Therapeutic Effects of Cannabinoids and Their Applications in COVID-19 Treatment
Abstract
:1. Introduction
2. Endocannabinoids
3. Cannabinoid Receptors
4. Phytocannabinoids
5. Clinical Evidence for Therapeutic Efficacies of Cannabinoids
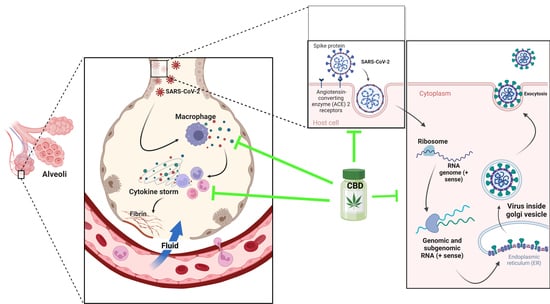
6. Cannabis and COVID-19: In Vitro Studies
7. Indispensable Proteins for the Development of Infection and CBD
8. Cytokine Storm and the Anti-Inflammatory Activity of CBD
9. Cannabis and COVID-19: In Vivo Studies
10. Cannabidiol and the Psychological Effects of COVID
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Plancarte-Sánchez, R.; Mansilla-Olivares, A.; De los Reyes-Pacheco, V.A.; Meneses-González, F. Aplicaciones terapéuticas por acción de los cannabinoides. Gac. Médica México 2019, 155, 307–318. [Google Scholar]
- Covarrubias-Torres, N. Uso medicinal de la Marihuana. Anest. En México 2019, 31, 49–58. [Google Scholar]
- Álvarez, L.G.; Gomar, J.J.; García-Portilla, M.P.; Bobes, J. Consumo de cannabis y alteraciones cognitivas en esquizofrenia y primeros episodios psicóticos. Adicciones 2019, 31, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.M.; Shalev, N.; Vinayaka, A.C.; Nadarajan, S.; Namdar, D.; Belausov, E.; Shoval, I.; Mani, K.A.; Mechrez, G.; Koltai, H. Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages. Sci. Rep. 2021, 11, 1462. [Google Scholar] [CrossRef]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef]
- El Biali, M.; Broers, B.; Besson, M.; Demeules, J. Cannabinoids and COVID-19. Med. Cannabis Cannabinoids 2020, 3, 111–115. [Google Scholar] [CrossRef]
- Esposito, G.; Pesce, M.; Seguella, L.; Sanseverino, W.; Lu, J.; Corpetti, C.; Sarnelli, G. The potential of cannabidiol in the COVID-19 pandemic. Br. J. Pharm. 2020, 177, 4967–4970. [Google Scholar] [CrossRef]
- Liu, D.X.; Liang, J.Q.; Fung, T.S. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae). Encycl. Virol. 2021, 2, 428–440. [Google Scholar] [CrossRef]
- Lucaciu, O.; Aghiorghiesei, O.; Petrescu, N.B.; Mirica, I.C.; Benea, H.R.C.; Apostu, D. In quest of a new therapeutic approach in COVID-19: The endocannabinoid system. Drug Metab. Rev. 2021, 53, 478–490. [Google Scholar] [CrossRef]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [Green Version]
- Galzerano Guida, J.; Orellana Navone, C.; Pérez, R.; Coitiño González, A.; Velázquez Ramos, P. Medicinal Cannabis as a therapeutic resource: Preliminary study. Rev. Med. Del Urug. 2019, 35, 113–137, 289–297. [Google Scholar]
- Kirkham, T. Endocannabinoids in the regulation of appetite and body weight. Behav. Pharmacol. 2005, 16, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.; Berrendero, F. Endogenous cannabinoid and opioid systems and their role in nicotine addiction. Curr. Drug Targets 2010, 11, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Parsons, L.H.; Hurd, Y.L. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015, 16, 579–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; De Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3216–3228. [Google Scholar] [CrossRef] [Green Version]
- Karhson, D.S.; Krasinska, K.M.; Dallaire, J.A.; Libove, R.A.; Phillips, J.M.; Chien, A.S.; Garner, J.P.; Hardan, A.Y.; Parker, K.J. Plasma anandamide concentrations are lower in children with autism spectrum disorder. Mol. Autism 2018, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mayo, L.M.; Asratian, A.; Lindé, J.; Holm, L.; Nätt, D.; Augier, G.; Stensson, N.; Vecchiarelli, H.A.; Balsevich, G.; Aukema, R.J. Protective effects of elevated anandamide on stress and fear-related behaviors: Translational evidence from humans and mice. Mol. Psychiatry 2020, 25, 993–1005. [Google Scholar] [CrossRef]
- Mayo, L.M.; Asratian, A.; Lindé, J.; Morena, M.; Haataja, R.; Hammar, V.; Augier, G.; Hill, M.N.; Heilig, M. Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: A randomized, controlled experimental medicine trial. Biol. Psychiatry 2020, 87, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Spohrs, J.; Ulrich, M.; Grön, G.; Prost, M.; Plener, P.L.; Fegert, J.M.; Bindila, L.; Abler, B. Fear extinction learning and anandamide: An fMRI study in healthy humans. Transl. Psychiatry 2021, 11, 161. [Google Scholar] [CrossRef]
- Correia-Sá, I.B.; Carvalho, C.M.; Serrão, P.V.; Loureiro, A.I.; Fernandes-Lopes, C.; Marques, M.; Vieira-Coelho, M.A. A new role for anandamide: Defective link between the systemic and skin endocannabinoid systems in hypertrophic human wound healing. Sci. Rep. 2020, 10, 11134. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Ermund, A.; Movahed, P.; Andersson, D.A.; Simonsen, C.; Jönsson, B.A.; Blomgren, A.; Birnir, B.; Bevan, S.; Eschalier, A.; et al. Monoacylglycerols activate TRPV1-a link between phospholipase C and TRPV1. PLoS ONE 2013, 8, e81618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, C.G. Editor From Cannabis to the Endogenous Cannabinoid System: New Perspectives in Neuroinflammation. Annals of the Royal Academy of Doctors of Spain. 2018, 3, 379–393. Available online: https://wwwpublicacionesradees/indexphp/arade/article/view/93/72 (accessed on 10 September 2022).
