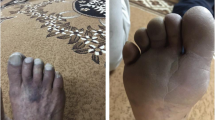
Abstract
Coronavirus disease 2019 (COVID-19) is a multi-system disease that can lead to various severe complications. Acute limb ischemia (ALI) has been increasingly recognized as a COVID-19-associated complication that often predicts a poor prognosis. However, the pathophysiology and molecular mechanisms underlying COVID-19-associated ALI remain poorly understood. Hypercoagulability and thrombosis are considered important mechanisms, but we also emphasize the roles of vasospasm, hypoxia, and acidosis in the pathogenesis of the disease. The angiotensin-converting enzyme 2 (ACE2) pathway, inflammation, and platelet activation may be important molecular mechanisms underlying these pathological changes induced by COVID-19. Furthermore, we discuss the hypotheses of risk factors for COVID-19-associated ALI from genetic, age, and gender perspectives based on our analysis of molecular mechanisms. Additionally, we summarize therapeutic approaches such as use of the interleukin-6 (IL-6) blocker tocilizumab, calcium channel blockers, and angiotensin-converting enzyme inhibitors, providing insights for the future treatment of coronavirus-associated limb ischemic diseases.
摘要
2019 冠状病毒病 (COVID-19) 作为一种多系统疾病, 可导致各种严重并发症。 急性肢体缺血 (ALI) 被认为是与 COVID-19 相关的一种并发症, 通常预示着不良预后。 但目前人们对 COVID-19 相关 ALI 的病理生理和分子机制仍知之甚少, 高凝状态和血栓形成被认为是重要机制。 本文强调了血管痉挛、缺氧和酸中毒在疾病发病中的作用, 血管紧张素转化酶2 (ACE2) 途径、炎症和血小板激活可能是 COVID-19 诱导上述病理变化的重要分子机制。 此外, 本文从遗传、年龄和性别角度对 COVID-19 相关 ALI 危险因素的假说进行了讨论和分析, 并对治疗方法如使用白细胞介素-6 (IL-6) 拮抗剂托珠单抗、钙通道拮抗剂和血管紧张素转化酶抑制剂等进行总结, 以期为治疗冠状病毒相关肢体缺血性疾病提供新见解。
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Abou-Ismail MY, Diamond A, Kapoor S, et al., 2020. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res, 194:101–115. https://doi.org/10.1016/j.thromres.2020.06.029
Agrawal A, 2013. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology, 59(5):421–426. https://doi.org/10.1159/000350536
Ahmetaj-Shala B, Peacock TP, Baillon L, et al., 2020. Resistance of endothelial cells to SARS-CoV-2 infection in vitro. bioRxiv, preprint. https://doi.org/10.1101/2020.11.08.372581
Aikawa T, Ogino J, Oyama-Manabe N, et al., 2022. Vasospastic angina: a cause of post-acute COVID-19 syndrome. Intern Med, 61(17):2693–2695. https://doi.org/10.2169/internalmedicine.0137-22
Al Yacoub R, Patel J, Solanky N, et al., 2022. Acute limb ischaemia due to vasospasm: a rare presentation. BMJ Case Rep, 15(1):e246495. https://doi.org/10.1136/bcr-2021-246495
Algahtani H, Subahi A, Shirah B, 2016. Neurological complications of middle east respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep Neurol Med, 2016:3502683. https://doi.org/10.1155/2016/3502683
Ali YM, Ferrari M, Lynch NJ, et al., 2021. Lectin pathway mediates complement activation by SARS-CoV-2 proteins. Front Immunol, 12:714511. https://doi.org/10.3389/fimmu.2021.714511
Alitter QT, Jarrar A, Alsoud F, et al., 2023. Unilateral limb ischemia in a COVID-19 patient: a case report. Cureus, 15(1):e34464. https://doi.org/10.7759/cureus.34464
Alonso MN, Mata-Forte T, García-León N, et al., 2020. Incidence, characteristics, laboratory findings and outcomes in acro-ischemia in COVID-19 patients. Vasc Health Risk Manag, 16:467–478. https://doi.org/10.2147/VHRM.S276530
Anderson CL, Chacko GW, Osborne JM, et al., 1995. The Fc receptor for immunoglobulin G (FcγRII) on human platelets. Semin Thromb Hemost, 21(1):1–9. https://doi.org/10.1055/s-2007-1000374
