Abstract
Background
Several reports have been published about the impact of coronavirus disease 2019 (COVID-19) vaccines on human health, and each vaccine has a different safety and efficacy profile. The aim of this study was to reveal the nature and classification of reported adverse drug reactions (ADRs) of the two COVID-19 vaccines (tozinameran and ChAdOx1) among citizens and residents living in Saudi Arabia, and show possible differences between the two vaccines and the differences between each batch on the health of populations.
Methods
A cross-sectional study was conducted in Saudi Arabia between December 2020 and March 2021. Saudi citizens and residents aged ≥ 16 years who had at least one dose of any batch of either of the two approved COVID-19 vaccines (tozinameran and ChAdOx1) and who reported at least one ADR from the vaccines were included. The study excluded people who reported ADRs after receiving tozinameran or ChAdOx1 vaccines but no information was provided about the vaccine’s batch number.
Results
During the study period, 12,868 vaccinated people, including a high-risk group (i.e., those with chronic illness or pregnant women), reported COVID-19 vaccine ADRs that had been documented in the General Directorate of Medical Consultations, Saudi Ministry of Health. The study reported several ADRs associated with COVID-19 vaccines, with the most common (> 25%) being fever/chills, general pain/weakness, headache, and injection site reactions. Among healthy and high-risk people, the median onset of all reported ADRs for tozinameran and ChAdOx1 vaccine batches were 1.96 and 1.64 days, respectively (p < 0.01). Furthermore, significant differences (p < 0.05) were recorded between the two studied vaccines in regard to fever/chills, gastrointestinal symptoms, headache, general pain/weakness, and neurological symptoms, with higher incidence rates of these ADRs observed with the ChAdOx1 vaccine than the tozinameran vaccine. However, the tozinameran vaccine was found to cause significantly (p < 0.05) more palpitation, blood pressure variations, upper respiratory tract symptoms, lymph node swelling, and other unspecified ADRs than the ChAdOx1 vaccine. Among patients vaccinated with seven different batches of the tozinameran vaccine, people vaccinated with the T4 and T5 batches reported the most ADRs.
Conclusion
There were significant differences regarding most of the reported ADRs and their onset among tozinameran and ChAdOx1 vaccines on both healthy people and high-risk individuals living in Saudi Arabia. Moreover, the study found that the frequencies of most listed ADRs were statistically different when seven batches of tozinameran vaccine were compared.
Similar content being viewed by others
The study reported several adverse drug reactions (ADRs) associated with coronavirus disease 2019 (COVID-19) vaccines, with the most common being fever/chills, general pain/weakness, headache, and injection site reactions. |
The median onset of all reported ADRs for the tozinameran and ChAdOx1 vaccine batches was 1.96 and 1.64 days, respectively (p < 0.01). |
Statistically, ChAdOx1-vaccinated people complained more of fever/chills, headache, general pain/weakness, and neurological symptoms compared with the tozinameran group. |
More ADRs were detected in people vaccinated with the T4 and T5 batches, followed by the T2, and T1, T7, T3, and T6 batches, respectively. |
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious pandemic disease that has resulted in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The global human death counts due to COVID-19 exceeded 3.9 million to June 2021 [2]. Although the mortality rate of COVID-19 is lower than middle east respiratory syndrome (MERS), the momentum of spreading COVID-19 is much more [3].
Various COVID-19 vaccines have been approved for use among people to control SARS-CoV-2 virus transmission and mortality. These COVID-19 vaccines depend mainly on three pharmaceutical techniques: messenger RNA (mRNA), adenovirus vector, or inactivated SARS-CoV-2 genes [4]. The vaccine based on mRNA (tozinameran) first appeared in 2020 during the COVID-19 pandemic. The mRNA vaccines work by delivering a specific mRNA sequence to human cells to produce spike protein, a part of SARS-CoV-2. The immune system stimulation due to the produced spike will protect the body from SARS-CoV-2 infection [5]. The first developed mRNA vaccine (tozinameran) was the result of a partnership between American and German pharmaceutical companies, and it was the first approved vaccine against COVID-19 [6]. The tozinameran vaccine was approved by the Saudi Food and Drug Authority (SFDA) in December 2020 [7].
