Abstract
Purpose
Diabetes has several adverse effects on patients with coronavirus disease 2019 (COVID-19); however, the determinants of this effect are still poorly understood. It is tried in current study to evaluate impacts of type 2 diabetes, with and without other comorbidities, on the clinical, para-clinical, and outcome parameters among COVID-19 patients.
Methods
A case series was applied, which involved 406 COVID-19 patients admitted in the city of Shiraz, south-central Iran, from February 20 to April 29, 2020. Demographic data, medical history, laboratory finding, chest computed tomography (CT) scan reports, and clinical outcomes of patients with and without type 2 diabetes were compared.
Results
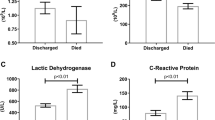
Results of the above-mentioned comparison showed that comorbidities such as HTN (35.5% vs. 13.7%, p < 0.001) and CVDs (26.2% vs. 13.4%, P = 0.002) were significantly more prevalent among the diabetic patients. Also, there was not any considerable difference between the chest CT severity parameters of both groups. After excluding all of the comorbidities except diabetes, it was found that the diabetic COVID-19 patients without other comorbidities had lower oxygen saturation level (P < 0.001), higher AST level (P = 0.037), higher BUN (P = 0.005), higher WBC counts (P = 0.025), lower lymphocyte counts (P = 0.029), and longer ICU admission duration (0.72 ± 2.83 vs. 1.71 ± 4.68, P = 0.046).
Conclusion
The diabetic COVID patients are at higher risks of hypoxemia, longer ICU stays, and more renal and hepatic dysfunction. These achievements could be useful in order to prevent the deterioration of clinical conditions among diabetic COVID-19 patients; also, they have to be considered in the management strategies.
Similar content being viewed by others
Introduction
A novel coronavirus (nCoV) was identified in late 2019 as the cause of a cluster of pneumonia cases in Wuhan, a city in Hubei Province of China. Then, it rapidly spread and led to an epidemic throughout China followed by an increasing number of cases in other countries across the world. In February 2020, the World Health Organization (WHO) designated coronavirus disease 2019 (COVID-19), which was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1].
In February 19, the first official report of the cases with COVID-19 in the city of Qom, Iran, was correspondingly released. COVID-19 outbreak has infected at least 32 million people in the world since September 25, 2020 [2]. According to the WHO statistics, more than 435,000 cases and 25,000 deaths have been reported in Iran so far. Based on a report represented by Shiraz University of Medical Sciences, Shiraz, Iran, COVID-19 cases in Fars Province have surpassed 50,000 people since September 20, 2020; moreover, the mortality rate has crossed 940 patients [2, 3].
According to the findings, the symptomatic infections range from mild to critical and most of the infections are not severe [4,5,6]. Pneumonia seems to be the most frequent serious symptom, which is primarily characterized by fever, cough, dyspnea, and bilateral infiltrates on the chest imaging tests [7,8,9]. Also, the upper respiratory tract infection symptoms, myalgia, diarrhea, and loss of smell or taste are prevalent [10, 11].
The acute respiratory distress syndrome (ARDS), which could be manifested shortly after the dyspnea, is considered as the main complication in patients with the severe type of the disease. Furthermore, other side effects are arrhythmias, acute cardiac injury, thromboembolic complications, and shock [7, 9, 12,13,14].
There are various mortality rates, comorbidities, and other difficult conditions associated with the severe cases of the disease. The severe condition can occur in healthy individuals of any age; however, it is predominantly observed in older adults or those with underlying medical comorbidities such as cardiovascular diseases (CVDs), diabetes mellitus (DM), hypertension (HTN), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), various types of cancer, obesity, and smoking [10, 15,16,17,18,19,20].