Althaus K, Marini I, Zlamal J, et al., 2021. Antibody-induced procoagulant platelets in severe COVID-19 infection. Blood, 137(8):1061–1071. https://doi.org/10.1182/blood.2020008762
Angeli F, Reboldi G, Verdecchia P, 2021a. Ageing, ACE2 deficiency and bad outcome in COVID-19. Clin Chem Lab Med, 59(10):1607–1609. https://doi.org/10.1515/cclm-2021-0658
Angeli F, Spanevello A, Reboldi G, et al., 2021b. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med, 88:1–8. https://doi.org/10.1016/j.ejim.2021.04.019
Antoniak S, Mackman N, 2014. Multiple roles of the coagulation protease cascade during virus infection. Blood, 123(17):2605–2613. https://doi.org/10.1182/blood-2013-09-526277
Arman M, Krauel K, Tilley DO, et al., 2014. Amplification of bacteria-induced platelet activation is triggered by FcγRIIA, integrin αIIbβ3, and platelet factor 4. Blood, 123(20):3166–3174. https://doi.org/10.1182/blood-2013-11-540526
Arning C, Schrattenholzer A, Lachenmayer L, 1998. Cervical carotid artery vasospasms causing cerebral ischemia: detection by immediate vascular ultrasonographic investigation. Stroke, 29(5):1063–1066. https://doi.org/10.1161/01.str.29.5.1063
Assinger A, Kral JB, Yaiw KC, et al., 2014. Human cytomegalovirus-platelet interaction triggers Toll-like receptor 2-dependent proinflammatory and proangiogenic responses. Arterioscler Thromb Vasc Biol, 34(4):801–809. https://doi.org/10.1161/ATVBAHA.114.303287
Baker AT, Boyd RJ, Sarkar D, et al., 2021. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci Adv, 7(49): eabl8213. https://doi.org/10.1126/sciadv.abl8213
Bautista-Vargas M, Bonilla-Abadía F, Cañas CA, 2020. Potential role for tissue factor in the pathogenesis of hyper-coagulability associated with in COVID-19. J Thromb Thrombolysis, 50(3):479–483. https://doi.org/10.1007/s11239-020-02172-x
Bellosta R, Luzzani L, Natalini G, et al., 2020. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg, 72(6):1864–1872. https://doi.org/10.1016/j.jvs.2020.04.483
Bellosta R, Piffaretti G, Bonardelli S, 2021. Regional survey in lombardy, northern italy, on vascular surgery intervention outcomes during the COVID-19 pandemic. Eur J Vasc Endovasc Surg, 61(4):688–697. https://doi.org/10.1016/j.ejvs.2021.01.037
Blanco-Rivero J, Cachofeiro V, Lahera V, et al., 2005. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension, 46(1):107–112. https://doi.org/10.1161/01.HYP.0000171479.36880.17
Bory M, Mattei M, Egre A, et al., 1979. Acute ischemic syndrome and apparently spontaneous spasms of the lower leg arteries. Coeur Med Interne, 18(4):607–611.
Bouck EG, Denorme F, Holle LA, et al., 2021. COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler Thromb Vasc Biol, 41(1):401–414. https://doi.org/10.1161/ATVBAHA.120.315338
Brill A, Fuchs TA, Savchenko AS, et al., 2012. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost, 10(1):136–144. https://doi.org/10.1111/j.1538-7836.2011.04544.x
Buie JNJ, Oates JC, 2014. Role of interferon alpha in endothelial dysfunction: insights into endothelial nitric oxide synthase-related mechanisms. Am J Med Sci, 348(2):168–175. https://doi.org/10.1097/MAJ.0000000000000284
Cappel, MA, Cappel, JA, Wetter, DA, 2021. Pernio (chilblains), SARS-CoV-2, and COVID toes unified through cutaneous and systemic mechanisms. Mayo Clin Proc, 96(4): 989–1005. https://doi.org/10.1016/j.mayocp.2021.01.009
Centers for Disease Control and Prevention, 2023. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fscience%2Fscience-briefs%2Funderlying-evidence-table.html [Accessed on Apr. 5, 2023].