On the other hand, an adenovirus vector technique uses a mutated adenovirus, which is able to induce the human cells to make spike protein and trigger the immune system response to provoke specific T cells for preventing COVID-19. The adenovirus vector vaccine (ChAdOx1) manufactured by a British–Swedish company with the assistance of Oxford University was approved in Saudi Arabia by the SFDA in February 2021 [7, 8]. Unlike the mRNA and adenovirus vector vaccines, the inactivated SARS-CoV-2 vaccine was produced by inactivating a specific variant of SARS-CoV-2 by using β-propiolactone, in which this drug is able to bind to the virus genes and block their replication activity, but the viral proteins are kept intact. Therefore, injecting these inactivated viruses to the body will trigger an immune response without causing the disease [9]. There are currently no inactivated SARS-CoV-2 vaccines approved by the SFDA.
Several reports of the impact of COVID-19 vaccines on human health have been published, and each vaccine has a different safety and efficacy profile. These reports were variable depending on the type of vaccine investigated and population characteristics. This variation is possible due to the different ethnic groups and genetic make-up [10,11,12]. The reported adverse drug reactions (ADRs) of the vaccines included pain at the injection site, body weakness, myalgia, shivering, headache, tachycardia, and symptoms or signs of upper respiratory inflammation [13, 14]. This study aims to reveal the nature and classification of reported ADRs of the two COVID-19 vaccines (tozinameran and ChAdOx1) among citizens and residents living in Saudi Arabia and assess the possible differences between the two COVID-19 vaccines and each batch of the vaccines on the health of populations.
Research design and methods
Study design and population
A cross-sectional study was conducted in Saudi Arabia between December 2020 and March 2021. Saudi citizens and residents aged ≥ 16 years who had at least one dose of any batch of any of the two approved COVID-19 vaccines (tozinameran and ChAdOx1 vaccines) and who reported at least one ADR from the vaccines were included. The study excluded people who reported ADRs after receiving tozinameran or ChAdOx1 vaccines but no information was provided about the vaccine’s batch number.
The studied subjects were divided into healthy and non-healthy (high-risk) groups. The high-risk group included participants with chronic illness, including diabetes mellitus (DM), hypertension (HTN), chronic kidney disease (CKD), allergy, respiratory disease (e.g., asthma), cardiovascular disease (e.g., coronary artery disease), cerebrovascular disease (e.g., stroke), sickle cell anemia (SCA), or immune deficiency (e.g., cancer or organ transplant), and pregnant women.
Outcomes
The primary outcomes of this study were to assess the different ADRs that were classified depending on the nature (e.g., upper respiratory symptoms, lower respiratory symptoms, neurological symptoms, and cardiovascular symptoms) and onset of ADRs caused by the two COVID-19 vaccines (mRNA vaccine and adenovirus vector vaccine) among citizens and residents living in Saudi Arabia. The study also compared ADRs between the two vaccines and between different batches of each vaccine, and the differences in ADRs in healthy and high-risk people.
Data collection tools and analysis
A database of ADRs reported by vaccinated citizens and residents themselves or by health care providers in different medical facilities was secondarily analyzed in this study. The database contained the source of the report, type of vaccine, vaccine’s batch number, ADR, the onset of ADR, and the diagnosed chronic health condition. Documentation of the reported ADRs in the database is performed via the Ministry of Health call center that is operated through the General Directorate of Medical Consultations in the Ministry of Health.
Data analysis was conducted using SPSS version 25 (IBM Corporation, Armonk, NY, USA), and a p-value < 0.05 was considered statistically significant. Human subjects were divided into healthy and high-risk participants (who have underlying conditions, including pregnant women). The frequency distribution of the reported ADRs of both studied vaccines and their batches were determined. Comparison between healthy and high-risk subjects and between both studied vaccines regarding the development of ADRs was conducted using appropriate statistical tests.
Ethical consideration
This study was reviewed and approved by the Institutional Review Board Committees of the Saudi Ministry of Health (IRB Log Number: 21-91 M) and King Fahad Medical City (IRB Log Number: 21-359E). The confidentiality and anonymity of the participants’ data were preserved.