DM and poor glycemic control are considered as the major risk factors for infections, especially influenza and pneumonia [21]. In addition, studies on patients with DM showed that viral respiratory infections may cause a more severe type of the disease compared with non-diabetic cases [22]. Indeed, DM has been widely investigated in previous epidemics and pandemics such as influenza A virus subtype H1N1 (A/H1N1), SARS, and Middle East respiratory syndrome-related coronavirus (MER-SCoV). It has been further identified as an important risk factor for mortality and morbidity [23,24,25].There are data regarding the association between DM and COVID-19, which indicated that DM has to be considered as a major risk factor for rapid progression and poor prognosis of COVID‐19 [22, 26]. Also, they demonstrate a significant association between DM status and higher mortality rate in diabetic patients with COVID-19 [17]. The importance of investigating the relationship between diabetes and glycemic control in COVID-19 patients could be reflected through reports on better clinical outcomes among COVID-19 patients with type 2 diabetes who showed better blood glucose control [27]. Therefore, evaluating the role of diabetes in COVID-19 patients, the possible pathophysiological mechanisms, and its management are still being carried out; however, there are not any appropriate conclusions based on current evidence [28, 29].
Current study was carried out on a population of COVID-19 patients in order to assess impacts of type 2 diabetes, with and without other comorbidities, on the clinical, para-clinical, and outcome parameters among COVID-19 patients.
Materials and methods
Design and settings
In current cross sectional study, it was tried to evaluate 406 adult COVID-19 patients who were more than 18 years of age and admitted to two hospitals for COVID-19, which were affiliated to Shiraz University of Medical Sciences, from February 20 to April 29, 2020. The exclusion criteria were denial or withdrawal of informed consent, pregnancy, and being under 18 years of age. Furthermore, COVID-19 management protocols among adult hospitalized patients were on the basis of the interim guidance, which was issued by Centers for Disease Control and Prevention and World Health Organization guidelines [1, 30].
Data collection
COVID-19 was diagnosed in the above-mentioned patients through the positive reverse-transcription polymerase chain reaction (RT-PCR) of the upper respiratory tract, or by an expert team that noticed the clinical symptoms and high-resolution computed tomography (HRCT) scan reports. These individuals were selected using the convenience sampling method. Specific questionnaires were distributed between all of the investigated COVID-19 patients, which contained the demographic data, exposure history, and the disease symptoms at the presentation time. In addition, the presence of comorbidities that were defined as the positive medical history of type 2 diabetes, hypertension, cardiovascular diseases, chronic kidney disease, hypothyroidism, malignancy, and respiratory failure was included in the questionnaire. The complete medication history was also self-reported.
All of the COVID-19 patients were classified into four distinct groups. First, they were divided in to two groups of diabetic and non-diabetic COVID-19 patients and then, patients with comorbidities other than diabetes were excluded. The remaining patients were divided into two groups of diabetic and non-diabetic COVID-19 patients without other comorbidities. It is noteworthy that diabetes diagnosis was confirmed with regard to receiving insulin or oral hypoglycemic agents and ADA criteria [28].
Laboratory measures
Throat‐swab specimens achieved from the upper respiratory tract of the patients upon their admission were stored in a viral‐transport medium (VTM). Total ribonucleic acid (RNA) was further extracted using QIAamp™ viral RNA mini kit from Qiagen™ according to the instructions of the manufacturer. With E-gene and Rdrp-gene probe/primer and superscript™ III platinum, the one-step qRT-PCR kit of Invitrogen company mixtures was prepared. The mixtures were transferred to Roche Light cycler™ 96 and applied Biosystem ABI step one plus™ real time thermal cyclers with positive control and no template control (NTC), as well as an internal control. After 45 cycles and observing the produced graphs, the rises after the noise and before cycle 32 were considered as positive for COVID-19 [31, 32]. Blood samples were collected for differential cell blood count in tubes with EDTA and immediately became processed using an automated hematology analyzer called SYSMEX XN-1000.
BUN (Blood Urea Nitrogen), creatinine, Sodium (Na), Potassium (K), Blood Sugar (BS), and Liver function tests (ALT, AST and Alkaline phosphatase), CRP (C-reactive protein), D-dimer, Troponin, CPK, and LDH were measured for each patient. Troponin I was measured by Chemiluminescence Microparticle Immune-Assay (CMIA) (Cobas c601), while other chemical assessment were measured by Roche Cobas c501 chemistry analyzer (RocheDiagnostics).
