- 1Department of Radiation Oncology, All India Institute of Medical Sciences, Patna, India
- 2Department of Radio-diagnosis, All India Institute of Medical Sciences, Patna, India
- 3Department of Anaesthesiology, All India Institute of Medical Sciences, Patna, India
- 4Department of Pulmonary Medicine, All India Institute of Medical Sciences, Patna, India
- 5Department of General Medicine, All India Institute of Medical Sciences, Patna, India
- 6Department of Community and Family Medicine, All India Institute of Medical Sciences, Patna, India
Background: Treatment for coronavirus disease 2019 (COVID-19) pneumonia remains largely supportive till date and multiple clinical trials took place within the short span of time to evaluate the role of investigational therapies. The anti-inflammatory effect of low dose whole lung radiation in treating pneumonia has been documented earlier. This clinical trial analyzed the effect of low dose radiation therapy (LDRT) in a moderately affected COVID-19 pneumonia patient cohort and has evaluated its effect in stopping the conversion of moderate disease into severe disease.
Methods: Patients with moderate COVID-19 pneumonia as characterized by the Ministry of Health and Family Welfare (MOHFW), Government of India, were randomized (1:1) to low dose whole lung radiation versus no radiation. All treatment of patients was concurrently being given as per institutional protocol. Patients were followed up with clinical and laboratory parameters monitored on Days 1, 3, 7, and 14. Computed tomography scan (CT scan) of thorax was performed on Days 1 and 7. Patients were evaluated for conversion of moderate into severe disease as per National Early Warning Score-2 (NEWS-2 score) as the primary end point. The secondary endpoints included changes in ratio between peripheral capillary oxygen saturation and fraction of inspired oxygen (SpO2/FiO2), biochemical markers, 25-point CT severity score, and radiation induced acute pulmonary toxicities.
Findings: At the interim analysis, there were seven patients in the radiation arm and six in the control. A whole lung LDRT improved the outcome of SpO2/FiO2 at Day 3; however it did not convert into a statistically significant improvement for the NEWS-2 score. The serum levels of LDH, CRP, Ferritin and D-dimer were significantly reduced on 14 days in the LDRT arm in comparison to the baseline value but were not significant between the two groups.
Interpretation: LDRT seems to have the potential to prevent moderate COVID-19 pneumonia from a deteriorating to severe category. However, further randomized clinical trial with an adequate number of such patients is warranted to establish the definitive role of LDRT in the management of COVID-19 pneumonia.
Funding: An intramural research project bearing code: I-27/621, was sanctioned from the All India Institute of Medical Sciences, Patna, India.
Clinical Trial Registration: Clinical Trials Registry-India (CTRI/2021/06/033912, 25th May 2021) ctri.nic.in/Clinicaltrials/login.php
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which primarily affects the respiratory system results in a wide spectrum of symptoms, starting from mild cough to acute respiratory distress syndrome (ARDS) and multi organ failure. Epidemiological studies have observed that 6 to 10% of patients may develop a severe form of COVID-19 infection and require high quality intensive care unit (ICU) support including Mechanical ventilation (MV) (1). Mortality rates reported in patients with severe symptoms in the ICU may range from 25 to 65% with regional, institutional, and ethnic variations (2–4). However, treatment remains largely supportive till date and multiple clinical trials took place within the short span of time to evaluate the role of investigational therapies or ‘off-label’ medications such as remdesivir, therapeutic plasma exchange, convalescent plasma, and mesenchymal stem cell therapies to reduce the disease severity in COVID-19. These therapies are extensively administered worldwide after getting the emergency use authorization (EUA) but unfortunately failed to produce significant clinical recovery and reduction of mortality rate (5, 6). In this context, the role of low dose radiotherapy (LDRT) has re-emerged as a potent anti-inflammatory and immune modulator which can effectively reduce the severity of lung damage caused by the SARS-CoV-2. In this prospective, randomized clinical trial, we have studied the role of LDRT on the moderately severe COVID-19 pneumonia patients with the evaluation of clinical, biochemical, and radiological parameters.
The primary objective was to evaluate changes from moderate to severe grade of the disease in radiation and observation group with the National Early Warning Score (NEWS-2) at days 3, 7, and 14 (7, 8).