Results
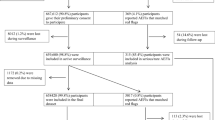
During December 2020 and March 2021, 12,868 of 4,432,572 vaccinated people reported ADRs from COVID-19 vaccines, representing a frequency of 0.3%. However, this number only represents those who reported ADRs that were documented in the General Directorate of Medical Consultations, Saudi Ministry of Health. There were 9629 healthy people (75% of the total; 3292 in the tozinameran vaccine group [34%] and 6337 [66%] in the ChAdOx1 vaccine group), and 3239 high-risk people (1808 in the tozinameran vaccine group and 1431 in the ChAdOx1 vaccine group). Table 1 describes the baseline characteristics of the studied vaccinated participants. More than 35% of those vaccinated with the tozinameran vaccine were high-risk people compared with 18% among those vaccinated with the ChAdOx1 vaccine, with a statistically significant difference between both groups (p < 0.01). In addition, a significantly (p < 0.01) higher proportion of people vaccinated with tozinameran had chronic illness compared with those vaccinated with ChAdOx1. No significant differences were recorded between both groups regarding SCA (0.9% and 0.6% for tozinameran and ChAdOx1, respectively) and pregnant women (0.04% for both vaccines).
The study reported several ADRs that had been sorted into 17 categories (Table 1), including fever/chills, injection site reactions (e.g., itching or inflammation at the injection site), gastrointestinal symptoms (e.g. nausea or vomiting), headache, general pain/weakness, lower respiratory tract symptoms (e.g., shortness of breath), anxiety, neurological symptoms (e.g., drowsiness or sleep disorder), palpitation, cardiovascular events (e.g., angina), blood pressure variations, bleeding events (e.g., epistaxis), upper respiratory tract symptoms (e.g., cough, sore throat, or nasal congestion), lymph node swelling, general allergic reactions (e.g., itching in different sites of the body), metabolic symptoms (e.g., increased blood glucose), and other unspecified ADRs (e.g., change in breast size). The most common ADRs (> 25%) associated with any of the two COVID-19 vaccines included fever/chills, injection site reactions, headache, and general pain/weakness (Table 2). The median onset of all reported ADRs for the tozinameran and ChAdOx1 vaccine batches were 1.96 and 1.64 days, respectively, and the difference between these vaccines regarding the onset of ADRs was significant (p < 0.01) (Table 3).
When the incidence of ADRs was compared between the two studied vaccines, statistically significant differences in fever/chills (p < 0.01), gastrointestinal symptoms (p < 0.05), headache (p < 0.01), general pain/weakness (p < 0.01), and neurological symptoms (p < 0.05) were observed, with higher percentages of people vaccinated with ChAdOx1 reporting these ADRs compared with those vaccinated with tozinameran (Table 2). However, tozinameran was significantly found to cause more palpitations (p < 0.05), blood pressure variations (p < 0.05), upper respiratory tract symptoms (p < 0.01), lymph node swelling (p < 0.01), and other unspecified ADRs (p < 0.05) than the ChAdOx1 vaccine (Table 2). There were no statistically significant differences between both vaccines regarding other categories of ADRs, including injection site reactions, lower respiratory tract symptoms, anxiety, cardiovascular events, bleeding events, and metabolic symptoms (Table 2). Although more significant people diagnosed with previous allergic reactions were found in the ChAdOx1 group, the frequency of general allergic reactions and lymph node swelling ADRs were more statistically detected in tozinameran-vaccinated people.
Regarding the ADRs reported for different batches of the tozinameran vaccine, including T1, T2, T3, T4, T5, T6, and T7, the study revealed significant differences between these batches regarding the ADR categories of fever/chills (p < 0.01), injection site reactions (p < 0.01), gastrointestinal symptoms (p < 0.01), headache (p < 0.01), general pain/weakness (p < 0.01), cardiovascular events (p < 0.05), blood pressure variations (p < 0.01), upper respiratory tract symptoms (p < 0.01), lymph node swelling (p < 0.01), and general allergic reactions (p < 0.01). By contrast, no significant differences were recorded between ChAdOx1 vaccine batches (C1 and C2) regarding these ADRs (p > 0.05) (Table 4).