- Turcotte, C.; Archambault, A.S.; Dumais, É.; Martin, C.; Blanchet, M.R.; Bissonnette, E.; Ohashi, N.; Yamamoto, K.; Itoh, T.; Laviolette, M. Endocannabinoid hydrolysis inhibition unmasks that unsaturated fatty acids induce a robust biosynthesis of 2-arachidonoyl-glycerol and its congeners in human myeloid leukocytes. FASEB J. 2020, 34, 4253–4265. [Google Scholar] [CrossRef] [PubMed]
- Prospéro García, O.E.; Ruiz Contreras, A.E.; Cortés Morelos, J.; Herrera Solís, A.; Méndez Díaz, M. Marihuana: Legalization and Medical Care. Rev. De La Fac. De Med. 2019, 62, 6–23. [Google Scholar] [CrossRef]
- Raïch, I.; Rivas-Santisteban, R.; Lillo, A.; Lillo, J.; Reyes-Resina, I.; Nadal, X.; Ferreiro-Vera, C.; de Medina, V.S.; Majellaro, M.; Sotelo, E.; et al. Similarities and differences upon binding of naturally occurring Δ(9)-tetrahydrocannabinol-derivatives to cannabinoid CB(1) and CB(2) receptors. Pharmacol. Res. 2021, 174, 105970. [Google Scholar] [CrossRef]
- Olianas, M.C.; Dedoni, S.; Onali, P. Cannabinoid CB(1) and CB(2) receptors differentially regulate TNF-α-induced apoptosis and LPA(1)-mediated pro-survival signaling in HT22 hippocampal cells. Life Sci. 2021, 276, 119407. [Google Scholar] [CrossRef]
- Köfalvi, A.; Moreno, E.; Cordomí, A.; Cai, N.S.; Fernández-Dueñas, V.; Ferreira, S.G.; Guixà-González, R.; Sánchez-Soto, M.; Yano, H.; Casadó-Anguera, V.; et al. Control of glutamate release by complexes of adenosine and cannabinoid receptors. BMC Biol. 2020, 18, 9. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. The pharmacology of cannabinoid receptors and their ligands: An overview. Int. J. Obes. 2006, 30, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.; Fatehi, K.S.; Menon, N.N.; Chaturvedi, P. Review of Medicinal Use of Cannabis Derivatives and the Societal Impact of Legalization. Indian J. Palliat. Care 2020, 26, 369–380. [Google Scholar] [CrossRef]
- Ceni, C.; Benko, M.J.; Mohamed, K.A.; Poli, G.; Di Stefano, M.; Tuccinardi, T.; Digiacomo, M.; Valoti, M.; Laprairie, R.B.; Macchia, M.; et al. Novel Potent and Selective Agonists of the GPR55 Receptor Based on the 3-Benzylquinolin-2(1H)-One Scaffold. Pharmaceuticals 2022, 15, 768. [Google Scholar] [CrossRef]
- Johns, D.; Behm, D.; Walker, D.; Ao, Z.; Shapland, E.; Daniels, D.; Riddick, M.; Dowell, S.; Staton, P.; Green, P. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br. J. Pharmacol. 2007, 152, 825–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 16511–16516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henstridge, C.M.; Balenga, N.A.; Ford, L.A.; Ross, R.A.; Waldhoer, M.; Irving, A.J. The GPR55 ligand L-α-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009, 23, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, C.M.; Balenga, N.A.; Schröder, R.; Kargl, J.K.; Platzer, W.; Martini, L.; Arthur, S.; Penman, J.; Whistler, J.L.; Kostenis, E. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br. J. Pharmacol. 2010, 160, 604–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.-Y.; Lu, H.-C.; Hille, B.; Mackie, K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A. Novel cannabinoid receptors. Br. J. Pharmacol. 2007, 152, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.S.; Golub, V.M. The pharmacological basis of cannabis therapy for epilepsy. J. Pharmacol. Exp. Ther. 2016, 357, 45–55. [Google Scholar] [CrossRef]
- Maccarrone, M.; Lorenzon, T.; Bari, M.; Melino, G.; Finazzi-Agro, A. Anandamide induces apoptosis in human cells via vanilloid receptors: Evidence for a protective role of cannabinoid receptors. J. Biol. Chem. 2000, 275, 31938–31945. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.D. Potential adverse drug events with tetrahydrocannabinol (THC) due to drug–drug interactions. J. Clin. Med. 2020, 9, 919. [Google Scholar] [CrossRef] [Green Version]
- Maroon, J.; Bost, J. Review of the neurological benefits of phytocannabinoids. Surg. Neurol. Int. 2018, 9, 91. [Google Scholar] [CrossRef]
- Mead, A. The legal status of cannabis (marijuana) and cannabidiol (CBD) under US law. Epilepsy Behav. 2017, 70, 288–291. [Google Scholar] [CrossRef] [Green Version]
- Suero-García, C.; Martín-Banderas, L.; Holgado, M. Efecto neuroprotector de los cannabinoides en las enfermedades neurodegenerativas. Ars Pharm. 2015, 56, 77–87. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food & Drug Administration Fel. Marinol® Data Sheet or Summary of Product Characteristics 06/12/2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf (accessed on 10 September 2022).