Secondary objectives of this trial include evaluation of changes in physiological parameters, namely, ratio between peripheral capillary oxygen saturation and fraction of inspired oxygen (SpO2/FiO2), changes in hematological and biochemical parameters such as Complete blood count (CBC), neutrophil to lymphocyte ratio (NLR), erythrocyte sedimentation rate (ESR), D-dimer, Lactate dehydrogenase (LDH), Ferritin, Interleukin-6 (IL-6), C-Reactive protein (CRP), arterial blood gases analysis (ABG), prothrombin time (PT), international normalized ratio (INR), D-dimer, IL-6 (interleukin-6) and changes in 25 point CT severity scoring on days 7 and 28. Radiation induced acute pulmonary toxicity is monitored on basis of the Common Terminology Criteria for Adverse Events (CTCAE v 5.0) scale (9).
Methods
This prospective, double arm, interventional, randomized controlled trial was conducted from May 2021 to August 2021 at the All India Institute of Medical Sciences (AIIMS), Patna, India after obtaining clearance from the institutional ethical committee and registration with the Central Drugs standard control Organization India (ECR/1387/Ins/BR/2020) and the Clinical Trials Registry-India (CTRI/2021/06/033912, May 25, 2021). Thirteen patients aged between 18 and 70 years, with confirmed SARS-CoV2 infection by RT-PCR from nasopharyngeal swab and or broncho-alveolar lavage (BAL) were recruited. Patients had moderate disease as per the criteria laid by the Ministry of Health and Family Welfare (MOHFW), Government of India which included SpO2 <94% on Room Air and Respiratory rate >20 breaths per minute at room air with fewer than 10 days of symptom onset (10). Patients were randomly assigned to a radiotherapy (RT) arm and a non-RT arm with 1:1 ratio by computer-generated random numbers. All patients were receiving standard medication as per institute protocol for COVID-19 pneumonia. Patients with severe COVID-19 disease, hemodynamically unstable, autoimmune disease, under mechanical ventilation, and pregnancy were excluded from the study.
The study was performed with strict compliance with institutional guidelines for infection prevention, namely, the use of personal protective equipment (PPE) by the entire team and the patients, dedicated patient transport corridor, and decontamination measures. Patients were transported from their dedicated COVID-19 wards to the department of radiotherapy under continuous monitoring by one physician and two paramedical personnel (Figure 1A) with supplementary oxygen support. After being shifted to the CT simulation room, a non-contrast CT scan of thorax with 5 mm slice thickness was done in supine position along with a 4-clamp thermoplastic immobilization cast and head rest (Figure 1B). Radiopaque markers were used to verify the coincidence of the isocentre marking on the cast with the isocentre defined in the planning CT scan. Average imaging parameters were calculated as 110 kVp and 130 mAs for most of the patients. The CT images were imported into the Monaco 5.11 treatment planning system. Window width and window level settings were adjusted to better visualize pulmonary tissue and window level/width equal to −400–600 HU/400–1, 600 HU was mostly applied before contouring. Clinical target volume (CTV) which included bilateral lung tissue was contoured promptly by the radiation oncologist with interpolation and manual edit. No organs at risk (OARs) were delineated during the same settings to reduce the overall treatment time. Heart, spinal cord, thyroid gland, head of humerus, liver, esophagus and breast tissue for female subjects were contoured later on for dosimetric purpose. No dose constraint was given to any other structure. The planning target volume (PTV) was generated by adding 0.7 mm margin around the CTV. The medical physics team planned a 3D conformal radiotherapy with anterior and posterior portals (AP/PA) with 6 MV and or 10 MV photon beams and equal weightage. The prescribed dose was 0.7 Gray (Gy) to bilateral lung fields for single fraction, with at least 95% of PTV receiving 95% of prescription dose. The overall required time since the entry of the patient and plan approval by the principal investigator was 15 min. The delivery of LDRT was executed with patient setup on Elekta Versa HD (Elekta, Stockholm, Sweden) under continuous monitoring with one of the in-room cameras directed to the monitor of the patient for remote surveillance (Figure 1C).

Figure 1 (A) (left) and (B) (right): Show the transfer and CT Simulation of the COVID 19 patients enrolled in our trial, maintaining proper personal protection. (C) Shows the continuous Surveillance and Monitoring of the patient during the delivery of LDRT.