Imaging evaluation
Non- contrast CT scan was conducted in a supine position during the full inspiration. Scanning was performed from thoracic inlet to the upper abdomen. The CT scans were performed on a scanner (GE Medical Systems, Milwaukee, WI, and USA) 120Kvp with the thickness of 1.25-2 mm and 1.25 mm interval. The CT images were reviewed by cardiothoracic radiologist. To further assess the abnormalities in CT images, the following parameters were analyzed: Ground-glass opacity, local patchy shadowing, bilateral patchy shadowing, and interstitial abnormalities.
Study outcomes
The hospital course and clinical outcomes during the period of admission to discharge or death were followed for each patient. All of the comorbidities such as CKD, CVDs, or respiratory failure were additionally recorded.
Ethical considerations
This study was approved by the Ethics Committee of Shiraz University of Medical Sciences (No.IR.SUMS.REC.1399.076), Shiraz, Iran. Written informed consent was achieved from each patient.
Statistical analysis
The qualitative and quantitative data were respectively described by frequency (percentage) and mean ± standard deviation (SD). Differences between two groups were analyzed using the × 2 test or Fischer exact test for categorical variables; moreover, the Student t test or Mann–Whitney U test were applied for the continuous variables. After the exclusion of patients with comorbidities except diabetes, similar analyses were carried out in order to achieve the laboratory test results and status of the diabetic patients. These findings were then become compared with non-diabetic COVID patients without other comorbidities. To make a comparison between the hospital mortality of diabetic and non-diabetic groups through the log rank test, Kaplan-Meier curves were plotted. All statistical analyses were performed using the SPSS Statistics software (version 19) for windows. Also, a < 0.05 p-value was considered to statistically be significant.
Results
There were 241 (59.4%) male cases among all of the 406 COVID-19 patients. As it could be seen in Table 1, the prevalence of preexisting type 2 DM, HTN, and CVDs, were respectively 26.4% (107 cases), 19.5% (79 cases), and 16.7% (68 cases). Furthermore, DM prevalence was found to be 49.5% among the cases aged more than 65 (p < 0.001), which was the highest ratio. Results showed that the comorbidities of HTN (35.5% vs. 13.7%, p < 0.001) and CVDs (26.2% vs. 13.4%, P = 0.002) were significantly more prevalent among diabetic COVID-19 cases compared to non-diabetic ones (Table 1).
Signs and symptoms
Table 1 represents the demographic data, and signs and symptoms on the presentation. The most common symptoms were dyspnea (63.8%), dry cough (57.4%), and fever (28.8%). Diabetic cases had lower oxygen (O2) saturation than non-diabetic ones on the admission day (P = 0.001). Other signs and symptoms at the presentation time were not statistically different between diabetics and non-diabetics. In addition, we did not find any significant difference in CT findings of the mentioned groups.
The mean durations of hospital stay were respectively found to be 7.64 ± 4.53 and 6.06 ± 4.53 days for diabetic and non-diabetic cases (P = 0.006). Correspondingly, the mean durations of ICU admission were 1.71 ± 4.69 and 1.00 ± 3.68 days for the diabetic and non-diabetic cases (P = 0.160), respectively. Although the in-hospital death rate was higher in patients with pre-existing DM compared to the non-diabetic individuals (11.3% vs. 6.3%), this difference was not statistically significant (P = 0.100).
Laboratory measurements
Table 2 represents the biochemical findings among diabetic and non-diabetic patients. Results of comparing the laboratory test results for patients on the admission day showed that serum sodium (Na) level was lower among the diabetic cases (P = 0.003).
Considering the diabetic group, the lymphocyte count was lower and levels of PMN, CRP, LDH, AST, ALT, and creatinine were higher. However, these differences were not statistically significant.
For further evaluation, patients with comorbidities other than DM were excluded in order to avoid their possible impacts on the results. The demographic data, laboratory measures, and outcomes among diabetic and non-diabetic COVID-19 patients without other comorbidities were provided in Table 3.