- Gloss, D. An overview of products and bias in research. Neurotherapeutics 2015, 12, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F.; Müller-Vahl, K. Medicinal uses of marijuana and cannabinoids. Crit. Rev. Plant Sci. 2016, 35, 378–405. [Google Scholar] [CrossRef]
- Gover, J. Sativex 2.7 mg/2.5 mg Oral Spray Solution. GW Pharma Ltd. 2018. Available online: https://cima.aemps.es/cima/dochtml/ft/72544/FT_72544.html (accessed on 25 July 2021).
- Abu-Sawwa, R.; Scutt, B.; Park, Y. Emerging use of epidiolex (cannabidiol) in epilepsy. J. Pediatr. Pharmacol. Ther. 2020, 25, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Custodio, N. Peruvian patients use cannabidiol for refractory epilepsy: Apropos of the evidence-based indication of marijuana-derived products. J. Neuro-Psychiatry 2019, 82, 101–103. [Google Scholar]
- Antonio, L.; De Los Milagros, P. Marihuana Medicinal en el Tratamiento de la Epilepsia. Rev. Médica Hosp. Hipólito Unanue Tacna 2018, 11, 38–42. [Google Scholar]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.H.; Devinsky, O.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Laux, L.; Wright, S. Cannabidiol (CBD) Reduces Convulsive Seizure Frequency in Dravet Syndrome: Results of a Multi-Center, Randomized, Controlled Trial (GWPCARE1)(CT. 001); AAN Enterprises: Barcelona, España, 2017. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef]
- Rajan, T.S.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 2016, 112, 104–115. [Google Scholar] [CrossRef]
- Rizzo, M.D.; Crawford, R.B.; Henriquez, J.E.; Aldhamen, Y.A.; Gulick, P.; Amalfitano, A.; Kaminski, N.E. HIV-infected cannabis users have lower circulating CD16+ monocytes and IFN-γ-inducible protein 10 levels compared with nonusing HIV patients. Aids 2018, 32, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vázquez, O.; Rojas, A.T.; Fleury, A. Neuroinflammation and epilepsy. TIP J. Spec. Chem. Biol. Sci. 2016, 19, 24–31. [Google Scholar]
- Fernández-Ruiz, J.; García, C.; Sagredo, O.; Gómez-Ruiz, M.; de Lago, E. The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin. Ther. Targets 2010, 14, 387–404. [Google Scholar] [CrossRef]
- Kaplan, J.S.; Stella, N.; Catterall, W.A.; Westenbroek, R.E. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 11229–11234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Thiele, E.A.; Wong, M.H.; Appleton, R.; Harden, C.L.; Greenwood, S.; Morrison, G.; Sommerville, K.; Group, G.P.A.S. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 2018, 90, e1204–e1211. [Google Scholar] [CrossRef] [Green Version]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef] [Green Version]
- Szaflarski, J.P.; Bebin, E.M.; Cutter, G.; DeWolfe, J.; Dure, L.S.; Gaston, T.E.; Kankirawatana, P.; Liu, Y.; Singh, R.; Standaert, D.G. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open-label add-on prospective study. Epilepsy Behav. 2018, 87, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Karaźniewicz-Łada, M.; Główka, A.K.; Mikulska, A.A.; Główka, F.K. Pharmacokinetic Drug-Drug Interactions among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients. Int. J. Mol. Sci. 2021, 22, 9582. [Google Scholar] [CrossRef]
- OMS. Panel de Control de la Enfermedad por Coronavirus (COVID-19) [En Línea]. 2022. Available online: https://covid19.who.int/ (accessed on 18 May 2022).