Patients in the non-RT arm were shifted to their respective COVID-19 ward just after CT simulation. The average time from entering to exit the radiation treatment area in the RT arm was 25 min whereas it was 10 min in the non-RT arm. The day of LDRT delivery or day of CT simulation was considered as day 1 and CT scan thorax was again done on days 7 and 28 for comparison of CT severity score of both groups. All the clinical records (general consciousness, temperature, pulse, blood pressure (BP), SpO2 at room air, SpO2 with oxygen, device used to maintain SpO2, FiO2, hematological and biochemical parameters were recorded on days 1, 3, 7, and 14 for both arms. These clinical and biochemical parameters were used to calculate the NEWS-2 score on respective days at a fixed 9:00 h.
The Statistical Package for Social Sciences version 25 (SPSS version 25.0) was used for the data analysis. Descriptive statistics was performed for clinical characteristics and demographic data. The primary endpoint was to compare the NEWS-2 score in both of the groups. Mean [ ± standard deviation (SD)] or median for continuous variables and frequency for categorical variables were reported. Continuous and the categorical data were compared using a 2-tailed Fisher’s exact test. A p-value <0.05 was considered statistically significant.
Results
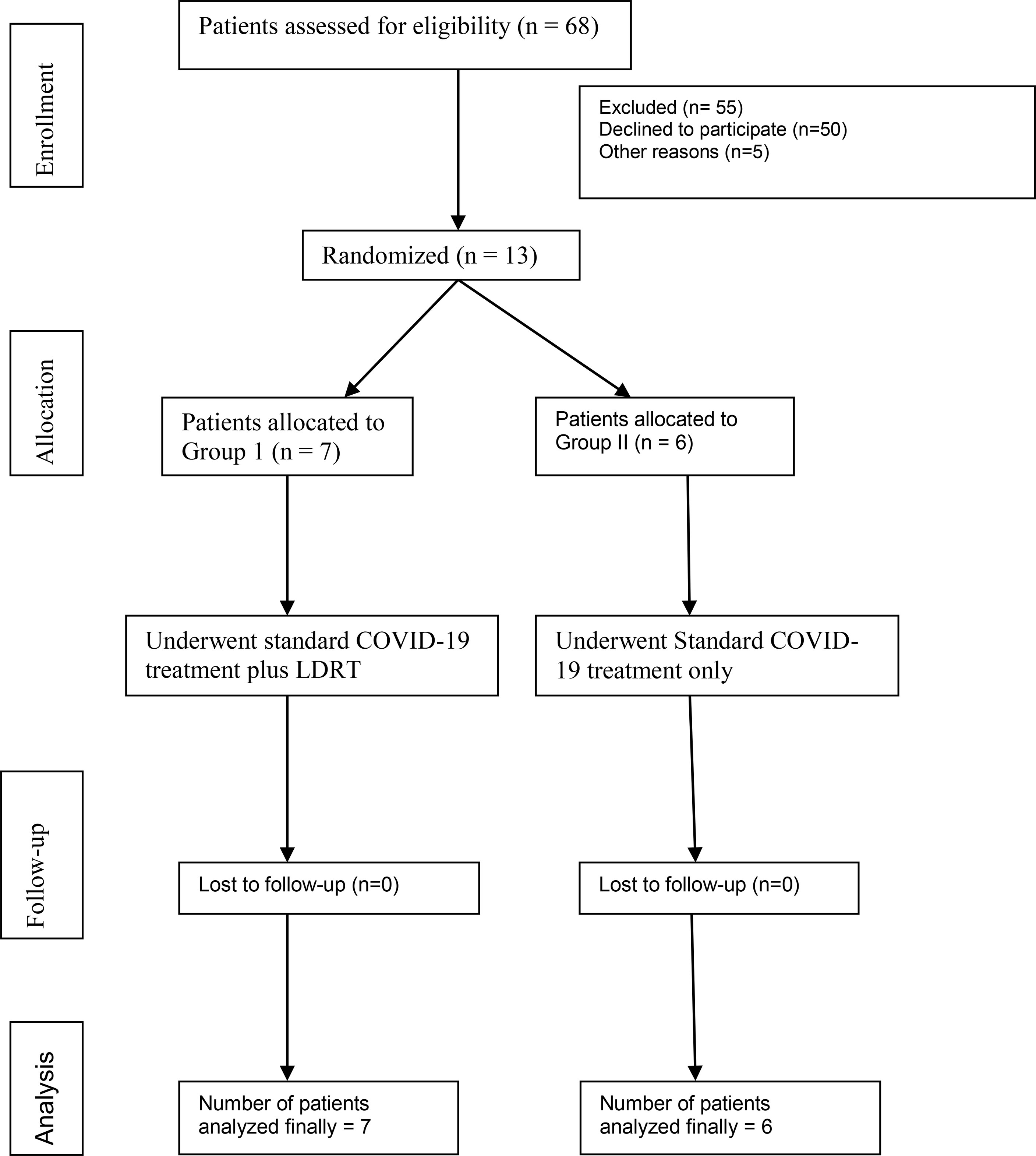
Investigators, residents of the department of radiotherapy, and primary physicians of the COVID-19 ward screened the patients who were admitted for management of COVID-19 at our institute and a total 68 patients with moderate COVID-19 pneumonia were screened for the trial. Patients and their family members were thoroughly explained about the possible side effects including the calculated attributable risk of second malignancy and the ‘off-label’ characteristic of LDRT in the treatment of COVID-19. Difficulty in obtaining consent was the major reason for poor accrual; however at the time of interim analysis we included seven patients in the RT arm and six patients in the non-RT arm (Figure 1). All enrolled patients had the history of less than 10 days since the onset of flu symptoms.
