Incidence of corneal transplant rejection following BNT162b2 SARS-CoV-2 messenger RNA vaccination
Article Information
Hisataka Fujimoto1*, Junichi Kiryu1
Department of Ophthalmology, Kawasaki Medical School, 577 Matsushima, Kurashiki 701-0192 Okayama, Japan
*Corresponding author: Hisataka Fujimoto, Department of Ophthalmology, Kawasaki Medical School, 577 Matsushima, Kurashiki, 701-0192 Okayama, Japan
Received: 12 September 2021; Accepted: 22 September 2021; Published: 27 September 2021
Citation: Hisataka Fujimoto, Junichi Kiryu. Incidence of corneal transplant rejection following BNT162b2 SARS-CoV-2 messenger RNA vaccination. Journal of Ophthalmology and Research 4 (2021): 279-388.
View / Download Pdf Share at FacebookAbstract
Purpose: We report seven cases of corneal allograft rejection following immunization with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA (mRNA) vaccine BNT162b2, and describe the implications for the management of transplant recipients post-vaccination for coronavirus disease 2019 (COVID-19). Methods: This retrospective observational study included 66 patients post-keratoplasty between 98 and 2054 days following transplantation. Data on the incidence of rejection, time series of the onset of rejection, corneal transplantation, and vaccination with the SARS-CoV-2 mRNA vaccine BNT162b2 were collected. In addition, the use of ophthalmic solutions including topical steroids and immunosuppressants at the time of rejection, and background including systemic diseases, such as autoimmune or allergic disease, were collected. Results: The incidence of rejection following BNT162b2 vaccination was observed in seven cases (observational period following vaccination was 74.0 ± 28.4 days), while no vaccination was observed in 2 cases (observational period following keratoplasty was 938.3 ± 1501.5 days). The odds ratio of rejection related to vaccination was 5.57 (95% confidence interval [CI], 1.18–25.57; p =0.036; Fisher’s exact test). The relative risk of rejection related to vaccination is 4.47 (95% CI, 1.15–18.34). Conclusion: A temporal association between corneal transplant rejection following immunization against COVID-19 was suspected. A possible mechanism could be the allogeneic response initiated by the host antibody following vaccination.
Keywords
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) messenger RNA (mRNA) vaccine; BNT162b2; Rejection; Keratoplasty; Corneal transplantation
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) messenger RNA (mRNA) vaccine articles; BNT162b2 articles; Rejection articles; Keratoplasty articles; Corneal transplantation articles
Article Details
Introduction
Corneal transplantation is a widely used transplant therapy performed worldwide. Although the cornea is an immune-privileged site, the most frequent cause of graft failure is allogeneic rejection [1]. Of the different types of corneal transplant procedures, rejection is reported in penetrating keratoplasty (PK), Descemet’s stripping automated endothelial keratoplasty (DSAEK), anterior lamellar keratoplasty (ALK), deep anterior lamellar keratoplasty (DALK), and most frequently following corneal limbal transplantation (LT), in which the transplanted donor tissue, including transplanted epithelial stem cells into the corneal limbs, accompanies relatively strong immune responses [2-4] Irrespective of whether the transplanted donor cornea is full or partial thickness, rejection episodes are possible. Even though reversed by treatment, rejection causes irreversible damage to the donor, including endothelial cells, which maintain corneal transparency. Progressive loss of endothelial cells results in decompensation and persistent stromal edema, with a reduction in visual acuity that forces re-transplantation. Vaccination-associated corneal graft rejection is a rare event [5]. Only 12 individuals reported this complication, most of whom recovered with topical corticosteroid therapy.
The coronavirus disease 2019 (COVID-19) pandemic has seen the rapid introduction of immunization directed against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in an effort to limit the spread of the disease and reduce its associated morbidity and mortality [6]. With the systematic vaccination efforts adopted by countries worldwide and by Japan even though relatively delayed compared to other developed countries, a very large numbers of patients with corneal transplants have had, or are determined to have, SARS-CoV-2 vaccines. The BNT162b2 messenger RNA (mRNA) vaccine has been administered in Japan since April 1, 2021, for patients in addition to the medical staff. We describe seven cases of rejection following COVID-19 immunization and propose the possibility of a causal association.