- Costiniuk, C.T.; Jenabian, M.A. Acute inflammation and pathogenesis of SARS-CoV-2 infection: Cannabidiol as a potential anti-inflammatory treatment? Cytokine Growth Factor Rev. 2020, 53, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.D.; Henriquez, J.E.; Blevins, L.K.; Bach, A.; Crawford, R.B.; Kaminski, N.E. Targeting Cannabinoid Receptor 2 on Peripheral Leukocytes to Attenuate Inflammatory Mechanisms Implicated in HIV-Associated Neurocognitive Disorder. J. Neuroimmune Pharmacol. 2020, 15, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Tortora, C.; Argenziano, M.; Di Paola, A.; Punzo, F. Cannabinoid Receptor Type 2: A Possible Target in SARS-CoV-2 (CoV-19) Infection? Int. J. Mol. Sci. 2020, 21, 3809. [Google Scholar] [CrossRef]
- Salles, É.L.; Khodadadi, H.; Jarrahi, A.; Ahluwalia, M.; Paffaro, V.A., Jr.; Costigliola, V.; Yu, J.C.; Hess, D.C.; Dhandapani, K.M.; Baban, B. Cannabidiol (CBD) modulation of apelin in acute respiratory distress syndrome. J. Cell. Mol. Med. 2020, 24, 12869–12872. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Manuzak, J.A.; Gott, T.M.; Kirkwood, J.S.; Coronado, E.; Hensley-McBain, T.; Miller, C.; Cheu, R.K.; Collier, A.C.; Funderburg, N.T.; Martin, J.N.; et al. Heavy Cannabis Use Associated With Reduction in Activated and Inflammatory Immune Cell Frequencies in Antiretroviral Therapy-Treated Human Immunodeficiency Virus-Infected Individuals. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 66, 1872–1882. [Google Scholar] [CrossRef] [Green Version]
- Park, M.D. Macrophages: A Trojan horse in COVID-19? Nat. Rev. Immunol. 2020, 20, 351. [Google Scholar] [CrossRef]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Hasenberg, M.; Stegemann-Koniszewski, S.; Gunzer, M. Cellular immune reactions in the lung. Immunol. Rev. 2013, 251, 189–214. [Google Scholar] [CrossRef] [PubMed]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Vardhana, S.A.; Wolchok, J.D. The many faces of the anti-COVID immune response. J. Exp. Med. 2020, 217, e20200678. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, Y.; Cao, L.; Wang, D.; Guo, M.; Jiang, A.; Guo, D.; Hu, W.; Yang, J.; Tang, Z.; et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 2020, 27, 883–890.e882. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [Green Version]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e1003. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Daniloski, Z.; Jordan, T.X.; Wessels, H.H.; Hoagland, D.A.; Kasela, S.; Legut, M.; Maniatis, S.; Mimitou, E.P.; Lu, L.; Geller, E.; et al. Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells. Cell 2021, 184, 92–105.e116. [Google Scholar] [CrossRef]
- Schneider, W.M.; Luna, J.M.; Hoffmann, H.H.; Sánchez-Rivera, F.J.; Leal, A.A.; Ashbrook, A.W.; Le Pen, J.; Ricardo-Lax, I.; Michailidis, E.; Peace, A.; et al. Genome-Scale Identification of SARS-CoV-2 and Pan-coronavirus Host Factor Networks. Cell 2021, 184, 120–132.e114. [Google Scholar] [CrossRef]
- Wang, R.; Simoneau, C.R.; Kulsuptrakul, J.; Bouhaddou, M.; Travisano, K.A.; Hayashi, J.M.; Carlson-Stevermer, J.; Zengel, J.R.; Richards, C.M.; Fozouni, P.; et al. Genetic Screens Identify Host Factors for SARS-CoV-2 and Common Cold Coronaviruses. Cell 2021, 184, 106–119.e114. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Tan, K.S.; Wang, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020, 41, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, A.; Tavakoli-Yaraki, M.; Salimi, V. Opioids/cannabinoids as a potential therapeutic approach in COVID-19 patients. Expert Rev. Respir. Med. 2020, 14, 965–967. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Matsabisa, M.G.; Muhammad, A.; Abarshi, M.M.; Amaku, J.F.; Katsayal, S.B.; Nde, A.L. Targeting of Protein's Messenger RNA for Viral Replication, Assembly and Release in SARS-CoV-2 Using Whole Genomic Data From South Africa: Therapeutic Potentials of Cannabis Sativa L. Front. Pharmacol. 2021, 12, 736511. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V. Purines, Pyrimidines and Nucleotides and the Chemistry of Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Attia, Y.A.; Alagawany, M.M.; Farag, M.R.; Alkhatib, F.M.; Khafaga, A.F.; Abdel-Moneim, A.E.; Asiry, K.A.; Mesalam, N.M.; Shafi, M.E.; Al-Harthi, M.A.; et al. Phytogenic Products and Phytochemicals as a Candidate Strategy to Improve Tolerance to Coronavirus. Front. Vet. Sci. 2020, 7, 573159. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.K.; Kaur, R.; Bhardwaj, A.; Bhardwaj, V.; Ohri, P.; Sharma, A.; Ahmad, A.; Bhardwaj, R.; Ahmad, P. Herbal immune-boosters: Substantial warriors of pandemic COVID-19 battle. Phytomed. Int. J. Phytother. Phytopharm. 2021, 85, 153361. [Google Scholar] [CrossRef]