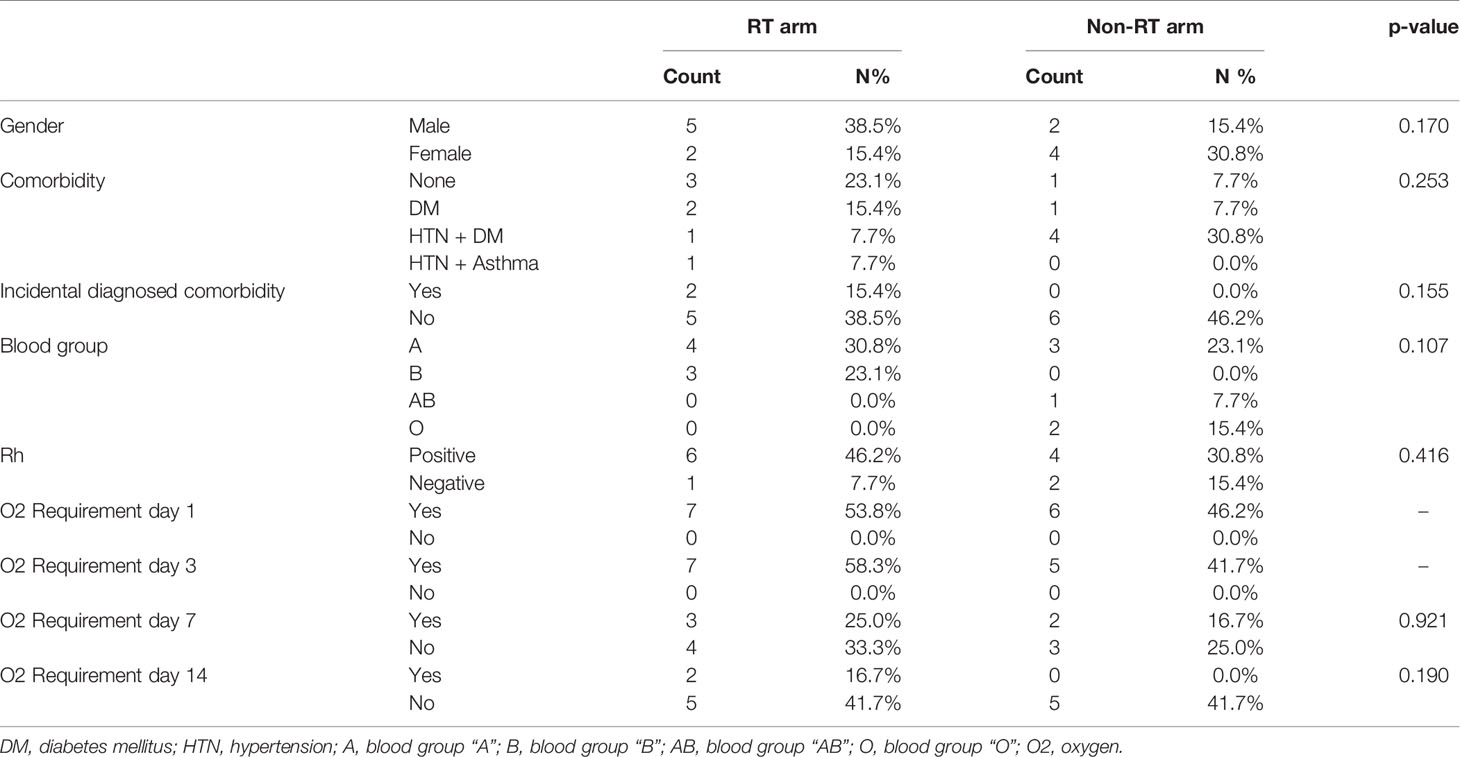
There were two female patients in the RT arm, whereas four female patients were in the non-RT arm. Approximately one-fourth of the patients had given history of smoking in both the arms. Two patients were diagnosed with diabetes mellitus (DM) during the hospital stay for management of COVID-19 while nine (69.2%) patients had known comorbidities (hypertension (HTN), DM, Asthma). Blood group A and Rh positivity were most commonly found in both arms (Table 1).
