIM use was interrupted. Significantly more patients had an in-person outpatient visit (60.2% versus 53.7%, P < 0.0001) or virtual visit (14.7% versus 12.7%, P < 0.0001) in the uninterrupted versus interrupted TIM use cohorts.
The likelihood of being hospitalized was 23% less (aOR = 0.77, 95% CI 0.63–0.94; P = 0.01; Supplemental Fig. 2B) in patients with a dermatologic condition in the uninterrupted versus interrupted TIM use cohort. There was no difference in the likelihood of having an ED visit (aOR = 1.01, 95% CI 0.89–1.15; P = 0.83) in the TIM use cohorts. In contrast, the likelihood of having an in-person outpatient visit or a virtual visit was 28% greater (aOR = 1.28, 95% CI 1.22–1.35; P < 0.0001) or 17% greater (aOR = 1.17, 95% CI 1.09–1.25; P < 0.0001), respectively, in the uninterrupted versus interrupted TIM use cohorts.
Gastrointestinal Conditions
A total of 21,208 patients had a gastrointestinal condition, including 17,547 (82.7%) with uninterrupted TIM use and 3661 (17.3%) with interrupted TIM use during the study period (Supplemental Table 6). Patients with a gastrointestinal condition were younger in the uninterrupted TIM use cohort than those in the interrupted TIM use cohort (40.0 ± 15.4 versus 42.3 ± 16.1 years, P < 0.0001). A larger proportion of patents in the uninterrupted versus interrupted TIM use cohort were female (50.6% versus 48.0%, P < 0.01). Crohn’s disease was more prevalent (69.7% versus 66.6%, in the uninterrupted TIM use versus interrupted TIM use cohort, respectively, P < 0.001) than ulcerative colitis (39.3% versus 42.4%, respectively, P < 0.001). Patients with a gastrointestinal condition in the uninterrupted TIM use cohort had a lower comorbidity burden than those in the interrupted TIM use cohort (CCI: 0.38 ± 0.90 versus 0.50 ± 1.12; P < 0.0001), and more than half of the patients in each cohort were being treated with a tumor necrosis factor inhibitor (74.9% versus 72.6%; P < 0.01), respectively.
During the 90-day study period, the percentage of patients with a gastrointestinal condition who were hospitalized or had an ED visit was similar in the uninterrupted and interrupted TIM use cohorts (Supplemental Fig. 1C). There were 0.3% patients in the uninterrupted TIM use cohort and 0.1% of patients in the interrupted TIM use cohort who had a respiratory outcome. Significantly more patients had an in-person outpatient visit (75.7% versus 43.7%, P < 0.0001) or virtual visit (19.2% versus 11.3%, P < 0.0001) in the uninterrupted versus interrupted TIM use cohort.
The likelihood of being hospitalized was 15% less (aOR = 0.85, 95% CI 0.69–1.06; P = 0.15; Supplemental Fig. 2C) in patients with a gastrointestinal condition in the uninterrupted versus interrupted TIM use cohort. In contrast, the likelihood of having an ED visit was 12% greater (aOR = 1.12, 95% CI 0.93–1.34; P = 0.23) in patients in the uninterrupted TIM use cohort compared with the interrupted TIM use cohort. The likelihood of having an in-person outpatient visit or a virtual visit was 309% greater (aOR = 4.09, 95% CI 3.79–4.41; P < 0.0001) or 87% greater (aOR = 1.87, 95% CI 1.68–2.09; P < 0.0001), respectively, in the uninterrupted versus interrupted TIM use cohort.
Discussion
This novel study demonstrates that patients with immune-mediated conditions and uninterrupted TIM use did not have an increase in hospitalizations, ED visits, or respiratory outcomes compared to patients with interrupted TIM use irrespective of COVID-19 diagnosis. Patients with uninterrupted TIM use were more likely to have an in-person outpatient visit than patients with interrupted TIM use. More frequent in-person outpatient or virtual visits in both COVID-19 and non-COVID-19 patients may be explained by the need for routine and perhaps heightened monitoring during treatment of their condition. Overall, these findings were generally consistent across rheumatology, dermatology, and gastroenterology indications in non-COVID-19 patients. It is reasonable to be concerned that patients who have an immune-mediated condition may be at greater risk for COVID-19 infection and thus more severe COVID-19 disease than the general population given their immunocompromised state resulting from their underlying condition as well as use of TIMs [14]. However, the overall findings of this research suggest that uninterrupted use of TIMs regardless of COVID-19 diagnosis is not associated with higher risks of hospitalizations, ED visits, and/or respiratory outcomes compared with interrupted TIM use.