2. Materials and Methods
2.1 Study design
This retrospective observational study included 66 patients who underwent keratoplasty. The study was approved by the Institutional Review Board of Kawasaki Medical School Hospital (approval number 3572-00). Informed consent was obtained from all the study participants, and the study adhered to the tenets of the Declaration of Helsinki and was performed according to Good Clinical Practice (GCP). This study did not involve patients or the public in its design, participant recruitment, or conduct.
2.2 Data collection
This retrospective observational study included 66 post-keratoplasty patients at 12–7456 days following transplantation. Data on the incidence of rejection and rejection onset date, transplantation date, vaccination date of SARS-CoV-2 mRNA vaccine BNT162b2, use of ophthalmic solutions, including topical steroids and immunosuppressants, following keratoplasty, use of systemic immunosuppressants or steroids following keratoplasty, and background including systemic diseases, such as autoimmune or allergic disease, were collected.
The study observation period was defined as the date from which the patient started receiving care at the study site until the last data point, which was measured and was between April 1, 2001, and September 1, 2021. To ensure uniform data collection, assessment, and compliance with the GCP, on-site training was conducted. Patient data records were selected based on the study inclusion and exclusion criteria, and patients receiving care between April 1, 2001, and September 1, 2021, were included in the study. To minimize bias, the eligible patients were enrolled in one of the two study groups (SARS-CoV-2 vaccination group and control group with no SARS-CoV-2 vaccination) allotted in a consecutive series. In all the cases, the SARS-CoV-2 vaccine was SARS-CoV-2 mRNA vaccine BNT162b2 (Pfizer-BioNTech), and the vaccination interval was 21 days.
The background data obtained included age, sex, observation period following keratoplasty (day), whether the cases were high-risk cases for rejection or not (high risk; post-inflammation, active inflammation including peripheral corneal ulcer caused by autoimmune disease, active perforation, recurrent herpes simplex virus infection, varicella-zoster virus infection, cytomegalovirus (CMV) infection, re-transplantation, and LT), operative procedure (PK, DSAEK, DALK, LT, or combinations of these ), post-keratoplasty topical steroid and/or ophthalmic solution use, and systemic immunosuppressants.
The best-corrected visual acuity (BCVA) was measured using the Landolt rings. Central corneal thickness (CCT) were obtained using anterior segment optical coherence tomography (AS-OCT) CASIA 2 (Tomey Corporation, Nagoya, Japan). Analysis software (Tomey Corporation, Nagoya, Japan) was used to identify and digitize the anterior corneal surfaces, posterior corneal surfaces, and tear meniscus areas. All digitization was confirmed by research in a masked manner. The intraocular pressure (IOP) was measured using a non-contact tonometer (NT-4000; NIDEK Co., Ltd, Japan) at every visit. IOP measurements were performed three times, and the IOP values were averaged. Corneal specialists diagnosed rejection based on slit lamp examination, BCVA, and AS-OCT.
2.3 Study participants and visits
2.3.1 Participants: A retrospective review of the clinical records (including image records) of 66 patients post-keratoplasty was included from a single center (Kawasaki Medical School) in Japan. Patients without active ocular surface disorders, such as epithelial disorders, infections, conjunctivitis, ocular inflammatory disease, and nasolacrimal duct obstruction, were included in the study. To minimize the intervention in this retrospective study, participants were enrolled in consecutive series with or without BNT162b2 vaccination history. BNT162b2 vaccination began on April 1, 2021, in Japan. The 66 patients comprised a consecutive series of 29 patients after BNT162b2 vaccination and 37 patients without BNT162b2 vaccination who met the above criteria.
2.4 Statistical analysis
The study was designed to assess the difference between patients after BNT162b2 vaccination and those without BNT162b2 vaccination based on allograft rejection of the transplanted cornea. The sample size was planned to examine the null hypothesis of no difference between the two groups (with/without BNT162b2 vaccination) with the specified probability. Data distributions were assessed for normality using the Shapiro-Wilk test. Following the assumption that all the data on the outcomes of interest followed a parametric distribution, the two-sample independent t-test, Pearson’s chi-squared test, Fisher’s exact test, and paired t-test were used to analyze the data. All the statistical analyses were performed using SPSS version 25.0 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, version 25.0. Armonk, NY: IBM Corp. USA).