Chang Y, Wei W, 2015. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin Exp Immunol, 179(2): 137–145. https://doi.org/10.1111/cei.12467
Chen T, Wu D, Chen HL, et al., 2020. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ, 368:m1091. https://doi.org/10.1136/bmj.m1091
Chen ZW, Tsai CH, Pan CT, et al., 2019. Endothelial dysfunction in primary aldosteronism. Int J Mol Sci, 20(20):5214. https://doi.org/10.3390/ijms20205214
Chou CH, Hung CS, Liao CW, et al., 2018. IL-6 trans-signalling contributes to aldosterone-induced cardiac fibrosis. Cardiovasc Res, 114(5):690–702. https://doi.org/10.1093/cvr/cvy013
Conway EM, Mackman N, Warren RQ, et al., 2022. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol, 22(10):639–649. https://doi.org/10.1038/s41577-022-00762-9
Dang EV, Barbi J, Yang HY, et al., 2011. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell, 146(5):772–784. https://doi.org/10.1016/j.cell.2011.07.033
de Masson A, Bouaziz JD, Sulimovic L, et al., 2020. Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from france. J Am Acad Dermatol, 83(2):667–670. https://doi.org/10.1016/j.jaad.2020.04.161
Demir Ş, Akin Ş, Tercan F, et al., 2010. Ergotamine-induced lower extremity arterial vasospasm presenting as acute limb ischemia. Diagn Interv Radiol, 16(2):165–167. https://doi.org/10.4261/1305-3825.DIR.1931-08.2
Deng Y, Liu W, Liu K, et al., 2020. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl), 133(11):1261–1267. https://doi.org/10.1097/CM9.0000000000000824
Deshotels MR, Xia HJ, Sriramula S, et al., 2014. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension, 64(6):1368–1375. https://doi.org/10.1161/HYPERTENSIONAHA.114.03743
Döring Y, Soehnlein O, Weber C, 2017. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res, 120(4):736–743. https://doi.org/10.1161/CIRCRESAHA.116.309692
Dotan A, Shoenfeld Y, 2021. Perspectives on vaccine induced thrombotic thrombocytopenia. J Autoimmun, 121:102663. https://doi.org/10.1016/j.jaut.2021.102663
Escher R, Breakey N, Lämmle B, 2020. Severe COVID-19 infection associated with endothelial activation. Thromb Res, 190:62. https://doi.org/10.1016/j.thromres.2020.04.014
Fan BE, 2020. Hematologic parameters in patients with COVID-19 infection: a reply. Am J Hematol, 95(8):E215. https://doi.org/10.1002/ajh.25847
Fiebeler A, Schmidt F, Müller DN, et al., 2001. Mineralocorticoid receptor affects AP-1 and nuclear factor-κB activation in angiotensin II-induced cardiac injury. Hypertension, 37(2):787–793. https://doi.org/10.1161/01.HYP.37.2.787
Foley JH, Conway EM, 2016. Cross talk pathways between coagulation and inflammation. Circ Res, 118(9):1392–1408. https://doi.org/10.1161/CIRCRESAHA.116.306853
Forrester SJ, Booz GW, Sigmund CD, et al., 2018. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev, 98(3):1627–1738. https://doi.org/10.1152/physrev.00038.2017
Galván Casas C, Català A, Carretero Hernández G, et al., 2020. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol, 183(1):71–77. https://doi.org/10.1111/bjd.19163
Gheblawi M, Wang KM, Viveiros A, et al., 2020. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res, 126(10):1456–1474. https://doi.org/10.1161/CIRCRESAHA.120.317015
Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al., 2020. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe, 27(6): 992–1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
Giannis D, Ziogas IA, Gianni P, 2020. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol, 127: 104362. https://doi.org/10.1016/j.jcv.2020.104362
Goeijenbier M, van Wissen M, van de Weg C, et al., 2012. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol, 84(10):1680–1696. https://doi.org/10.1002/jmv.23354
Goldman IA, Ye K, Scheinfeld MH, 2020. Lower-extremity arterial thrombosis associated with COVID-19 is characterized by greater thrombus burden and increased rate of amputation and death. Radiology, 297(2):E263–E269. https://doi.org/10.1148/radiol.2020202348
Gomez-Arbelaez D, Ibarra-Sanchez G, Garcia-Gutierrez A, et al., 2020. COVID-19-related aortic thrombosis: a report of four cases. Ann Vasc Surg, 67:10–13. https://doi.org/10.1016/j.avsg.2020.05.031
Gould TJ, Vu TT, Swystun LL, et al., 2014. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol, 34(9):1977–1984. https://doi.org/10.1161/ATVBAHA.114.304114
Herman EW, Kezis JS, Silvers DN, 1981. A distinctive variant of pernio: clinical and histopathologic study of nine cases. Arch Dermatol, 117(1):26–28. https://doi.org/10.1001/archderm.1981.01650010032019
Hermidorff MM, de Assis LVM, Isoldi MC, 2017. Genomic and rapid effects of aldosterone: what we know and do not know thus far. Heart Fail Rev, 22(1):65–89. https://doi.org/10.1007/s10741-016-9591-2
Holter JC, Pischke SE, de Boer E, et al., 2020. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci USA, 117(40):25018–25025. https://doi.org/10.1073/pnas.2010540117
Hubiche T, Le Duff F, Chiaverini C, et al., 2021. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis, 21(3):315–316. https://doi.org/10.1016/S1473-3099(20)30518-1
Iba T, Levy JH, 2022. The roles of platelets in COVID-19-associated coagulopathy and vaccine-induced immune thrombotic thrombocytopenia. Trends Cardiovasc Med, 32(1):1–9. https://doi.org/10.1016/j.tcm.2021.08.012
Iba T, Levy JH, Levi M, et al., 2020. Coagulopathy in COVID-19. J Thromb Haemost, 18(9):2103–2109. https://doi.org/10.1111/jth.14975
Inoue K, Makita N, Matsuo K, et al., 2009. OE-045 Clinical significance of aldosterone levels and C-reactive protein in patients with coronary vasospasm (OE08, atherosclerosis (clinical/diagnosis) (IHD), oral presentation (English), the 73rd Annual Scientific Meeting of the Japanese Circulation Society). Circ J, 73:185.