- Asai, A.; Konno, M.; Ozaki, M.; Otsuka, C.; Vecchione, A.; Arai, T.; Kitagawa, T.; Ofusa, K.; Yabumoto, M.; Hirotsu, T.; et al. COVID-19 Drug Discovery Using Intensive Approaches. Int. J. Mol. Sci. 2020, 21, 2839. [Google Scholar] [CrossRef] [Green Version]
- Peng, M. Outbreak of COVID-19: An emerging global pandemic threat. Biomed. Pharmacother. = Biomed. Pharmacother. 2020, 129, 110499. [Google Scholar] [CrossRef]
- Tu, Y.F.; Chien, C.S.; Yarmishyn, A.A.; Lin, Y.Y.; Luo, Y.H.; Lin, Y.T.; Lai, W.Y.; Yang, D.M.; Chou, S.J.; Yang, Y.P.; et al. A Review of SARS-CoV-2 and the Ongoing Clinical Trials. Int. J. Mol. Sci. 2020, 21, 2657. [Google Scholar] [CrossRef] [Green Version]
- Weisberg, E.; Sattler, M.; Yang, P.L.; Parent, A.; Gray, N.; Griffin, J.D. Current therapies under investigation for COVID-19: Potential COVID-19 treatments. Can. J. Physiol. Pharmacol. 2020, 98, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.C.; Yang, D.; Nicolaescu, V.; Best, T.J.; Ohtsuki, T.; Chen, S.N.; Friesen, J.B.; Drayman, N.; Mohamed, A.; Dann, C.; et al. Cannabidiol Inhibits SARS-CoV-2 Replication and Promotes the Host Innate Immune Response. bioRxiv 2021. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e1039. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Kochs, G.; Weber, F. The interferon response circuit: Induction and suppression by pathogenic viruses. Virology 2006, 344, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration. Application Number 210365Orig1s000. In Clinical Pharmacology and Biopharmaceutical Reviews; FDA: Silver Spring, MD, USA, 2017. [Google Scholar]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e899. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Muchiri, R.N.; Bates, T.A.; Weinstein, J.B.; Leier, H.C.; Farley, S.; Tafesse, F.G. Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants. J. Nat. Prod. 2022, 85, 176–184. [Google Scholar] [CrossRef]
- Raj, V.; Park, J.G.; Cho, K.H.; Choi, P.; Kim, T.; Ham, J.; Lee, J. Assessment of antiviral potencies of cannabinoids against SARS-CoV-2 using computational and in vitro approaches. Int. J. Biol. Macromol. 2021, 168, 474–485. [Google Scholar] [CrossRef]
- Muus, C.; Luecken, M.D.; Eraslan, G.; Waghray, A.; Heimberg, G.; Sikkema, L.; Kobayashi, Y.; Vaishnav, E.D.; Subramanian, A.; Smilie, C.; et al. Integrated single-cell atlas analyses reveal associations of age, gender, and smoking with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlight inflammatory programs in putative target cells. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Malinowska, B.; Baranowska-Kuczko, M.; Kicman, A.; Schlicker, E. Opportunities, Challenges and Pitfalls of Using Cannabidiol as an Adjuvant Drug in COVID-19. Int. J. Mol. Sci. 2021, 22, 1986. [Google Scholar] [CrossRef]
- Wang, B.; Kovalchuk, A.; Li, D.; Rodriguez-Juarez, R.; Ilnytskyy, Y.; Kovalchuk, I.; Kovalchuk, O. In search of preventive strategies: Novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging 2020, 12, 22425–22444. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.; Fiselier, A.; Kovalchuk, I.; Kovalchuk, O. New AKT-dependent mechanisms of anti-COVID-19 action of high-CBD Cannabis sativa extracts. Cell Death Discov. 2022, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, A.; Vartak, R.; Patki, M.; Patel, K. Cannabidiol Inhibits In Vitro Human Liver Microsomal Metabolism of Remdesivir: A Promising Adjuvant for COVID-19 Treatment. Cannabis Cannabinoid Res. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, T.; Montes, C.; Harui, A.; Beedanagari, S.R.; Kiertscher, S.; Stripecke, R.; Hossepian, D.; Kitchen, C.; Kern, R.; Belperio, J.; et al. Clarifying CB2 receptor-dependent and independent effects of THC on human lung epithelial cells. Toxicol. Appl. Pharmacol. 2008, 231, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janecki, M.; Graczyk, M.; Lewandowska, A.A.; Pawlak, Ł. Anti-Inflammatory and Antiviral Effects of Cannabinoids in Inhibiting and Preventing SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 4170. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef]