3. Results
The study comprised a total of 35 males and 31 females with an average age of 72.03 ± 16.50 years and 64.88 ± 18.36 years for the with BNT162b2 and without BNT162b2 groups, respectively. There were no significant differences in the baseline parameters between the study groups (Table 1), including operative procedure, risk for rejection, age, sex, topical steroid and immunosuppressant use, and systemic immunosuppressant use (at rejection in rejection-induced cases).
|
BNT162B2 vaccination (+) Average ± SD |
control vaccination (-) Average ± SD |
p- value vaccination (+) vs. |
|
|
Rejection incidence |
24.1% (7/29) |
5.4% (2/37) |
0.027^ |
|
Age (years) |
72.03 ± 16.50 |
64.88 ± 18.36 |
0.1 |
|
Male ratio |
0.48 |
0.56 |
0.49^ |
|
Observation period following keratoplasty (day) |
678.3 ± 659.6 |
938.3 ± 1501.5 |
0.36 |
|
Observation period following vaccination (day) |
74.0 ± 28.4 |
||
|
High risk case for rejection |
31.0% (9/29) |
27.0% (10/37) |
0.72^ |
|
PK |
69.0% (20/29) |
68.4% (25/37) |
0.90^ |
|
DSAEK |
10.3% (3/29) |
16.2% (6/37) |
0.49^ |
|
ALK or DALK |
10.3% (3/29) |
13.5% (5/37) |
0.70^ |
|
LT |
10.3% (3/29) |
5.4% (2/37) |
0.45^ |
|
0.1% betamethasone topical use per day |
1.79 ± 1.92 |
1.64 ± 1.74 |
0.74 |
|
0.1% fluorometholone topical use per day |
1.07 ± 1.58 |
0.94 ± 1.19 |
0.74 |
|
0.1% tacrolimus topical use per day |
0.62 ± 0.94 |
0.44 ± 0.84 |
0.44 |
|
Immunosuppressants systemic use (oral) |
10.3% (3/29) |
2.7% (1/37) |
0.19^ |
Table 1: Comparison between the BNT162B2 vaccination (+) and control [SARS-CoV-2 vaccination (-)] group.
Data are expressed as mean ± standard deviation. P values were calculated using a two-sample independent t-test in all instances except for the female ratio and ophthalmic solution use ratio, where the Pearson chi-squared test was used (^). SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ALK, anterior lamellar keratoplasty; DALK, deep anterior lamellar keratoplasty; DSAEK, Descemet stripping automated endothelial keratoplasty; LT, corneal limbal transplantation; PK, penetrating keratoplasty; SD, standard deviation.
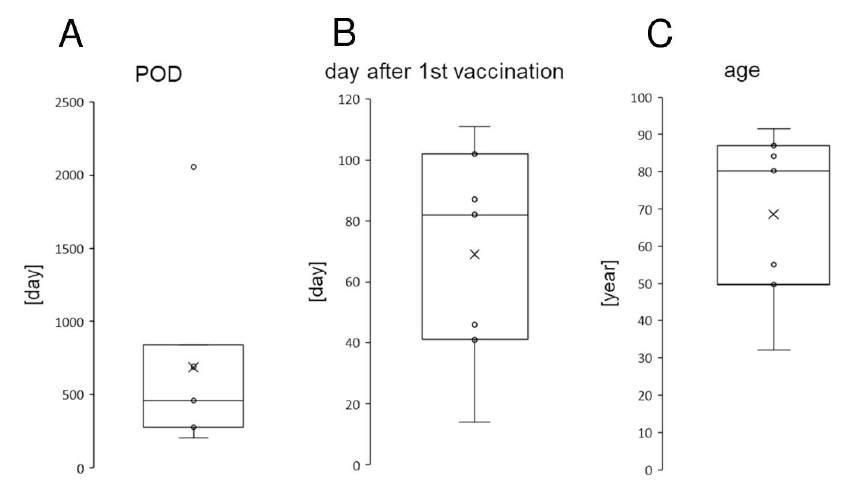
Seven eyes of seven patients were reported to have expedient rejection following BNT162b2 vaccination (Table 2). These patients comprised a total of five men and two women. The average age was 68.54 ± 22.81 years (minimum 32 years; maximum, 91 years; Figure 1, Table 2). The average period from the first BNT162b2 vaccination to the onset of rejection was 69.0 ± 35.8 days (minimum, 14 days; maximum, 111 days; Figure 1, Table 2). The average period from the corneal transplantations to the onset of rejection was 687.4 ± 647.47 days (minimum, 204 days; maximum, 2056 days; Figure 1, Table 2).