Jabalameli N, Rajabi F, Firooz A, et al., 2022. The overlap between genetic susceptibility to COVID-19 and skin diseases. Immunol Invest, 51(4):1087–1094. https://doi.org/10.1080/08820139.2021.1876086
Jahani M, Dokaneheifard S, Mansouri K, 2020. Hypoxia: a key feature of COVID-19 launching activation of HIF-1 and cytokine storm. J Inflamm, 17(1):33. https://doi.org/10.1186/s12950-020-00263-3
Jain A, Reddy A, Murugesan R, et al., 2022. Outcomes of patients with acute limb ischemia in patients with COVID-19: a systemic review and meta-analysis. Cureus, 14(7):e27370. https://doi.org/10.7759/cureus.27370
Kambas K, Chrysanthopoulou A, Vassilopoulos D, et al., 2014. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis, 73(10):1854–1863. https://doi.org/10.1136/annrheumdis-2013-203430
Kaneyama J, Kawarada O, Sakamoto S, et al., 2014. Vasospastic limb ischemia presenting acute and chronic limb ischemia. Ann Vasc Dis, 7(2):169–172. https://doi.org/10.3400/avd.cr.13-00113
Kang SJ, Kishimoto T, 2021. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp Mol Med, 53(7):1116–1123. https://doi.org/10.1038/s12276-021-00649-0
Kang SJ, Tanaka T, Inoue H, et al., 2020. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci USA, 117(36):22351–22356. https://doi.org/10.1073/pnas.2010229117
Kartikasari U, Djajalaksana S, Martini H, 2021. Acute limb ischemia in a patient with Covid-19 pneumonia: a case report. J Thromb Thrombolysis, 52(3):974–979. https://doi.org/10.1007/s11239-021-02434-2
Keser Z, Suarez-Cedeno G, Saha RK, et al., 2018. An atypical presentation of varicella zoster (VZV) vasculopathy. J Vasc Interv Neurol, 10(1):23–24.
Khattab K, Kempa AT, Atas R, et al., 2021. Peripheral ischemic limb necrosis (Acro-ischemia) associated with severe COVID-19 patients (COVID-19 limbs): a report of three cases. Lung India, 38(S1):S58–S60. https://doi.org/10.4103/lungindia.lungindia_470_20
Kim OY, Chae JS, Paik JK, et al., 2012. Effects of aging and menopause on serum interleukin-6 levels and peripheral blood mononuclear cell cytokine production in healthy nonobese women. AGE, 34(2):415–425. https://doi.org/10.1007/s11357-011-9244-2
Klinger MH, Wilhelm D, Bubel S, et al., 1995. Immunocytochemical localization of the chemokines RANTES and MIP-1α within human platelets and their release during storage. Int Arch Allergy Immunol, 107(4):541–546. https://doi.org/10.1159/000237097
Klok FA, Kruip MJHA, van der Meer NJM, et al., 2020. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res, 191:145–147. https://doi.org/10.1016/j.thromres.2020.04.013
Kolivras A, Dehavay F, Delplace D, et al., 2020. Coronavirus (COVID-19) infection-induced chilblains: a case report with histopathologic findings. JAAD Case Rep, 6(6):489–492. https://doi.org/10.1016/j.jdcr.2020.04.011
Kuchynkova S, Chochola M, Varejka P, et al., 2017. A rare cause of acute limb ischemia of both upper and lower limbs caused by prolonged vasospasm. Cor Vasa, 59(5):e503–e506. https://doi.org/10.1016/j.crvasa.2017.01.005
Kyriazopoulou E, Leventogiannis K, Norrby-Teglund A, et al., 2017. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med, 15:172. https://doi.org/10.1186/s12916-017-0930-5
Lari E, Lari A, Alqinai S, et al., 2020. Severe ischemic complications in Covid-19—a case series. Int J Surg Case Rep, 75:131–135. https://doi.org/10.1016/j.ijscr.2020.09.009
Lee N, Hui D, Wu AL, et al., 2003. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med, 348(20):1986–1994. https://doi.org/10.1056/NEJMoa030685
Li X, Wang LW, Yan SN, et al., 2020. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis, 94:128–132. https://doi.org/10.1016/j.ijid.2020.03.053
Liao MF, Liu Y, Yuan J, et al., 2020. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med, 26(6):842–844. https://doi.org/10.1038/s41591-020-0901-9
