DOI: 10.31038/CST.2022732
Abstract
We present a case of a 33 years old patient with glioblastoma who was diagnosed with Coronavirus disease 2019 (COVID-19) while undergoing chemotherapy. The patient did not have any other medical comorbidities. Due to active infection and leucopenia, his chemotherapy with temozolomide was on hold for 1 month. Temozolomide was resumed after resolution of leucopenia, improvement of COVID symptoms and a negative COVID-PCR (polymerase chain reaction) test. Patient continued to do well after administration of subsequent temozolomide cycles. A repeat computed tomography (CT) chest 2 months post infection revealed resolution of consolidation and no new areas of consolidation. Temozolomide was safely administered in this patient without reactivation of COVID-19 infection. Patient did not have any thrombotic events.
Keywords
Glioblastoma, COVID-19 pneumonia, Temozolomide, Chemotherapy resumption
Case Report
We present a case of a previously healthy 33 years old patient who presented with severe headaches and was found to have a right parietal lobe heterogeneously enhancing intra-axial neoplasm on magnetic resonance imaging (MRI) of the brain. He underwent a complete resection of the enhancing tumor volume. Pathology revealed a WHO grade IV astrocytoma, isocitrate dehydrogenase enzyme (IDH wild-type), O6-methylguanine-DNA methyltransferase (MGMT) unmethylated, positive CDKN2A loss (cyclin-dependent kinase inhibitor). He was subsequently treated as per the Stupp protocol with concurrent temozolomide and radiation followed by maintenance temozolomide. He was non-compliant with tumor treating fields which he self-discontinued after few months of treatment.
After completion of 3 out of 6 temozolomide cycles, he was admitted for fever and upper abdominal pain. A COVID-19 polymerase chain reaction (PCR) test was positive. He acquired this infection in the pre-COVID-19 vaccine era. Laboratory tests revealed normal WBC count of 4.8 K/UL, normal absolute neutrophil count (3.5 K/MM3), grade 2 lymphopenia with low absolute lymphocyte count (0.7 K/MM3). Computed tomography (CT) of the chest revealed patchy peripheral bibasilar ground glass and consolidative opacities compatible with pulmonary infection, with viral etiology such as COVID-19 (Figure 1A). He did not have pulmonary symptoms including shortness of breath or cough. Fever and abdominal pain resolved after 2 weeks of supportive care. However, due to the active COVID-19 infection and leucopenia, temozolomide dosing was held. It was subsequently resumed (cycle 4) after resolution of clinical symptoms and signs of COVID, normalization of hematological parameters (resolution of lymphopenia) and a negative COVID-19 PCR test. He tolerated his chemotherapy well through the completion of 6 cycles of temozolomide. A repeat CT chest 2 months after the initial COVID-19 infection revealed resolution of the lung consolidation (Figure 1B). Patient did not have any thrombotic events.
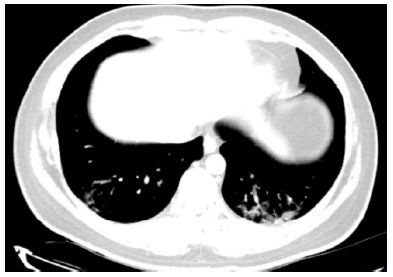
Figure 1A: Non contrast computed tomography chest showing bibasilar ground glass and consolidative opacities
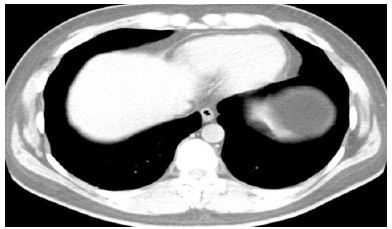
Figure 1B: resolution of bibasilar ground glass and consolidative opacities after 2 months
Discussion
COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which was declared as a global pandemic in March 2020 [1].
Impact of anti-cancer therapies on the course of COVID-19, and risk factors affecting the COVID-19 outcome for patients with primary brain tumors in particular, have not yet been elucidated. The aggravated immune response to COVID infection mediated by endothelial cells cytokines can potentially change the permeability of the blood brain barrier (BBB) causing exposure of the cerebral tissue to viral proteins [2].