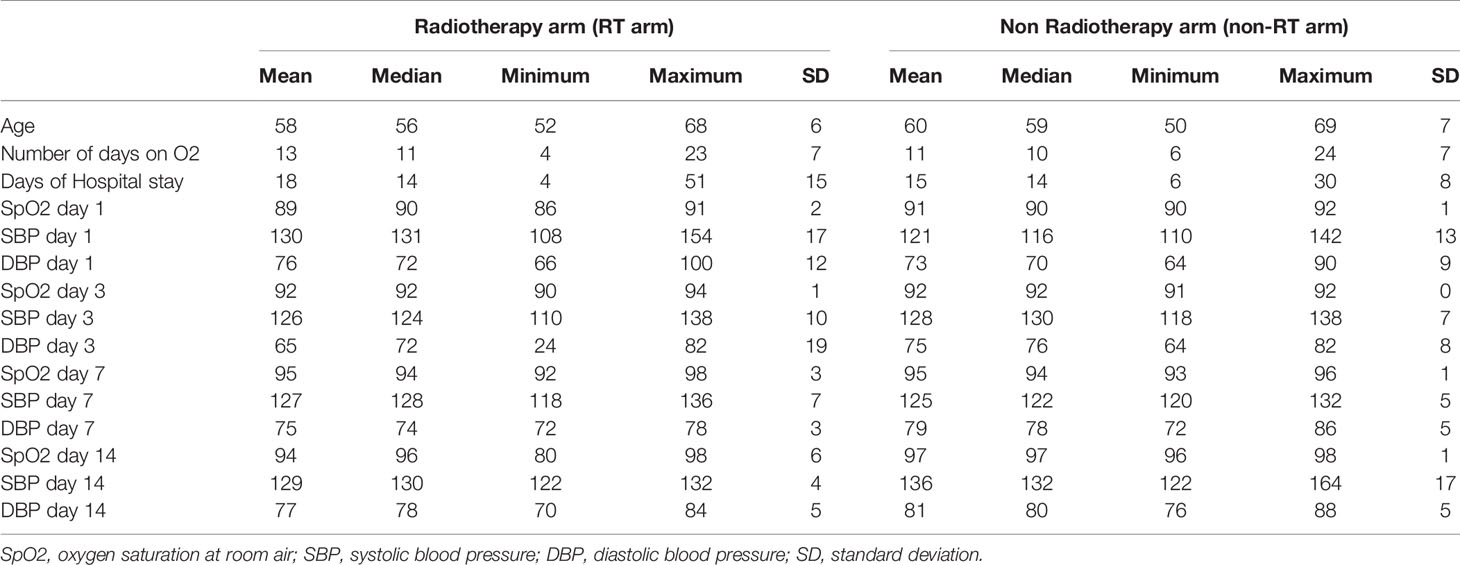
The median age of patients was 56 (52–68) years in the RT arm and 59 (50–69) years in the non-RT arm. The median days to get admitted after onset of symptoms were 5 (3–9 days). The median duration of hospital stay (14 days for both arms) and median duration on oxygen support (11 and 10 days in RT and Non RT arms respectively) were almost equal. The median SpO2 of patients at admission was 90% at room air. Other clinical parameters on days 1, 3, 7, and 14 are depicted in Table 2.
The dose prescribed and delivered to bilateral lungs was 0.7 Gy with 3D-CRT technique. The mean dose received by the OAR, namely, heart, esophagus, thyroid, liver, and spine were 67 cGy,64 cGy, 44 cGy, and 45 cGy, respectively. For female patients mean dose to right and left breast are 46 cGy and 51 cGy respectively.