At the admission time, diabetic cases had lower oxygen saturation than non-diabetics (p < 0.001). Laboratory results established that as it was expected, the blood glucose level was much higher in the diabetic group compared with cases without comorbidities (p < 0.001). Also, the increased AST (P = 0.037) and decreased kidney function (P = 0.005) were found to be more frequent in the diabetic patients than non-diabetics in the cases without other comorbidities. Furthermore, DM patients had reduced lymphocyte count (P = 0.029) and increased WBC count (P = 0.025) significantly.
The clinical outcomes achieved from current study demonstrated in the Kaplan–Meier survival curve (Fig. 1), did not show any significant difference in overall survival time between diabetic and non-diabetic patients.
Discussion
It has been acknowledged that DM with its high prevalence is an important comorbidity in patients with COVID-19. Compared to non-diabetic patients, DM cases are more vulnerable to the infection and development of poor prognosis [33].
The United States Centers for Disease Control and Prevention (CDC) created a list of certain comorbidities associated with severe COVID-19. Among these comorbidities, DM was introduced as an established risk factor for the progression of the disease [25, 27, 34]. Also, CDC declared increased mortality in DM cases (2.3% overall and 7.3% patients with DM) [15]. The main findings of this study were in line with this statement. In this report, the diabetic patients were presented with advanced age, lower oxygen saturation, and signs of greater liver and kidney dysfunction.
In addition, type 2 DM was associated with the activation of the renin-angiotensin system in different tissues that may contribute to higher adverse risks in diabetic COVID-19 patients [35]. By binding to angiotensin-converting enzyme 2 (ACE2), the virus would enter into the cells and reduce the expression of ACE2 [36]. Therefore, the wide distribution of ACE2 level in the lungs, vascular endothelium, heart, kidney, and intestines could partially explain the underlying mechanism of multi-organ failure, especially myocardial, kidney, and liver injury in COVID-19 patients [26, 34].
Results showed that there was a bidirectional relationship between COVID-19 and DM [37]. DM was associated with an increased risk of severe COVID-19; also, COVID-19 could influence the pathophysiology of DM with transient insulin resistance and worsening of blood glucose control [23, 38]. This could be due to the cytokine storm, direct virus-mediated beta-cell damage, and special medications prescribed in the treatment course of viral infections such as anti-viral drugs or corticosteroids [34, 39].
Poor glycemic control was generally specified in various studies as a factor that influences the poor recovery of hospitalized patients [27, 40, 41]. This process was explained by a wide variety of immune response alterations associated with hyperglycemia. It was also specified that even transient stress hyperglycemia could cause innate immune response dysfunctions such as impairments in polymorphonuclear and monocytic cell chemotaxis and phagocytosis, complement function, and cytokine dysregulation [27, 41]. Accordingly, the investigated diabetic individuals with higher blood glucose level at the admission time had complications including lower oxygen saturation and lymphocyte count, abnormal liver enzyme levels and signs of renal function impairment.
Besides, The idiosyncratic effect of COVID-19 on the respiratory control system was also proposed in studies [42]. Patients with coronavirus disease (COVID-19) are described as exhibiting oxygen levels incompatible with life without dyspnea. The hypoxic ventilator response was diminished by more than 50% in diabetic patients. The diabetic cases had a 1.8-fold reduced ability to perceive respiratory sensations [43]. This is in line with this study that the patients with diabetes had lower oxygen (O2) saturation despite similar level of dyspnea and chest CT severity parameters at presentation in comparison with non-diabetic patients.
There are still some controversies regarding the effect of DM on mortality rate in COVID-19 patients; however, the bulk of studies have reported this association [26, 27, 44, 45]. It was found in current study that although the in-hospital death rate was higher in patients with pre-existing DM compared to non-diabetic cases, the difference was not statistically significant. It seemed that respiratory failures such as the lung volumes, pulmonary diffusing capacity, bronchomotor tone, and bronchial neuroadrenergic innervation led to the increase of mortality among diabetic COVID-19 patients [46]. The clinical outcomes achieved from current study analyzed by Kaplan–Meier survival curve, did not show any significant difference in overall survival time between diabetic and non-diabetic patients. Some other investigations revealed that the mortality rate was higher among DM patients [47, 48].