The study also reported several significant variations between healthy and high-risk people regarding the frequency of ADRs. For the tozinameran-vaccinated group, healthy people had significantly more ADRs relating to gastrointestinal symptoms (p < 0.01), headache (p < 0.01), general pain/weakness (p < 0.05), and lymph node swelling (p < 0.01) compared with the high-risk group. Meanwhile, the high-risk group had statistically more blood pressure variations (p < 0.05), upper respiratory tract symptoms (p < 0.05), and metabolic symptoms (p < 0.01) than healthy subjects (Table 5). With regard to the ChAdOx1 vaccine, the incidence rate of fever/chills, lower respiratory tract symptoms, palpitations, lymph node swelling, and metabolic symptoms was significantly higher (p < 0.05) in the high-risk group than in the healthy group (Table 6).
Discussion
The current study investigated people reporting at least one ADR after receiving different batches of the tozinameran and ChAdOx1 vaccines from December 2020 through March 2021. Despite the baseline characteristics showing a significantly higher proportion of healthy people who received the ChAdOx1 vaccine than the tozinameran vaccine, the incidence of fever/chills, headache, general pain/weakness, and neurological symptoms was significantly higher in ChAdOx1-vaccinated people than those vaccinated with tozinameran. Furthermore, the onset of ADRs among tozinameran-vaccinated people was significantly delayed compared with ChAdOx1-vaccinated people, which may be correlated with more patients diagnosed with chronic diseases in the tozinameran group.
Among the included subjects who were vaccinated with the tozinameran and ChAdOx1 vaccines, several ADRs, such as fever, injection site reactions, and general weakness, were expected. However, the researchers in this study revealed that mild neurological-related ADRs were also commonly reported. The mechanisms responsible for these neurological issues associated with COVID-19 vaccines are not well understood, but some studies hypothesized that elevated blood levels of specific types of inflammatory cytokines could be correlated with intensive immune responses, causing some damage to the neurons [15, 16].
The nature of ADRs reported in the current study is generally similar to that reported in other studies. For instance, El-Shitany et al. tracked the short-term ADRs of the tozinameran vaccine in Saudi Arabia and found that the most common symptoms were injection site pain, headaches, flu-like symptoms, fever, and tiredness [17], while less common ADRs included fast heartbeat, whole body aches, difficulty breathing, joint pain, chills, and drowsiness. Rare ADRs include Bell’s palsy and lymph node swelling and tenderness [17]. In a large, randomized, double-blind, placebo-controlled phase I/II clinical trial that enrolled more than 43,000 participants, the most common ADRs associated with the ChAdOx1 vaccine were fever, fatigue, headache, muscle pain, chills, injection site pain, and fever [18]. Although the nature of ADRs reported in these studies are generally similar to that reported in the current study, the incidence of the reported ADRs is almost twofold higher in the current study. The anticipated reason for the highly reported ADRs in this study could be related to people described as a high-risk group, as more than 35% of the included subjects in the tozinameran group have chronic diseases, most commonly DM, HTN, and respiratory diseases.
Findings from other studies that compared the safety of the tozinameran versus ChAdOx1 vaccines were somewhat consistent with the findings from the current study. Almufty et al. reported that ADRs of fever, general pain/weakness, headache, and chills were detected significantly more often in people immunized with the ChAdOx1 vaccine compared with people who received the tozinameran vaccine. These findings were consistent with the results of the current study [19]. Al Khames Aga et al. also showed that ADRs of fatigue, body pain, headache, and general pain were reported more often among ChAdOx1-vaccinated people compared with those who received the tozinameran vaccine (significance not tested) [20]. Another randomized, cross-sectional study conducted in Jordan revealed that chills, upper respiratory tract symptoms, and sleepiness and laziness ADRs were more frequently presented among ChAdOx1-vaccinated people than tozinameran-vaccinated people (p < 0.05) [21]. In this study, injection site pain and swelling were reported more frequently with tozinameran than with ChAdOx1 [21]. In the study by Alhazmi et al., which evaluated ADRs relating to the tozinameran and ChAdOx1 vaccines in Saudi Arabia among 515 participants within 3 weeks through an online questionnaire, fatigue, pain at the site of injections, fever, and headache were among the most commonly reported ADRs [22]. Most of the participants reported having ADRs on the first day of receiving the vaccines, with the ADRs having a duration of 1 day [22]. The current study also mentioned that these ADRs were very common, however the incidence rates of the ADRs reported by Alhazmi et al. were much higher. Furthermore, unlike the onset of ADRs reported by the previous study, the current study found that ADRs from the ChAdOx1 vaccine were commonly reported on the second day of vaccination. The expected reasons for these variations are the mass difference in the sample size and the methods of reporting ADRs; the current study included 12,868 vaccinated people from different Saudi regions who reported their ADRs through official governmental channels (e.g., primary healthcare centers and telemedicine applications).