Limb J, Binning A, 2009. Thrombosis associated with varicella zoster in an adult. Int J Infect Dis, 13(6):e498–e500. https://doi.org/10.1016/j.ijid.2009.02.007
Liu JY, Liu Y, Xiang P, et al., 2020. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med, 18:206. https://doi.org/10.1186/s12967-020-02374-0
Liu YM, Wang HQ, Shao ZH, 2021. SARS-CoV-2 vaccines induced immune thrombotic thrombocytopenia. Chin J Hematol, 42(7):607–610 (in Chinese). https://doi.org/10.3760/cma.j.issn.0253-2727.2021.07.016
Mackman N, Grover SP, Antoniak S, 2021. Tissue factor expression, extracellular vesicles, and thrombosis after infection with the respiratory viruses influenza a virus and coronavirus. J Thromb Haemost, 19(11):2652–2658. https://doi.org/10.1111/jth.15509
Maggio M, Basaria S, Ble A, et al., 2006. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab, 91(1):345–347. https://doi.org/10.1210/jc.2005-1097
Manne BK, Denorme F, Middleton EA, et al., 2020. Platelet gene expression and function in patients with COVID-19. Blood, 136(11):1317–1329. https://doi.org/10.1182/blood.2020007214
Marceau F, Bawolak MT, Fortin JP, et al., 2018. Bifunctional ligands of the bradykinin B2 and B1 receptors: an exercise in peptide hormone plasticity. Peptides, 105:37–50. https://doi.org/10.1016/j.peptides.2018.05.007
Maseri A, L’Abbate A, Baroldi G, et al., 1978. Coronary vasospasm as a possible cause of myocardial infarction—a conclusion derived from the study of preinfarction angina. N Engl J Med, 299(23):1271–1277. https://doi.org/10.1056/NEJM197812072992303
Massberg S, Grahl L, von Bruehl ML, et al., 2010. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med, 16(8):887–896. https://doi.org/10.1038/nm.2184
Mast AE, Wolberg AS, Gailani D, et al., 2021. SARS-CoV-2 suppresses anticoagulant and fibrinolytic gene expression in the lung. eLife, 10:e64330. https://doi.org/10.7554/eLife.64330
Mazzotta F, Troccoli T, 2020. Acute acro-ischemia in the child at the time of COVID-19. Eur J Pediat Dermatol, 30(2):71–74. https://doi.org/10.26326/2281-9649.30.2.2102
Middleton EA, He XY, Denorme F, et al., 2020. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood, 136(10):1169–1179. https://doi.org/10.1182/blood.2020007008
Mohamedi N, Mirault T, Durivage A, et al., 2021. Ergotism with acute limb ischemia, provoked by HIV protease inhibitors interaction with ergotamine, rescued by multisite transluminal balloon angioplasty. J Med Vasc, 46(1): 13–21. https://doi.org/10.1016/j.jdmv.2020.12.002
Möhlendick B, Schönfelder K, Breuckmann K, et al., 2021. ACE2 polymorphism and susceptibility for SARS-CoV-2 infection and severity of COVID-19. Pharmacogenet Genomics, 31(8):165–171. https://doi.org/10.1097/FPC.0000000000000436
Monov D, Lilyanov N, Bogovsky S, et al., 2022. Cerebral vasospasm after subarachnoid hemorrhage from intracranial aneurysm rupture in SARS-CoV-2 positive patients. A retrospective review from two bulgarian hospitals. Biotechnol Biotechnol Equip, 36(1):413–417. https://doi.org/10.1080/13102818.2022.2092421
Nagata D, Takahashi M, Sawai K, et al., 2006. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension, 48(1):165–171. https://doi.org/10.1161/01.HYP.0000226054.53527.bb
Neumann FJ, Ott I, Marx N, et al., 1997. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler Thromb Vasc Biol, 17(12): 3399–3405. https://doi.org/10.1161/01.atv.17.12.3399
Ng KHL, Wu AKL, Cheng VCC, et al., 2005. Pulmonary artery thrombosis in a patient with severe acute respiratory syndrome. Postgrad Med J, 81(956):e3. https://doi.org/10.1136/pgmj.2004.030049
Nicholls JM, Poon LLM, Lee KC, et al., 2003. Lung pathology of fatal severe acute respiratory syndrome. Lancet, 361(9371):1773–1778. https://doi.org/10.1016/s0140-6736(03)13413-7