Rheumatologist and patient impressions of COVID-19 disease may influence their treatment decisions. In a national survey evaluating perceptions of rheumatologists regarding COVID-19 in the US, 48% believed that patients with rheumatic conditions were at higher risk of COVID-19 regardless of immunosuppressive regimen, but 50% disagreed that the pandemic led them to reduce the use, dosage, or frequency of biologic therapies [15]. From the patient perspective, a survey of 1517 adults with rheumatic conditions in the US revealed that levels of concern about COVID-19 were high, with 46% of respondents being extremely concerned and 34% moderately concerned [12]. Over half (56.6%) avoided doctor’s visits. Among patients who did not report a respiratory illness or COVID-19 infection and were being treated with a biologic or Janus kinase inhibitor, 16.5% stopped using their medication because of concerns over COVID-19 infection. Most of these interruptions occurred without physician recommendation [12].
Several published reports have indicated that patients with immune-mediated conditions have similar rates of COVID-19 infection and that the severity of COVID-19 disease and mortality rates in these patients are not significantly different from the general population [16,17,18,19,20,21]. In addition, a retrospective observational study in Madrid found that the likelihood of developing severe acute respiratory syndrome was less in patients with an immune-mediated condition compared with those who did not have an immune-mediated condition [22].
Individual physicians and medical organizations have supported treatment interruptions in the management of patients with immune-mediated conditions. A recent study by Lebwohl et al. [23] reported that some physicians may be concerned that the immunosuppressive or immunomodulating agents, including biologic agents used to treat immune-mediated conditions, may render patients more susceptible to COVID-19 infection, even though there is no evidence to support this hypothesis. Additionally, organizations such as the National Psoriasis Foundation, American Academy of Dermatology, Medical Dermatology Society, American College of Rheumatology, and American College of Gastroenterology have recommended discontinuation or postponement of TIMs; however, evidence that substantiates these recommendations is limited [7,8,9,10,11]. Ironically, despite guidance recommendations for discontinuation or postponement of TIMs, immunosuppressive agents are currently under investigation for the attenuation of cytokine release syndrome, which occurs during severe COVID-19-related disease [24,25,26,27,28,29].
In a study examining demographic and clinical factors associated with COVID-19 hospitalization status in patients with rheumatic conditions, treatment with biologic/targeted synthetic DMARD therapy was associated with lower odds of hospitalization compared with no DMARD therapy [5], and tumor necrosis factor inhibitor use was also associated with reduced odds of hospitalization [5]. These findings are consistent with the results obtained in this study.
One important strength of the current study is that data were leveraged from a large national database providing results that may be generalizable to the entire US population covered by commercial and Medicare Advantage plans. The results and interpretations of this study should be taken in context of potential limitations, one of which is the potential for unobserved confounders that are inherent to retrospective observational studies. Consistent with all retrospective studies of healthcare claims data, there is the possibility of coding errors or omissions in the diagnostic and procedure codes. Patients may not have used recorded medications as prescribed after filling a prescription. Causal relationships between treatment continuation and outcomes cannot be inferred. Due to the small sample size of COVID-19 patients, we were not able to stratify the results by the rheumatic, dermatologic, and gastrointestinal therapeutic areas. Finally, the reasons for a patient’s treatment discontinuation (e.g., lack of treatment response, treatment-related side effects, and patient preference) cannot be directly observed in claims data.
Conclusion
Overall, our findings demonstrate that uninterrupted TIM use was not associated with an increased likelihood of hospitalizations, ED visits, or respiratory outcomes compared to interrupted TIM use among patients with an immune-mediated condition, irrespective of COVID-19 diagnosis. These data suggest that future therapeutic approaches by clinicians should consider not interrupting TIM therapy when managing patients with immune-mediated conditions amid the COVID-19 pandemic. However, shared clinical decision-making between the clinician and the patient remains a hallmark in routine care management.
References
COVID-19 Coronavirus pandemic. https://www.worldometers.info/coronavirus/#countries. Accessed 22 June 2021.
Centers for Disease Control and Prevention. COVID data tracker. https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Accessed 19 Jan 2021.
Shah P, Zampella JG. Use of systemic immunomodulatory therapies during the coronavirus disease 2019 (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:e203–4.
Czeisler M, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19-Related Concerns—United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1250–7.
Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–66.
Gianfrancesco MA, Hyrich KL, Gossec L, et al. Rheumatic disease and COVID-19: initial data from the COVID-19 global rheumatology alliance provider registries. Lancet Rheumatol. 2020;2:e250–3.
American Academy of Dermatology Association. Guidance on the use of immunosuppressive agents. https://www.aad.org/member/practice/coronavirus/clinical-guidance/biologics Accessed 26 Feb 2021.
Mikuls TR, Johnson SR, Fraenkel L, et al. American College of Rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 1. Arthritis Rheumatol. 2020;72:1241–51.
Rubin DT, Feuerstein JD, Wang AY, Cohen RD. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology. 2020;159:350–7.
Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 task force guidance for management of psoriatic disease during the pandemic: Version 1. J Am Acad Dermatol. 2020;83:1704–16.
Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150–9.
George MD, Venkatachalam S, Banerjee S, et al. Concerns, healthcare use, and treatment interruptions in patients with common autoimmune rheumatic diseases during the COVID-19 pandemic. J Rheumatol. 2021;48:603–7.