To the best of our knowledge, this study is the first to compare ADRs from the tozinameran and ChAdOx1 COVID-19 vaccine batches. The significant variations in the frequencies of ADRs in the studied batches belonging to the tozinameran vaccine could be related to the vaccine’s quality or efficacy, which might be caused by issues regarding cold chain and storage. Based on recent studies, it is recommended to ensure high standards for logistics, cold chain, and vaccine storage. These recommendations are substantial to provide the maximum efficacy of COVID-19 vaccines that will help in reducing the morbidity and mortality rates related to the COVID-19 pandemic [23, 24].
Study limitations
Several ADRs were self-reported, which could not reflect the actual existence of these ADRs. Moreover, there were significant baseline differences between the tozinameran and ChAdOx1 groups in relation to healthy people and those with chronic disorders.
In addition, participants’ sociodemographic data (e.g., age, sex, and nationality) and order of the COVID-19 vaccines dose number (either first or second dose) at the time of reporting ADRs were not available; therefore, the researchers could not find many possible risk factors that may be associated with the development of the reported ADRs.
Conclusion
Statistically, ChAdOx1-vaccinated people complained more of fever/chills, headache, general pain/weakness, and neurological symptoms compared with the tozinameran group. Controversially, the frequency of palpitations, blood pressure variations, upper respiratory tract symptoms, and lymph node swelling was statistically more significant in tozinameran-vaccinated people. There were significant differences regarding most of the reported ADRs and their onset among tozinameran and ChAdOx1 vaccines in both healthy people and those at high-risk. Moreover, the study found that the frequencies of most listed ADRs were statistically different when seven batches of the tozinameran vaccine were compared. Further studies are needed to determine the long-term ADRs of the two vaccines and their efficacy in preventing and controlling SARS-CoV-2 infection.
References
Kandeel M, Yamamoto M, Tani H, et al. Discovery of new fusion inhibitor peptides against SARS-CoV-2 by targeting the spike s2 subunit. Biomol Ther. 2021;29(3):282–9.
Machingaidze S, Wiysonge CS. Understanding COVID-19 vaccine hesitancy. Nat Med. 2021;27:1338–9.
Pitlik SD. COVID-19 Compared to Other Pandemic Diseases. Rambam Maimonides med J. 2020;11(3):e0027.
Federico M. The conundrum of current anti-SARS-CoV-2 vaccines. Cytokine Growth Factor Rev. 2021;60:46–51.
Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383(20):1920–31.
Khehra N, Padda I, Jaferi U, et al. Tozinameran (BNT162b2) vaccine: the journey from preclinical research to clinical trials and authorization. AAPS PharmSciTech. 2021;22(5):172.
Assiri A, Al-Tawfiq JA, Alkhalifa M, et al. Launching COVID-19 vaccination in Saudi Arabia: lessons learned, and the way forward. Travel Med Infect Dis. 2021;43: 102119. https://doi.org/10.1016/j.tmaid.2021.102119.
Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–91.
Mirzaei R, Mohammadzadeh R, Mahdavi F, et al. Overview of the current promising approaches for the development of an effective severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. Int Immunopharmacol. 2020;88: 106928. https://doi.org/10.1016/j.intimp.2020.106928.
Nguyen LH, Joshi AD, Drew DA, et al. Racial and ethnic differences in COVID-19 vaccine hesitancy and uptake. medRxiv. 2021. https://doi.org/10.1101/2021.02.25.21252402.