Figure 1: Demographics of the seven cases of rejection for allograft corneal transplantation following severe acute respiratory syndrome coronavirus 2 messenger RNA (SARS-CoV-2 mRNA) vaccination BNT162b2 (Pfizer-BioNTech). The rejection onset date from the transplantation date (A, days), the rejection onset date from the first BNT162b2 vaccination date (B, days), and the ages at the rejection onset (C years) are presented. The horizontal lines in the box and whisker plots represent the median values, and the bottom and top of the boxes represent the lower and upper quartiles, respectively. The x represents the mean and the bars represent the minimum and maximum values within 1.5 times the lower and upper quartiles. POD, post operative day
Table 2: Demographics of the cases with rejection after SARS-CoV-2 messenger RNA (mRNA) BNT162b2 vaccination.
BCVA, best corrected visual acuity; BK, bullous keratopathy; CCT, central corneal thickness; DSAEK, Descemet’s stripping automated endothelial keratoplasty; GvHD, graft versus host disease; HM, hand motion; LSCD, limbal stem cell deficiency; LT, corneal limbal transplantation; PK, penetrating keratoplasty; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
At the time of rejection diagnosis, keratic precipitates or corneal stromal edema were observed in all eyes by slit lamp examination (Table 2). In all eyes, visual acuity deteriorated compared to the last examination before rejection diagnosis (Table 2). In all the eyes, CCT increased at the rejection (625.4 ± 76.3μm) compared to the last examination before the rejection (535.3 ± 61.7μm, p = 0.0002, paired t-test) (Table 2). IOP did not differ before (14.3 ± 3.8 mmHg) and after (13.7 ± 4.0 mmHg, p = 0.59, paired t-test) rejection. The interval between the rejection diagnosis and the last examination before the rejection was 41.6 ± 31.6 days (minimum, 6 days; maximum 93 days).
One patient had irreversible graft failure and bullous keratopathy, while the other 6 cases healed by topical steroid and/or immunosuppressant use within weeks, except for one patient who required a systemic steroid (prednisolone, maximum 20 mg/day) via oral administration for 4 weeks (Table 2).
The incidence of rejection following BNT162b2 vaccination was observed in seven cases (observational period following vaccination was 74.0 ± 28.4 days), while no vaccination was observed in 2 cases (observational period following keratoplasty was 938.3 ± 1501.5 days). The odds ratio of rejection related to vaccination was 5.57 (95% confidence interval [CI], 1.18–25.57; p=0.036; Fisher’s exact test, Table 1), which is underestimated since the observational period was shorter in the vaccination group. The relative risk of rejection related to vaccination was 4.47 (95% CI, 1.15-18.34).
4. Discussion
Vaccination in transplantation recipients has been demonstrated to be efficacious and safe since meta-analyses showed no evidence of rejection following vaccination [7]. Only a few cases of corneal graft rejection following vaccination have been described after influenza, hepatitis B, and tetanus toxoid immunizations [5,8,9]. Recently, multiple case reports revealed the possibility of rejection induced by the BNT162b2 vaccination [10,11]. Despite the immune privilege of the cornea, immune-mediated corneal allograft rejection does occur, especially following LT and PK in high rejection risk eyes. The mechanism of vaccination-induced corneal transplantation graft rejection is not well understood. Vaccinations may trigger a cascade of inflammatory responses either by the development of cross-reactivity with cellular antigens or because of nonspecific immune activation [5]. The BNT162b2 vaccine introduces genetic material to cells, which results in the generation of a systemic antibody response [12]. This vaccine has demonstrated high levels of neutralizing antibody titers and antigen-specific CD8+ and TH1-type CD4+ T-cell responses [13]. BNT162b2 vaccination increases the levels of proinflammatory cytokines, such as interferon gamma (IFNγ) [14], IFNγ-producing CD4+ T helper 1 (Th1) cells are thought to be a key cell type in corneal allograft rejection [15].