- Gertsch, J. Editorial: Lung macrophages high on cannabinoids: Jamming PAMs and taming TAMs? J. Leukoc. Biol. 2016, 99, 518–520. [Google Scholar] [CrossRef] [Green Version]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanus, L.O. Cannabidiol–recent advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Wang, B.; Li, D.; Rodriguez-Juarez, R.; Ilnytskyy, S.; Kovalchuk, I.; Kovalchuk, O. Fighting the storm: Could novel anti-TNFα and anti-IL-6 C. sativa cultivars tame cytokine storm in COVID-19? Aging 2021, 13, 1571–1590. [Google Scholar] [CrossRef]
- Vuolo, F.; Abreu, S.C.; Michels, M.; Xisto, D.G.; Blanco, N.G.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Reis, C.; Bahl, M.; et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur. J. Pharmacol. 2019, 843, 251–259. [Google Scholar] [CrossRef]
- Vuolo, F.; Petronilho, F.; Sonai, B.; Ritter, C.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Dal-Pizzol, F. Evaluation of Serum Cytokines Levels and the Role of Cannabidiol Treatment in Animal Model of Asthma. Mediat. Inflamm. 2015, 2015, 538670. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, B.L.; Springs, A.E.; Kaminski, N.E. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem. Pharm. 2008, 76, 726–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodadadi, H.; Salles, É.L.; Jarrahi, A.; Chibane, F.; Costigliola, V.; Yu, J.C.; Vaibhav, K.; Hess, D.C.; Dhandapani, K.M.; Baban, B. Cannabidiol Modulates Cytokine Storm in Acute Respiratory Distress Syndrome Induced by Simulated Viral Infection Using Synthetic RNA. Cannabis Cannabinoid Res. 2020, 5, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Salles, É.L.; Shin, E.; Jarrahi, A.; Costigliola, V.; Kumar, P.; Yu, J.C.; Morgan, J.C.; Hess, D.C.; Vaibhav, K.; et al. A potential role for cannabichromene in modulating TRP channels during acute respiratory distress syndrome. J. Cannabis. Res. 2021, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Milam, J.E.; Keshamouni, V.G.; Phan, S.H.; Hu, B.; Gangireddy, S.R.; Hogaboam, C.M.; Standiford, T.J.; Thannickal, V.J.; Reddy, R.C. PPAR-gamma agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L891–L901. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wan, T.; Pang, N.; Zhou, Y.; Jiang, X.; Li, B.; Gu, Y.; Huang, Y.; Ye, X.; Lian, H.; et al. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J. Cell. Physiol. 2019, 234, 21224–21234. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Almeida, V.I.; Costola-de-Souza, C.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Gimenes-Junior, J.A.; Akamine, A.T.; Crippa, J.A.; Tavares-de-Lima, W.; et al. Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury. Immunopharmacol. Immunotoxicol. 2015, 37, 35–41. [Google Scholar] [CrossRef]
- Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A(2A) receptor. Eur. J. Pharmacol. 2012, 678, 78–85. [Google Scholar] [CrossRef]
- Sadowska, O.; Baranowska-Kuczko, M.; Gromotowicz-Popławska, A.; Biernacki, M.; Kicman, A.; Malinowska, B.; Kasacka, I.; Krzyżewska, A.; Kozłowska, H. Cannabidiol Ameliorates Monocrotaline-Induced Pulmonary Hypertension in Rats. Int. J. Mol. Sci. 2020, 21, 7077. [Google Scholar] [CrossRef]
- Arruza, L.; Pazos, M.R.; Mohammed, N.; Escribano, N.; Lafuente, H.; Santos, M.; Alvarez-Díaz, F.J.; Hind, W.; Martínez-Orgado, J. Cannabidiol reduces lung injury induced by hypoxic-ischemic brain damage in newborn piglets. Pediatr. Res. 2017, 82, 79–86. [Google Scholar] [CrossRef]
- Crippa, J.A.S.; Pacheco, J.C.; Zuardi, A.W.; Guimarães, F.S.; Campos, A.C.; Osório, F.L.; Loureiro, S.R.; Dos Santos, R.G.; Souza, J.D.S.; Ushirohira, J.M.; et al. Cannabidiol for COVID-19 Patients with Mild to Moderate Symptoms (CANDIDATE Study): A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Cannabis Cannabinoid Res. 2021, 7, 658–669. [Google Scholar] [CrossRef]
- Whittaker, A.; Anson, M.; Harky, A. Neurological Manifestations of COVID-19: A systematic review and current update. Acta Neurol. Scand. 2020, 142, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Krizinger, A.K.A.; Hamel, L.; Brodie, M. KFF Health Follow-up Survey—Early April 2020: The Impact of Coronavirus on Life in the United States. Available online: https://www.kff.org/coronavirus-covid-19/report/kff-health-tracking-poll-early-april-2020/ (accessed on 10 August 2022).