Nowroozpoor A, Bank MA, Jafari D, 2021. Limb ischemia due to extensive arterial thrombosis in the absence of venous occlusion as an unusual complication of critical illness from COVID-19. Case Rep Acute Med, 4(1):23–31. https://doi.org/10.1159/000514291
Omar IM, Weaver JS, Samet JD, et al., 2022. Musculoskeletal manifestations of COVID-19: currently described clinical symptoms and multimodality imaging findings. RadioGraphics, 42(5):1415–1432. https://doi.org/10.1148/rg.220036
Orrapin S, Reanpang T, Orrapin S, et al., 2015. Case series of HIV infection-associated arteriopathy: diagnosis, management, and outcome over a 5-year period at Maharaj Nakorn Chiang Mai Hospital, Chiang Mai University. Int J Low Extrem Wounds, 14(3):251–261. https://doi.org/10.1177/1534734615598226
Ozbey R, Algan MF, 2022. Acro-ischemic lesions in COVID-19 patients: a case series. J Cosmet Dermatol, 21(5):1822–1829. https://doi.org/10.1111/jocd.14893
Papamichalis P, Papadogoulas A, Katsiafylloudis P, et al., 2020. Combination of thrombolytic and immunosuppressive therapy for coronavirus disease 2019: a case report. Int J Infect Dis, 97:90–93. https://doi.org/10.1016/j.ijid.2020.05.118
Park SH, Kim TJ, Ko SB, et al., 2022. Transcranial doppler monitoring in subarachnoid hemorrhage. J Neurosonol Neuroimag, 14(1):1–9. https://doi.org/10.31728/jnn.2022.00115
Peerschke EI, Yin W, Ghebrehiwet B, 2010. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol, 47(13):2170–2175. https://doi.org/10.1016/j.molimm.2010.05.009
Perini P, Nabulsi B, Massoni CB, et al., 2020. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet, 395(10236):1546. https://doi.org/10.1016/S0140-6736(20)31051-5
Predenciuc A, Casian D, Culiuc V, 2023. Outcomes of surgical revascularization for acute limb ischemia in COVID-19 patients comparing to noninfected cohort: a single-center observational prospective study. Ann Vasc Surg, 91:81–89. https://doi.org/10.1016/j.avsg.2022.11.024
Putko RM, Bedrin MD, Clark DM, et al., 2021. SARS-CoV-2 and limb ischemia: a systematic review. J Clin Orthop Trauma, 12(1):194–199. https://doi.org/10.1016/j.jcot.2020.11.018
Ramos SG, da Cruz Rattis BA, Ottaviani G, et al., 2021. ACE2 down-regulation may act as a transient molecular disease causing RAAS dysregulation and tissue damage in the microcirculatory environment among COVID-19 patients. Am J Pathol, 191(7):1154–1164. https://doi.org/10.1016/j.ajpath.2021.04.010
Rieder M, Wirth L, Pollmeier L, et al., 2021. Serum ACE2, angiotensin II, and aldosterone levels are unchanged in patients with COVID-19. Am J Hypertens, 34(3):278–281. https://doi.org/10.1093/ajh/hpaa169
Rosas IO, Bräu N, Waters M, et al., 2021. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med, 384(16):1503–1516. https://doi.org/10.1056/NEJMoa2028700
Salama C, Han J, Yau L, et al., 2021. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med, 384(1):20–30. https://doi.org/10.1056/NEJMoa2030340
Schultz K, Wolf JM, 2020. Digital ischemia in COVID-19 patients: case report. J Hand Surg, 45(6):518–522. https://doi.org/10.1016/j.jhsa.2020.04.024
The Severe Covid-19 GWAS Group, 2020. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med, 383(16):1522–1534. https://doi.org/10.1056/NEJMoa2020283
Shahi V, Wetter DA, Cappel JA, et al., 2015. Vasospasm is a consistent finding in pernio (chilblains) and a possible clue to pathogenesis. Dermatology, 231(3):274–279. https://doi.org/10.1159/000437224
Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, et al., 2018. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol, 314(1):L17–L31. https://doi.org/10.1152/ajplung.00498.2016
Souyris M, Cenac C, Azar P, et al., 2018. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol, 3(19):eaap8855. https://doi.org/10.1126/sciimmunol.aap8855
Stelzer M, Henes J, Saur S, 2021. The role of antiphospholipid antibodies in COVID-19. Curr Rheumatol Rep, 23(9):72. https://doi.org/10.1007/s11926-021-01041-7