The institutional management protocol for COVID-19 pneumonia was similar for both groups. All patients in our study received intravenous dexamethasone, enoxaparin, and azithromycin. Dose and duration of the medications were decided by the primary physician at the COVID ward. Remdesivir was given to two patients in the RT arm and four patients in the non-RT arm.
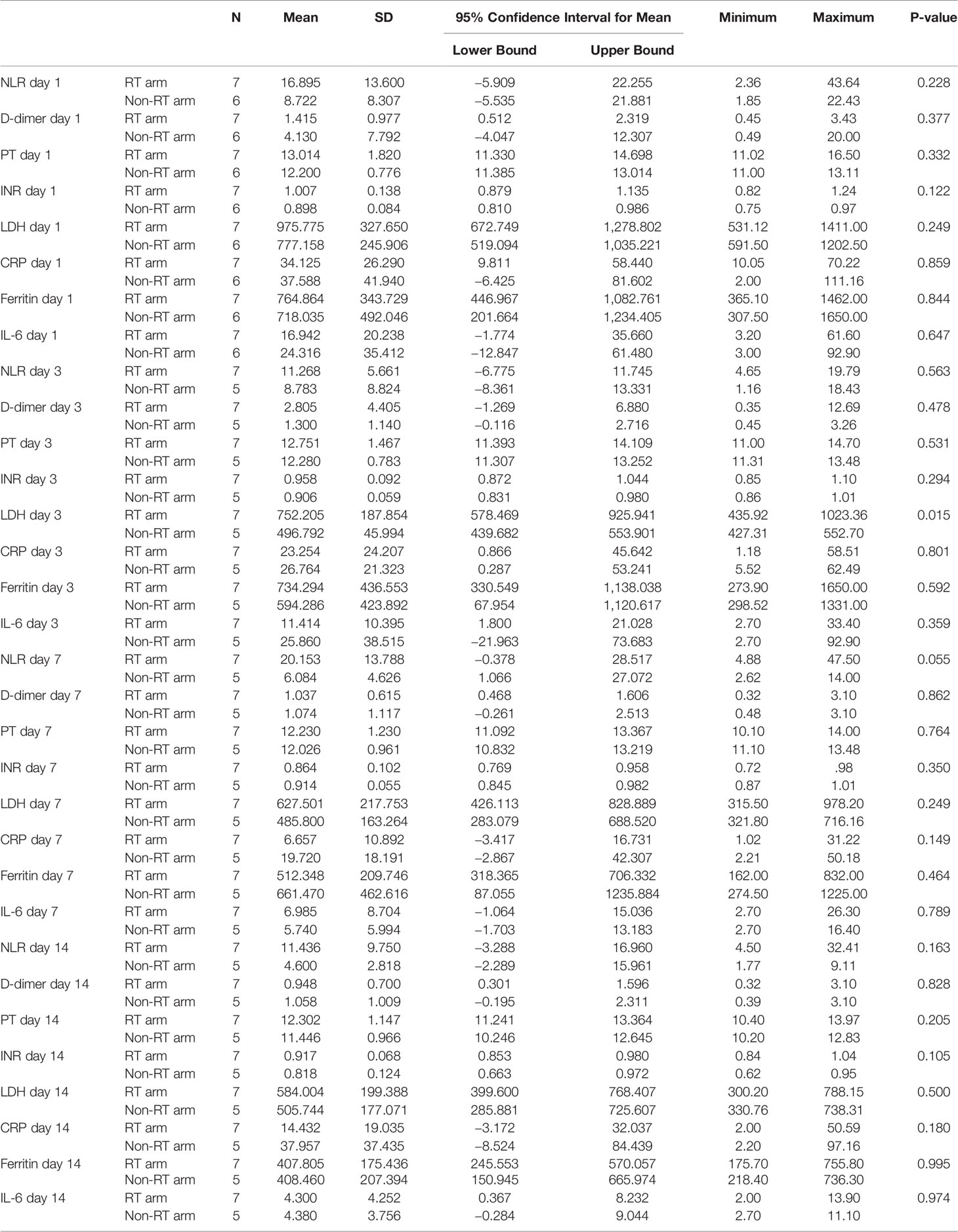
On day 1, the mean value of total leucocyte count (TLC) and neutrophil to lymphocyte ratio (NLR) were 9,160/ml and 16.895 in the RT arm and 12,630/ml and 8.722 in the non-RT arm respectively which showed no statistically significant difference at day 1. Other biochemical parameters, namely, d-dimer, PT, INR, CRP, LDH, IL-6, and ferritin similarly showed no statistically significant difference at day 1 (p >0.05) (Table 3).