- Fiani, B.; Sarhadi, K.J.; Soula, M.; Zafar, A.; Quadri, S.A. Current application of cannabidiol (CBD) in the management and treatment of neurological disorders. Neurol. Sci. 2020, 41, 3085–3098. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Glass, M.; Pertwee, R.G. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: Interspecies differences. Br. J. Pharm. 2007, 152, 583–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breivogel, C.S.; Sim-Selley, L.J. Basic neuroanatomy and neuropharmacology of cannabinoids. Int. Rev. Psychiatry 2009, 21, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.R.; Núñez, E.; Benito, C.; Tolón, R.M.; Romero, J. Functional neuroanatomy of the endocannabinoid system. Pharmacol. Biochem. Behav. 2005, 81, 239–247. [Google Scholar] [CrossRef]
- Neumeister, A.; Normandin, M.D.; Pietrzak, R.H.; Piomelli, D.; Zheng, M.Q.; Gujarro-Anton, A.; Potenza, M.N.; Bailey, C.R.; Lin, S.F.; Najafzadeh, S.; et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: A positron emission tomography study. Mol. Psychiatry 2013, 18, 1034–1040. [Google Scholar] [CrossRef] [Green Version]
- Thibaut, F.; van Wijngaarden-Cremers, P.J.M. Women’s Mental Health in the Time of COVID-19 Pandemic. Front. Glob. Women's Health 2020, 1, 588372. [Google Scholar] [CrossRef]
- Yan, S.; Xu, R.; Stratton, T.D.; Kavcic, V.; Luo, D.; Hou, F.; Bi, F.; Jiao, R.; Song, K.; Jiang, Y. Sex differences and psychological stress: Responses to the COVID-19 pandemic in China. BMC Public Health 2021, 21, 79. [Google Scholar] [CrossRef]
- Solowij, N.; Broyd, S.J.; Beale, C.; Prick, J.A.; Greenwood, L.M.; van Hell, H.; Suo, C.; Galettis, P.; Pai, N.; Fu, S.; et al. Therapeutic Effects of Prolonged Cannabidiol Treatment on Psychological Symptoms and Cognitive Function in Regular Cannabis Users: A Pragmatic Open-Label Clinical Trial. Cannabis Cannabinoid Res. 2018, 3, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Crippa, J.A.; Zuardi, A.W.; Garrido, G.E.; Wichert-Ana, L.; Guarnieri, R.; Ferrari, L.; Azevedo-Marques, P.M.; Hallak, J.E.; McGuire, P.K.; Filho Busatto, G. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 2004, 29, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Zuardi, A.W.; Cosme, R.A.; Graeff, F.G.; Guimarães, F.S. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol. 1993, 7, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W.; Rodrigues, N.P.; Silva, A.L.; Bernardo, S.A.; Hallak, J.E.C.; Guimarães, F.S.; Crippa, J.A.S. Inverted U-Shaped Dose-Response Curve of the Anxiolytic Effect of Cannabidiol during Public Speaking in Real Life. Front. Pharmacol. 2017, 8, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamaschi, M.M.; Queiroz, R.H.; Chagas, M.H.; de Oliveira, D.C.; De Martinis, B.S.; Kapczinski, F.; Quevedo, J.; Roesler, R.; Schröder, N.; Nardi, A.E.; et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology 2011, 36, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.; Derenusson, G.N.; Ferrari, T.B.; Wichert-Ana, L.; Duran, F.L.; Martin-Santos, R.; Simões, M.V.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011, 25, 121–130. [Google Scholar] [CrossRef]
- Elms, L.; Shannon, S.; Hughes, S.; Lewis, N. Cannabidiol in the Treatment of Post-Traumatic Stress Disorder: A Case Series. J. Altern. Complement. Med. 2019, 25, 392–397. [Google Scholar] [CrossRef]
- Shannon, S.; Lewis, N.; Lee, H.; Hughes, S. Cannabidiol in anxiety and sleep: A large case series. Perm. J. 2019, 23, 18–41. [Google Scholar] [CrossRef] [Green Version]
- Appiah-Kusi, E.; Petros, N.; Wilson, R.; Colizzi, M.; Bossong, M.G.; Valmaggia, L.; Mondelli, V.; McGuire, P.; Bhattacharyya, S. Effects of short-term cannabidiol treatment on response to social stress in subjects at clinical high risk of developing psychosis. Psychopharmacology 2020, 237, 1121–1130. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.; Bossong, M.G.; Appiah-Kusi, E.; Petros, N.; Brammer, M.; Perez, J.; Allen, P.; McGuire, P.; Bhattacharyya, S. Cannabidiol attenuates insular dysfunction during motivational salience processing in subjects at clinical high risk for psychosis. Transl. Psychiatry 2019, 9, 203. [Google Scholar] [CrossRef] [Green Version]
- Crippa, J.A.S.; Zuardi, A.W.; Guimarães, F.S.; Campos, A.C.; de Lima Osório, F.; Loureiro, S.R.; Dos Santos, R.G.; Souza, J.D.S.; Ushirohira, J.M.; Pacheco, J.C.; et al. Efficacy and Safety of Cannabidiol Plus Standard Care vs. Standard Care Alone for the Treatment of Emotional Exhaustion and Burnout Among Frontline Health Care Workers During the COVID-19 Pandemic: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2120603. [Google Scholar] [CrossRef]