Tanaka K, Koike Y, Shimura T, et al., 2014. In vivo characterization of neutrophil extracellular traps in various organs of a murine sepsis model. PLoS ONE, 9(11):e111888. https://doi.org/10.1371/journal.pone.0111888
Tang N, Li DJ, Wang X, et al., 2020. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost, 18(4):844–847. https://doi.org/10.1111/jth.14768
Teixeira JG, Pitta GBB, da Silva CRA, et al., 2022. Diagnosis and management of patients with acute limb ischemia after Covid-19 infection: a case series. J Vasc Bras, 21: e20220044. https://doi.org/10.1590/1677-5449.202200441
Tiede A, Sachs UJ, Czwalinna A, et al., 2021. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood, 138(4):350–353. https://doi.org/10.1182/blood.2021011958
Tobaiqy M, Elkout H, MacLure K, 2021. Analysis of thrombotic adverse reactions of COVID-19 astrazeneca vaccine reported to eudravigilance database. Vaccines, 9(4):393. https://doi.org/10.3390/vaccines9040393
Tukiainen T, Villani AC, Yen A, et al., 2017. Landscape of X chromosome inactivation across human tissues. Nature, 550(7675):244–248. https://doi.org/10.1038/nature24265
van de Veerdonk F, Netea MG, van Deuren M, et al., 2020. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach. Preprints.org, preprint. https://doi.org/10.20944/preprints202004.0023.v1
Vickers C, Hales P, Kaushik V, et al., 2002. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem, 277(17):14838–14843. https://doi.org/10.1074/jbc.M200581200
Villard O, Morquin D, Molinari N, et al., 2020. The plasmatic aldosterone and C-reactive protein levels, and the severity of Covid-19: the Dyhor-19 study. J Clin Med, 9(7):2315. https://doi.org/10.3390/jcm9072315
Walls AC, Park YJ, Tortorici MA, et al., 2020. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell, 181(2):281–292.e6. https://doi.org/10.1016/j.cell.2020.02.058
Wang DW, Hu B, Hu C, et al., 2020. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA, 323(11): 1061–1069. https://doi.org/10.1001/jama.2020.1585
Wang RY, Lee JH, Kim J, et al., 2023. SARS-CoV-2 restructures host chromatin architecture. Nat Microbiol, 8(4):679–694. https://doi.org/10.1038/s41564-023-01344-8
Winckiewicz M, Stanišić MG, Szajkowski R, et al., 2007. Acute lower limb ischemia in a young woman with arterial hypoplasia: a case report. Angiology, 58(4):494–497. https://doi.org/10.1177/0003319706291154
Wu ZY, Hu R, Zhang CZ, et al., 2020. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit Care, 24:290. https://doi.org/10.1186/s13054-020-03015-0
Xavier FE, Aras-López R, Arroyo-Villa I, et al., 2008. Aldosterone induces endothelial dysfunction in resistance arteries from normotensive and hypertensive rats by increasing thromboxane A2 and prostacyclin. Br J Pharmacol, 154(6):1225–1235. https://doi.org/10.1038/bjp.2008.200
Xie BW, Semaan DB, Binko MA, et al., 2023. COVID-associated acute limb ischemia during the delta surge and the effect of vaccines. J Vasc Surg, 77(4):1165–1173.e1. https://doi.org/10.1016/j.jvs.2022.12.002
Xie XD, Chen JZ, Wang XX, et al., 2006. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci, 78(19):2166–2171. https://doi.org/10.1016/j.lfs.2005.09.038
Xing DQ, Nozell S, Chen YF, et al., 2009. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol, 29(3):289–295. https://doi.org/10.1161/ATVBAHA.108.182279
Yeaman MR, 2014. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol, 12(6):426–437. https://doi.org/10.1038/nrmicro3269
Yoon HE, Kim EN, Kim MY, et al., 2016. Age-associated changes in the vascular renin-angiotensin system in mice. Oxid Med Cell Longev, 2016:6731093. https://doi.org/10.1155/2016/6731093
Yu J, Yuan X, Chen H, et al., 2020. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor d inhibition. Blood, 136(18): 2080–2089. https://doi.org/10.1182/blood.2020008248