Reid JA, Mabhala MA. Ethnic and minority group differences in engagement with COVID-19 vaccination programmes—at pandemic pace; when vaccine confidence in mass rollout meets local vaccine hesitancy. Isr J Health Policy Res. 2021;10(1):33.
Green MS, Abdullah R, Vered S, et al. A study of ethnic, gender and educational differences in attitudes toward COVID-19 vaccines in Israel—implications for vaccination implementation policies. Isr J Health Policy Res. 2021;10(1):26.
Riad A, Pokorná A, Attia S, et al. Prevalence of COVID-19 vaccine ADRs among healthcare workers in the Czech Republic. J Clin Med. 2021;10(7):1428.
Kaur SP, Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res. 2020;288:198114. https://doi.org/10.1016/j.virusres.2020.198114.
Lu L, Xiong W, Mu J, et al. The potential neurological effect of the COVID-19 vaccines: a review. Acta Neurol Scand. 2021;144(1):3–12.
Hsiao YT, Tsai MJ, Chen YH, et al. Acute transverse myelitis after COVID-19 vaccination. Medicina. 2021;57(10):1010.
El-Shitany NA, Harakeh S, Badr-Eldin SM, et al. Minor to moderate ADRs of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. Int J Gen Med. 2021;14:1389–401.
Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–78.
Almufty HB, Mohammed SA, Abdullah AM, Merza MA. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes Metab Syndr Clin Res Rev. 2021;15(5):102207.
Al Khames Aga QA, Alkhaffaf WH, Hatem TH, et al. Safety of COVID-19 vaccines. J Med Virol. 2021;93(12):6588–94.
Hatmal MM, Al-Hatamleh MA, Olaimat AN, et al. Side effects and perceptions following COVID-19 vaccination in Jordan: a randomized, cross-sectional study implementing machine learning for predicting severity of side effects. Vaccines. 2021;9(6):556.
Alhazmi A, Alamer E, Daws D, et al. Evaluation of ADRs associated with COVID-19 vaccines in Saudi Arabia. Vaccines. 2021;9:674.
Das MK. COVID-19 vaccine and the cold chain implications for global adoption. Indian J Public Health. 2021;65(3):307–10.
Hanson CM, George AM, Sawadogo A, Schreiber B. Is freezing in the vaccine cold chain an ongoing issue? A literature review. Vaccine. 2017;35(17):2127–33.
Acknowledgments
The authors would like to thank the Data Governance Team at the Saudi Ministry of Health for their assistance in completing this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No funding was received to assist in the preparation of this study.
Competing interests
Amjad Alfaleh, Abdullah Alkattan, Nashwa Radwan, Mona Elzohri, Abrar Alzaher, Mona Ibrahim, Eman Alsalameen, Amani Alsultan, Dina Alhabib, Alanood Alshelwah, Nagla Mahmoud, Khlood Sagor, and Khaled Alabdulkareem have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript, including employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethical approval and consent to participate
This study was reviewed and approved by the Institutional Review Board Committees of the Saudi Ministry of Health (IRB Log Number: 21- 91 M) and King Fahad Medical City (IRB Log Number: 21-359E). The confidentially and anonymity of the participants’ data were preserved. This study was dependent on anonymous secondary data, therefore no consent for participation was required.
Consent for publication
This study was dependent on anonymous secondary data, therefore no consent for participation or publication was required.
Availability of data and material (data transparency)
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability (software application or custom code)
Not applicable.
Authors’ contributions
AF, AK, AZ, and MI contributed to the study conception and design. Data collection and analysis was performed by AF, AK, AZ, EA, AS, MZ, AS, DH, and KS. The first draft of the manuscript was written by AK, NR, NM, and KA. All authors commented on previous versions of the manuscript, and all authors read, reviewed, and approved the final manuscript.
Clinical trials registration number
Not applicable.
Electronic supplementary material
Not applicable.
Rights and permissions
About this article
Cite this article
Alfaleh, A., Alkattan, A., Radwan, N. et al. Adverse drug reactions from two COVID-19 vaccines reported in Saudi Arabia. Drugs Ther Perspect 38, 84–92 (2022). https://doi.org/10.1007/s40267-022-00893-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-022-00893-y