- Murillo-Rodríguez, E.; Millán-Aldaco, D.; Palomero-Rivero, M.; Mechoulam, R.; Drucker-Colín, R. The nonpsychoactive Cannabis constituent cannabidiol is a wake-inducing agent. Behav. Neurosci. 2008, 122, 1378–1382. [Google Scholar] [CrossRef]





| Commercial Name | Active Ingredient | Therapeutic Applications | Countries, in Which Its Use Is Approved |
|---|---|---|---|
| MARINOL® [44,45,46] | Capsules of 2.5 mg, 5 mg or 10 mg of dronabinol (THC) dissolved in sesame oil. | Treatment of nausea and vomiting due to chemotherapy, in adults and pediatric patients. Treatment of anorexia/cachexia in adults with AIDS or terminal cancer. | United States, Canada, South Africa, Denmark. |
| SATIVEX® [45,46,47] | Spray of a standardized extract of Cannabis. Each spray releases a fixed dose of 2.7 mg of THC and 2.5 mg of CBD. | Adjuvant treatment for: 1. moderate or severe spasticity due to Multiple Sclerosis (MS); 2. symptomatic pain relief neuropathic in adult patients with MS; 3. in adult cancer patients suffering from moderate to severe pain. | Approved in 27 countries (European Nations and Asia, New Zealand, Canada and Australia). |
| Epidiolex® [45,46,48] | Oral solution (100 mL of 100 mg/mL CBD | Reduces seizure frequency in patients with end-stage Dravet syndrome, a rare and devastating form of epilepsy that is drug-resistant. | GW Pharmaceuticals (United Kingdom) |
| Nabiximol [49] | Mouth spray (50% CBD and 50% THC) | Treatment of spasticity in multiple sclerosis. | U.S., Canadian and U.K. regulatory agencies |
| Clinical Trial Number/Year | Patients | Description | Results | References |
|---|---|---|---|---|
| NCT02224560/2018 | Adults and children (2–55 years of age, n = 225) with Lennox-Gastaut syndrome | Randomized, double-blind trial 14-week study of two dose levels (10 mg/kg/day and 20 mg/kg/day), to evaluate the efficacy and safety of CBD (GWP42003-P) | It confirmed a reduction in the number of seizures | [59] |
| NCT02224690/2015 | Patients (2-55 years of age) with Lennox-Gastaut syndrome (n = 171) | GWP42003-P in a single dose of 20 mg/kg/day for 14 weeks | The results confirmed the reduction in seizure frequency after administration | [60] |
| NCT02091206/ | Children (4–10 years) suffering from Dravet syndrome (n = 34) | It was a safety and pharmacokinetic dose-ranging study of Epidiolex (5, 10 and 20 mg/kg/day) with a duration of 21 days | Results confirmed that Epidiolex is a safe drug; however, it caused more adverse effects than placebo did | [61] |
| NCT02091375/ 2015 | Children and adults (2–18 years) with Dravet syndrome (n = 120) | Evaluation of the antiepileptic efficacy of GWP42003-P | Results confirmed that CBD (20 mg/kg/day/14 weeks) resulted in 43% of the patients experiencing a reduction in seizure frequency of 50% or more. Three of these patients became seizure-free | [62] |
| NCT02700412/ 2015 | Eighty epileptic subjects between 18 and 99 years | This interventional study evaluated the safety and tolerability of Epidiolex at various doses between 5 and 50 mg/kg/day as an add-on drug for the treatment of drug-resistant debilitating epilepsy | Results confirmed Epidiolex seizure reduction in 63.6% of patients after 12 weeks of treatment | [63] |
| NCT02695537/ 2015 | Children and adults (1–19 years), n = 89 | Evaluation of the safety and tolerability of Epidiolex for the treatment of drug-resistant debilitating epilepsy | The results confirmed after administration of various doses between 5 and 50 mg/kg/day a seizure reduction of 63.6% after 12 weeks of treatment | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, R.; Glaser, T.; Villegas, C.; Burgos, V.; Ulrich, H.; Paz, C. Therapeutic Effects of Cannabinoids and Their Applications in COVID-19 Treatment. Life 2022, 12, 2117. https://doi.org/10.3390/life12122117
Pérez R, Glaser T, Villegas C, Burgos V, Ulrich H, Paz C. Therapeutic Effects of Cannabinoids and Their Applications in COVID-19 Treatment. Life. 2022; 12(12):2117. https://doi.org/10.3390/life12122117
Chicago/Turabian StylePérez, Rebeca, Talita Glaser, Cecilia Villegas, Viviana Burgos, Henning Ulrich, and Cristian Paz. 2022. "Therapeutic Effects of Cannabinoids and Their Applications in COVID-19 Treatment" Life 12, no. 12: 2117. https://doi.org/10.3390/life12122117