There was no statistically significant difference in NEWS-2 score in the RT and non-RT arms at day 3 (p = 0.360), day 7 (p = 0.617), and day 14 (p = 0.506). But there was a tendency of improvement of NEWS-2 score in the RT arm at day 3. There was a statistically significant difference in the improvement of FiO2 (p = 0.042) and SpO2/FiO2 (p = 0.028) at day 3 between the two arms, however this significant difference was gradually lost after the third day (Figure 2 and Table 4). The 25 point CT severity score at enrollment had a median value of 16 and 18 in the RT and non-RT arm respectively. There were no statistically significant differences in the CT severity score in either of the two arms at day 1 (p = 0.325) and day 7 (p = 0.354).
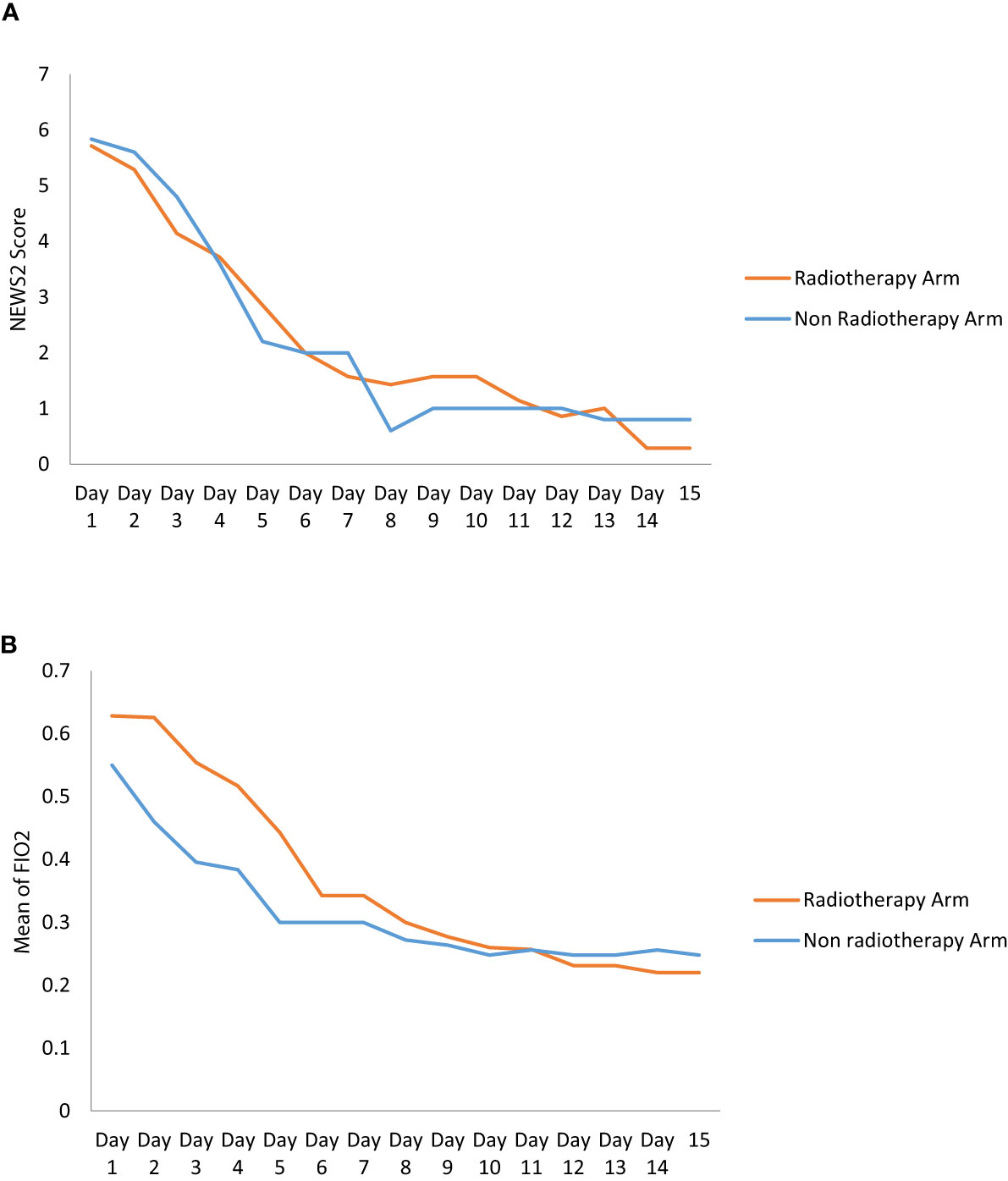
Figure 2 (A) The graph shows the change of NEWS2 Score in RT and Non RT arm on Day 1 and serial follow-up. (B) The Graph shows the change of mean of Fio2 in RT and Non RT arm on Day 1 and serial follow-up.