Zhang HB, Penninger JM, Li YM, et al., 2020. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med, 46(4):586–590. https://doi.org/10.1007/s00134-020-05985-9
Zhang S, Liu YY, Wang X F, et al., 2020. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol, 13:120. https://doi.org/10.1186/s13045-020-00954-7
Zhang X, Yu J, Pan LY, et al., 2020. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol Res, 158:104927. https://doi.org/10.1016/j.phrs.2020.104927
Zhang Y, Cao W, Xiao M, et al., 2020. Clinical and coagulation characteristics in 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Chin J Hematol, 41(4): 302–307 (in Chinese). https://doi.org/10.3760/cma.j.issn.0253-2727.2020.008
Zhao Y, Zhao ZX, Wang YJ, et al., 2020. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med, 202(5):756–759. https://doi.org/10.1164/rccm.202001-0179LE
Zhou F, Yu T, Du RH, et al., 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet, 395(10229):1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3
Zhu HB, Wei L, Niu P, 2020. The novel coronavirus outbreak in Wuhan, China. Glob Health Res Policy, 5:6. https://doi.org/10.1186/s41256-020-00135-6
Zhu LL, Liu L, Zhang Y, et al., 2018. High level of neutrophil extracellular traps correlates with poor prognosis of severe influenza A infection. J Infect Dis, 217(3):428–437. https://doi.org/10.1093/infdis/jix475
Zimmermann N, Wolf C, Schwenke R, et al., 2019. Assessment of clinical response to Janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol, 155(3):342–346. https://doi.org/10.1001/jamadermatol.2018.5077
Zuo Y, Yalavarthi S, Shi H, et al., 2020. Neutrophil extracellular traps in COVID-19. JCI Insight, 5(11):e138999. https://doi.org/10.1172/jci.insight.138999
Acknowledgments
This work was supported by the Zhejiang Provincial Medical Scientific Research Program (No. 2022RC136), China.
Author information
Authors and Affiliations
Contributions
Hui LU, Olga ALENIKOVA, Sahar Ahmed ABDALBARY and Zhenfeng LIU designed the study. Chengjun YAO, Yanzhao DONG, Haiying ZHOU, Xiaodi ZOU, Ahmad ALHASKAWI, Sohaib Hasan Abdullah EZZI, Zewei WANG, Jingtian LAI, Vishnu Goutham KOTA, and Mohamed Hasan Abdulla Hasan ABDULLA performed the literature collection and analyzed the results. Chengjun YAO, Yanzhao DONG, Haiying ZHOU, Xiaodi ZOU, and Ahmad ALHASKAWI drafted the manuscript. Chengjun YAO, Sahar Ahmed ABDALBARY, Zewei WANG, Jingtian LAI, Vishnu Goutham KOTA, and Mohamed Hasan Abdulla Hasan ABDULLA critically revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Chengjun YAO, Yanzhao DONG, Haiying ZHOU, Xiaodi ZOU, Ahmad ALHASKAWI, Sohaib Hasan Abdullah EZZI, Zewei WANG, Jingtian LAI, Vishnu Goutham KOTA, Mohamed Hasan Abdulla Hasan ABDULLA, Zhenfeng LIU, Sahar Ahmed ABDALBARY, Olga ALENIKOVA, and Hui LU declare that they have no conflicts of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (The First Affiliated Hospital of Zhejiang University School of Medicine, China) and with the Helsinki Declaration of 1975, as revised in 2013. Informed consent was obtained from all patients included in the study. Additional informed consent was obtained from all patients for whom identifying information is included in this article.
Rights and permissions
About this article
Cite this article
Yao, C., Dong, Y., Zhou, H. et al. COVID-19 and acute limb ischemia: latest hypotheses of pathophysiology and molecular mechanisms. J. Zhejiang Univ. Sci. B 26, 333–352 (2025). https://doi.org/10.1631/jzus.B2300512
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B2300512
Key words
- Acute limb ischemia (ALI)
- Coronavirus disease 2019 (COVID-19) infection complication
- Hypercoagulability
- Thrombosis
- Vasospasm
- Hypoxia-inducible factor 1α (HIF-1α)
- Angiotensin-converting enzyme 2 (ACE2)
- Type I interferon (IFN-I)
- Tocilizumab