Conclusion
Comparing the health conditions of diabetic and non-diabetic COVID-19 patients, it was found that the first group was at a higher risk of complications. The unusual clinical picture of the respiratory distress-caused hypoxia and chest CT finding in of DM cases emphasized the importance of identifying the subtle clinical signs that were often missed for these patients. More investigations are required in order to achieve a better understanding of the pathophysiological and molecular mechanisms of this clinical entity, to prevent the complications and mortality among diabetic COVID-19 patients.
Limitations
Current study was faced with some limitations. Data was achieved from admitted diabetic patients with COVID-19, and the ones in the outpatient settings were not included. In addition, the glycemic data of pre-hospital status of these individuals was not accessible, which could be significantly associated with numerous clinical parameters. Therefore, several large-scale prospective cohort studies are required to understand the importance and association of glycemic control in COVID-19 progression.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- ACE2:
-
Angiotensin-Converting Enzyme 2
- ALT:
-
Alanine Aminotransferase
- ARDS:
-
Acute Respiratory Distress Syndrome
- AST:
-
Aspartate Aminotransferase
- BUN:
-
Blood Urea Nitrogen
- CDC:
-
Centers for Disease Control and Prevention
- CKD:
-
Chronic Kidney Disease
- COPD:
-
Chronic Obstructive Pulmonary Disease
- COVID-19:
-
Coronavirus Disease 2019
- CRP:
-
C-Reactive Protein
- CT:
-
Computed Tomography
- CVDs:
-
Cardiovascular Diseases
- DM:
-
Diabetes Mellitus
- Hb:
-
Hemoglobin
- A/H1N1:
-
Influenza A virus subtype H1N1
- HRCT:
-
High-Resolution Computed Tomography
- HTN:
-
Hypertension
- ICU:
-
Intensive Care Unit
- LDH:
-
Lactate Dehydrogenase
- MERS-CoV:
-
Middle East Respiratory Syndrome-related Coronavirus
- NTC:
-
No Template Control
- PMN:
-
Polymorphonuclear
- RNA:
-
Ribonucleic Acid
- RT-PCR:
-
Reverse-Transcription Polymerase Chain Reaction
- SARS:
-
Severe Acute Respiratory Syndrome
- VTM:
-
Viral‐Transport Medium
- WBC:
-
White Blood Cell
- WHO:
-
World Health Organization
References
Organization WH. Novel Coronavirus (2019-nCoV) technical guidance. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/patient-management. Accessed 21 June 2020.
WHO. Corona virus disease (COVID-19) Situation report. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200621-covid-19-sitrep-153.pdf?sfvrsn=c896464d_2. Accessed 20 June 2020.
How Iran Became a New Epicenter of the Coronavirus Outbreak. https://www.newyorker.com/news/our-columnists/how-iran-became-a-new-epicenter-of-the-coronavirus-outbreak. Accessed 28 Feb 2020.
Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–23.
Bajema KL, Oster AM, McGovern OL, Lindstrom S, Stenger MR, Anderson TC, et al. Persons evaluated for 2019 novel coronavirus—United States, January 2020. Morb Mortal Wkly Rep. 2020;69(6):166.
Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020;8(5):475–81.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–9.
Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, Felix SEB et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. Morb Mortal Wkly Rep. 2020;69(24):759.
Guan W-j, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–4.
Cao J, Tu W-J, Cheng W, Yu L, Liu Y-K, Hu X et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):748–55.
Xie Y, Wang X, Yang P, Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiol Cardiothorac Imaging. 2020;2(2):e200067.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42.
Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. Bmj. 2020;369.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–62.
Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–7.
Covid C, COVID C, COVID C, Chow N, Fleming-Dutra K, Gierke R, et al. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. Morbid Mort Wkly Rep. 2020;69(13):382.
Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–91.