Reactivation of virus post cancer chemotherapy has been reported with hepatitis B [3,4]. Similar concern exists with resumption of chemotherapy after SARS-CoV-2 infection. The immunocompromised state of cancer patients makes them more vulnerable to infection and morbidity with COVID-19 [5-7]. This increased vulnerability is partially due to the chronic immunocompromised state which can be exacerbated by anti-cancer therapies. There are conflicting results on risk of mortality with cytotoxic chemotherapy in COVID-19 patients [8-12]. Poor outcomes of COVID-19 infection in patients with receipt of recent chemotherapy is likely related to other comorbidities (peri-COVID-19 lymphopenia, neutropenia, diabetes, hypertension, and cardiovascular diseases) [10,13]. American Society of Clinical Oncology guidelines suggest waiting for infected patients to become asymptomatic and register a negative COVID-19 test before resuming treatment [9,14]. It is crucial to avoid under-treatment and treatment delays.
A report from the Dutch Oncology COVID-19 Consortium included 30 primary brain tumor patients of which 60% were glioblastomas [15]. Most patients had received cancer treatment ≤90 days prior to COVID-19 diagnosis. There were no indications of a negative impact of systemic anti-cancer therapies on the outcome of COVID-19 [15]. The thoracic cancer international COVID-19 collaboration (TERAVOLT) registry is the first large dataset of patients with COVID-19 and thoracic malignancies, regardless of therapies administered. No statistically significant association was found between anti-cancer treatment with mortality rate of the patients with thoracic cancers who were infected with COVID-19 [16]. Safe resumption of chemotherapy after resolution of COVID symptoms (approximately a month after the positive PCR test) has been reported in a young breast cancer patient without significant comorbidities [17]. There was no re-activation of COVID-19 or pulmonary adverse events [17]. Similarly, chemotherapy was safely resumed (approximately a month after the positive PCR test) in a 60 years old lady with ovarian cancer who was infected with COVID-19 [18]. In both these cases chemotherapy was resumed after resolution of COVID symptoms and negative PCR test. A 29 years old gentleman with a poor risk germ cell tumor safely resumed cytotoxic chemotherapy despite positive SARS-CoV-2 nasopharyngeal swab (he did have resolution of COVID symptoms) [19]. Two patients from Poland had no complications with chemotherapy continuation after COVID infection [20]. One patient was a young gentleman with primary mediastinal B-cell lymphoma, other patient was 56 years old gentleman with sigmoid cancer with asymptomatic COVID infection when receiving chemotherapy [20]. A prospective multi-institutional study 39 cancer patients in China reported low risk of SARS-CoV-2 reactivation after chemotherapy administration [21]. This study involved patients with different cancer pathologies including a 41 years old patient with glioblastoma with no comorbidities [21]. A severe case of COVID pneumonia was reported due to viral re-activation after resumption of immunosuppressive therapy (lymphocyte- and antibody-depleting therapy) in a 55 years old lady with CD20+ B cell acute lymphoblastic leukemia as well as other comorbidities including diabetes and cardiovascular disease [22]. Chemotherapy in her case was resumed 2 months after the first positive COVID PCR test, she had complete resolution of COVID symptoms and a negative PCR test before initiation of chemotherapy [22]. Most of the above studies indicate safe resumption of chemotherapy after resolution of COVID infection in patients without significant comorbidities.
For brain tumor patients there is lack of reliable data on the optimal timing and safety of resumption of cytotoxic chemotherapy in patients infected with COVID-19. Malignant gliomas are aggressive tumors with poor survival rates. Delay or discontinuation of cytotoxic chemotherapy will result in tumor progression and neurologic adverse events. Safety of adjuvant chemotherapy with temozolomide for treating gliomas during covid-19 pandemic has been reported [23]. Nevertheless, risk and benefits of chemotherapy should be considered especially in systemic chemotherapy such as alkylating agents, moderate delay in systemic treatment is acceptable [24]. To the best of our knowledge, there has been no report of continuation of chemotherapy with temozolomide in patients with high grade glioma and symptomatic covid-19 infection. In the case reported here, successful resumption of standard temozolomide dosing was possible with resolution of the COVID-19 associated clinical symptoms and radiographic findings and normalization of the hematological parameters. This report may provide insight into timing of safe resumption of essential chemotherapy post COVID-19 infection in a high grade glioma patient without significant comorbidities.
References
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382: 1708-1720.