Bujang MA, Sa’at N, Sidik T, Joo LC. Sample size guidelines for logistic regression from observational studies with large population: emphasis on the accuracy between statistics and parameters based on real life clinical data. Malays J Med Sci. 2018;25:122–30.
Schett G, Sticherling M, Neurath MF. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol. 2020;20:271–2.
Mehta B, Jannat-Khah D, Mancuso CA, et al. Geographical variations in COVID-19 perceptions and patient management: a national survey of rheumatologists. Semin Arthritis Rheum. 2020;50:1049–54.
Brazzelli V, Isoletta E, Barak O, et al. Does therapy with biological drugs influence COVID-19 infection? Observational monocentric prevalence study on the clinical and epidemiological data of psoriatic patients treated with biological drugs or with topical drugs alone. Dermatol Ther. 2020;33:e14516.
Gianfrancesco M, Yazdany J, Robinson PC. Epidemiology and outcomes of novel coronavirus 2019 in patients with immune-mediated inflammatory diseases. Curr Opin Rheumatol. 2020;32:434–40.
Ansarin K, Taghizadieh A, Safiri S, et al. COVID-19 outcomes in patients with systemic autoimmune diseases treated with immunomodulatory drugs. Ann Rheum Dis. 2020.
Haberman R, Axelrad J, Chen A, et al. COVID-19 in immune-mediated inflammatory diseases—case series from New York. N Engl J Med. 2020;383:85–8.
Favalli EG, Bugatti S, Klersy C, et al. Impact of corticosteroids and immunosuppressive therapies on symptomatic SARS-CoV-2 infection in a large cohort of patients with chronic inflammatory arthritis. Arthritis Res Ther. 2020;22:290.
Sanchez-Piedra C, Diaz-Torne C, Manero J, et al. Clinical features and outcomes of COVID-19 in patients with rheumatic diseases treated with biological and synthetic targeted therapies. Ann Rheum Dis. 2020;79:988–90.
Monreal E, de la Maza SS, Fernández-Velasco JI, et al. The impact of Immunosuppression and autoimmune disease on severe outcomes in patients hospitalized with COVID-19. J Clin Immunol. 2021;41:315–23.
Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217–8.
Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R. COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun Rev. 2020;19:102523.
Sarzi-Puttini P, Giorgi V, Sirotti S, et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38:337–42.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4.
Campochiaro C, Della-Torre E, Cavalli G, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–9.
Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–31.
Della-Torre E, Campochiaro C, Cavalli G, et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020;79:1277–85.
Acknowledgements
Funding
Financial support for the study and the journal’s Rapid Service Fee were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the manuscript. All authors contributed to the development of the manuscript and maintained control over the final content. No honoraria or payments were made for authorship.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
MG and HBN contributed to analysis and interpretation of data and critical revision of the manuscript for important intellectual content. CDS, KJK, PAP, and VG contributed to study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; administrative, technical, or material support; and study supervision. SX contributed to analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; administrative, technical, or material support; and study supervision.
Medical writing Assistance
Medical writing assistance was provided by Joann Hettasch, PhD (Fishawack Facilitate Ltd., part of Fishawack Health, Conshohocken, PA), and funded by AbbVie Inc., North Chicago, IL.
Disclosures
Martin Bergman is a consultant/speaker for AbbVie, Amgen, BMS, Genentech, Gilead, Janssen, Merck, Novartis, Pfizer, Regeneron, Sanofi, and Sandoz and is a shareholder of JNJ (parent of Janssen). Haley B. Naik has received grant support from AbbVie, consulting fees from 23andme, AbbVie and DAVA Oncology, advisory board fees from Boehringer Ingelheim, and is an investigator for Pfizer. She is also an Associate Editor for JAMA Dermatology and an unpaid board member of the US Hidradenitis Suppurativa Foundation. Christopher D. Saffore, Pankaj A. Patel, Vishvas Garg, and Si Xuan are AbbVie employees and may own AbbVie stock or options. Katherine J. Kim is currently an employee of Acceleron and was an employee of AbbVie at the time this study was conducted.
Compliance with Ethics Guidelines
This was a retrospective study using anonymous data; therefore, Ethics Committee approval was not required. The data were de-identified following the statistical de-identification rules of the Health Insurance Portability and Accountability Act (HIPPA) and managed according to customer data use agreements.
Data Availability
All data generated or analyzed during this study are included in this published article or as supplementary files. The IBM® MarketScan® Research Databases are not publicly available and are subject to a data use agreement between the researcher and IBM. Information about the IBM® MarketScan® Research Databases including detailed descriptions and the process for obtaining access to them, is available at https://www.ibm.com/products/marketscan-research-databases/databases.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bergman, M., Saffore, C.D., Kim, K.J. et al. Healthcare Resource Use in Patients with Immune-Mediated Conditions Treated with Targeted Immunomodulators During COVID-19 Pandemic: A Retrospective Claims Analysis. Adv Ther 38, 5302–5316 (2021). https://doi.org/10.1007/s12325-021-01906-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01906-4