Valdez R, Narayan KM, Geiss LS, Engelgau MM. Impact of diabetes mellitus on mortality associated with pneumonia and influenza among non-Hispanic black and white US adults. Am J Public Health. 1999;89(11):1715–21. https://doi.org/10.2105/ajph.89.11.1715.
Bloomgarden ZT. Diabetes and COVID-19. J Diabetes. 2020;12(4):347–8. https://doi.org/10.1111/1753-0407.13027.
Yang J, Feng Y, Yuan M, Yuan S, Fu H, Wu B, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–8.
Alene KA, Viney K, Gray DJ, McBryde ES, Wagnew M, Clements AC. Mapping tuberculosis treatment outcomes in Ethiopia. BMC Infect Dis. 2019;19(1):474.
Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y, et al. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11(1):59.
Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020;36(7):e3319.
Zhu L, She Z-G, Cheng X, Qin J-J, Zhang X-J, Cai J et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–77. e3.
Association AD. 15. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S211–S20.
Organization WH. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: interim guidance, 19 March 2020. https://apps.who.int/iris/bitstream/handle/10665/331501/WHO-COVID-19-laboratory-2020.5-eng.pdf. Accessed 19 March 2020.
Lamontagne F, Agoritsas T, Macdonald H, Leo Y-S, Diaz J, Agarwal A et al. A living WHO guideline on drugs for covid-19. bmj. 2020;370.
Organization WH. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: interim guidance, 19 March 2020. https://apps.who.int/iris/bitstream/handle/10665/331501/WHO-COVID-19-laboratory-2020.5-eng.pdf. Accessed 19 March 2020.
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3):2000045.
Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):303–10.
Pal R, Bhadada SK. COVID-19 and diabetes mellitus: An unholy interaction of two pandemics. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):513–7.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80. e8.
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875–9.
Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH et al. New-onset diabetes in Covid-19. N Engl J Med. 2020;383(8):789–90.
Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4(20). https://doi.org/10.1172/jci.insight.131774.
Maddaloni E, Buzzetti R. Covid‐19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020;36(7):e33213321.
Zhou J, Tan J. Diabetes patients with COVID-19 need better blood glucose management in Wuhan, China. Metabolism. 2020;107:154216.
Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. Journal of Diabetes Science and Technology. 2020;14(4):813–21.
Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202(3):356–60.
O’Donnell CR, Friedman LS, Russomanno JH, Rose RM. Diminished perception of inspiratory-resistive loads in insulin-dependent diabetics. N Engl J Med. 1988;319(21):1369–73.
Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):535–45.
Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–25. https://doi.org/10.1016/j.jinf.2020.04.021.
Fuso L, Pitocco D, Antonelli‐Incalzi R. Diabetic lung, an underrated complication from restrictive functional pattern to pulmonary hypertension. Diabetes/metabolism research and reviews. 2019;35(6):e3159.
Shang J, Wang Q, Zhang H, Wang X, Wan J, Yan Y, et al. The relationship between diabetes mellitus and COVID-19 prognosis: a retrospective cohort study in Wuhan. China The American journal of medicine. 2021;134(1):e6–14.
Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001343.
Acknowledgements
The present study was supported by a grant from the Vice-chancellor for Research, Shiraz University of Medical Sciences, Shiraz, Iran.
Funding
The research grant was provided by the Research Deputy of Shiraz University of Medical Sciences. The funding body of the study did not play any role in its designation, collection, analysis, data interpretation, and writing the manuscript.
Author information
Authors and Affiliations
Contributions
MHD, STH and MJ contributed in designing the study, data analysis, result interpretation, and manuscript drafting. ARE contributed in data analysis and result interpretation. ARE, STH and MJ contributed in the result interpretation and manuscript drafting. The final version was confirmed by all authors for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Shiraz University of Medical Sciences.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Estedlal, A., Jeddi, M., Heydari, S.T. et al. Impacts of diabetes mellitus on clinical and para-clinical parameters among COVID-19 patients. J Diabetes Metab Disord 20, 1211–1219 (2021). https://doi.org/10.1007/s40200-021-00844-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-021-00844-w