In our study, seven individuals presented with corneal graft rejection 2–16 weeks following BNT162b2 vaccination. Neither patient had a previous history of SARS-CoV-2 or a history of uveitis or herpes infection. In addition, there were no previous episodes of corneal graft rejection in either patient. All patients responded well to treatment with topical and systemic corticosteroids.
One of the limitations of this study is its retrospective design and small sample size. The randomized design with configuration of placebo vaccination group is ethically inappropriate as the high BNT162b2 efficacy is elucidated. In addition, the odds ratio and relative risk were underestimated considering
that the observational period after vaccination was shorter in the vaccination group. The most promising statistical analysis for these types of studies could be a matched case-control study with a completely identical control study participant for each patient with a history of rejection suspected to be induced by the BNT162b2 vaccination. However, the limited number and the frequently complicated background of post-keratoplasty patients limit this approach. Further enrollment of the cases and the prolonged observation period would be needed in the future, considering that the population with BNT162b2 vaccination is still increasing in Japan.
5. Conclusions
In conclusion, BNT162b2 vaccination could be related to rejection following corneal transplantation. Ophthalmologists and patients should consider the possibility of rejection following vaccination, and suspected cases should be promptly examined, and intervened if necessary.
Acknowledgments
We thank K. Kiyohara, N. Okamoto, M. Yaida, Y. Ieki, K. Baba, A. Masuda, Y. Mito, and Y. Setoguchi for their discussions, advice, and criticism that greatly benefited this project. This work was supported by the Charitable Trust Fund for Ophthalmic Research in Commemoration of Santen Pharmaceutical’s Founder 2019 (to H.F.).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- Niederkorn JY, Larkin DFP. Immune privilege of corneal allografts. Ocular Immunology Inflammation 12 (2010): 162-171.
- Price DA, Kelley M, Price FW, et al. Five-Year graft survival of Descemet membrane endothelial keratoplasty (EK) versus Descemet stripping EK and the effect of donor sex matching. Ophthalmology 10 (2018): 1508-1514.
- Anshu A, Price MO, Price FW. Risk of corneal transplant rejection significantly reduced with Descemet's membrane endothelial keratoplasty. Ophthalmology 15 (2012): 536-540.
- Hos D, Matthaei M, Bock F, et al. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Progress in Retinal and Eye Research 16 (2019): 100768.
- Lockington D, Lee B, Jeng BH, et al. Survey of corneal surgeons’ attitudes regarding keratoplasty rejection risk associated with vaccinations. Cornea 11 (2021).
- Cook TM, Roberts JV. Impact of vaccination by priority group on UK deaths, hospital admissions and intensive care admissions from COVID-19. Anaesthesia 9 (2021): 608-616.
- Mulley WR, Dendle C, Ling JE, et al. Does vaccination in solid-organ transplant recipients result in adverse immunologic sequelae? A systematic review and meta-analysis. Journal of Heart and Lung Transplantation 28 (2018): 844-852.
- Solomon A, Frucht-Pery J. Bilateral simultaneous corneal graft rejection after influenza vaccination. American Journal of Ophthalmology 18 (1996): 708-709.
- Vignapiano R, Vicchio L, Favuzza E, et al. Corneal graft rejection afteryellow fever vaccine: a case report. Ocular Immunology Inflammation 24 (2021): 1-4.
- Wasser LM, Roditi E, Zadok D, et al. Keratoplasty rejection after the BNT162b2 messenger RNA Vaccine. Cornea 12 (2021): 1070-1072.
- Phylactou M, Li J-PO, Larkin DFP. Characteristics of endothelial corneal transplant rejection following immunisation with SARS-CoV-2 messenger RNA vaccine. British Journal of Ophthalmology 15 (2021): 893-896.
- Jhaveri R. The COVID-19 mRNA vaccines and the pandemic: do they represent the beginning of the end or the end of the beginning? Clinical Therapeutics 23 (2021): 549-556.
- Sahin U, Muik A, Vogler I, et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. MedRxiv (2020).
- Hegde S, Beauregard C, Mayhew E, et al. CD4(+) T-cell-mediated mechanisms of corneal allograft rejection: role of Fas-induced apoptosis. Transplantation 11 (2005): 23-31.
- Amouzegar A, Chauhan SK, Dana R. Alloimmunity and tolerance in corneal transplantation. Journal of Immunology 12 (2016): 3983-3991.


 Impact Factor: * 1.2
Impact Factor: * 1.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