- Ljubimov VA, Ramesh A, Davani S, Danielpour M, Breunig JJ, et al. (2022) Neurosurgery at the crossroads of immunology and nanotechnology. New reality in the COVID-19 pandemic. Adv Drug Deliv Rev 181: 114033. [crossref]
- Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, et al. (2000) Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol 62: 299-307. [crossref]
- Lubel JS, Angus PW (2010) Hepatitis B reactivation in patients receiving cytotoxic chemotherapy: diagnosis and management. J Gastroenterol Hepatol 25: 864-871. [crossref]
- Zhang L, Zhu F, Xie L, Wang C, Wang J, et al. (2020) Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol 31: 894-901. [crossref]
- Zhang H, Han H, He T, Labbe KE, Hernandez AV, et al. (2021) Clinical Characteristics and Outcomes of COVID-19-Infected Cancer Patients: A Systematic Review and Meta-Analysis. J Natl Cancer Inst 113: 371-380. [crossref]
- Liang W, Guan W, Chen R, Wang W, Li J, et al. (2020) Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 21: 335-337. [crossref]
- Cioffi R, Sabetta G, Rabaiotti E, Bergamini A, Bocciolone L, et al. (2021) Impact of COVID-19 on medical treatment patterns in gynecologic oncology: a MITO group survey. Int J Gynecol Cancer 31: 1363-1368. [crossref]
- Lievre A, Turpin A, Ray-Coquard I, Le Malicot K, Thariat J, et al. (2020) Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: A French nationwide cohort study (GCO-002 CACOVID-19). Eur J Cancer 141: 62-81. [crossref]
- Tian J, Yuan X, Xiao J, Zhong Q, Yang C, et al. (2020) Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol 21: 893-903.
- Yang K, Sheng Y, Huang C, Jin Y, Xiong N, et al. (2020) Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol 21: 904-913.
- Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, et al. (2020) COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 395: 1919-1926. [crossref]
- Jee J, Foote MB, Lumish M, Stonestrom AJ, Wills B, et al. (2020) Chemotherapy and COVID-19 Outcomes in Patients With Cancer. J Clin Oncol 38: 3538-3546. [crossref]
- ASCO, Cancer Treatment & Supportive Care.
- De Joode K, Taal W, Snijders TJ, Hanse M, Koekkoek JAF, et al. (2022) Patients with primary brain tumors and COVID-19: A report from the Dutch Oncology COVID-19 Consortium. Neuro Oncol 24: 326-328. [crossref]
- Garassino MC, Whisenant JG, Huang LC, Trama A, Torri V, et al. (2020) COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol 21: 914-922. [crossref]
- Horiguchi J, Nakashoji A, Kawahara N, Matsui A, Kinoshita T (2021) Chemotherapy resumption in breast cancer patient after COVID-19. Surg Case Rep 7: 170. [crossref]
- Liontos M, Kaparelou M, Karofylakis E, Kavatha D, Mentis A, et al. (2020) Chemotherapy resumption in ovarian cancer patient diagnosed with COVID-19. Gynecol Oncol Rep 33: 100615. [crossref]
- Pedrazzoli P, Rondonotti D, Cattrini C, Secondino S, Ravanini P, et al. (2021) Metastatic Mediastinal Germ-Cell Tumor and Concurrent COVID-19: When Chemotherapy Is Not Deferrable. Oncologist 26: e347-e349. [crossref]
- Wozniak K, Sachs W, Boguradzki P, Basak GW, Stec R (2021) Chemotherapy During Active SARS-CoV2 Infection: A Case Report and Review of the Literature. Front Oncol 11: 662211. [crossref]
- Bi J, Ma H, Zhang D, Huang J, Yang D, et al. (2020) Does chemotherapy reactivate SARS-CoV-2 in cancer patients recovered from prior COVID-19 infection? Eur Respir J 56: 2002672. [crossref]
- Lancman G, Mascarenhas J, Bar-Natan M (2020) Severe COVID-19 virus reactivation following treatment for B cell acute lymphoblastic leukemia. J Hematol Oncol 13: 131.
- Pessina F, Navarria P, Bellu L, Clerici E, Politi LS, et al. (2020) Treatment of patients with glioma during the COVID-19 pandemic: what we learned and what we take home for the future. Neurosurg Focus 49: E10. [crossref]
- Weller M, Preusser M (2020) How we treat patients with brain tumour during the COVID-19 pandemic. ESMO Open 4: e000789. [crossref]