Radiation induced acute pulmonary toxicity was not observed in any of the patients in the radiation arm. One patient of the RT arm required MV after initial clinical recovery because of the development of sinonasal mucormycosis infection and the patient died in the ICU. A sudden cardiac death was reported in the non-RT arm.
Discussion
Historically, X-ray therapy has been used for non-responsive pneumonia with promising results but had been abandoned with the advent of the newer antibiotic era (11–13). In the present scenario of COVID-19 pandemic, many studies have been re-initiated to evaluate the response of LDRT (0.5 to 1 Gray) to bilateral lungs and have reported rapid relief of respiratory symptoms in terms of decreased requirement of supplemental oxygenation, radiological, and biochemical responses (14–16). LDRT to the lung leads to the polarization of macrophages towards the anti-inflammatory M2 phenotype which helps to decrease the inflammatory process and possible evolvement into ARDS (17). It is therefore postulated that LDRT can also prevent cytokine storm by lymphocyte exhaustion and immune consumption. Reduction in adhesion of mononuclear cells to the endothelial cells and induction of apoptosis by LDRT may also lead to the modulation of inflammatory cell activity in COVID-19 (18, 19),. Hence LDRT delivered to both lungs in such patients is backed up by biological and clinical basis that justify its use as a possible therapeutic option (20). Many clinical studies have since evaluated the role of LDRT to lungs in COVID-19 pneumonia. Most of them are single arm studies and have documented feasibility, tolerability, and clinical benefit (16, 21, 22). However few studies such as that of Papachristofilou et al. demonstrated no benefit of LDRT to improve recovery in critically ill patients requiring MV for COVID-19 pneumonia (23). Available studies have also indicated the importance of the timing of LDRT to bilateral lung fields stating that there is a small window of opportunity after which radiation may not be adequately effective.
To the best of our knowledge this is the only randomized trial with moderate COVID-19 pneumonia patients and analyzed its role to halt the progression of a moderate disease to a severe one with NEWS-2 score. Although the primary physician was not blinded, we feel that the probable impact would have been minimal since all patients received the treatment according to the institutional protocol in the inpatient department as per their clinical severity. Moreover, the CT severity of both arms was scored by a single radiologist who was blinded to reduce bias. The other variables that were evaluated were laboratory tests which would not be impacted by the blinding process.
The dose for whole-lung LDRT was 0.7 Gy. According to a recent evaluation the lifetime attributable risk of cancer for a dose of 0.5 Gy for ages 20–80 can range from 0.29 to 1.7% for men and 0.5–4.9% for women (24). Cardiac events after 5 years of radiotherapy in the study by Darby et al. have shown a linear relationship with increasing dose by 7.4% per Gy (25). Taylor et al. have also shown a significant increase in cardiac mortality after 10 years of radiation to be 0.04% per Gy (26). The mean dose received by the heart in our study is approximately 0.67 Gy hence the probable effect of LDRT on the cardiovascular system needs to be monitored. We are following up the patients at 6 monthly intervals. The history of addictions like smoking in the background of co-morbidities increases the chance of attributable cancer and coronary heart disease. CT based planning with AP/PA portals helped in documenting the serial radiological response (Figure 3) with 25 points CT severity score and also to document the dosimetric profile of LDRT particularly to the adjacent organs such as the liver, heart, thyroid gland, spinal cord, and breasts for female patients. Therefore, we consider this trial as a unique opportunity to measure such dosimetric data which will serve as an important archive on longer follow up, if any recipient of LDRT develops any possible, attributable side effect in the lifetime.
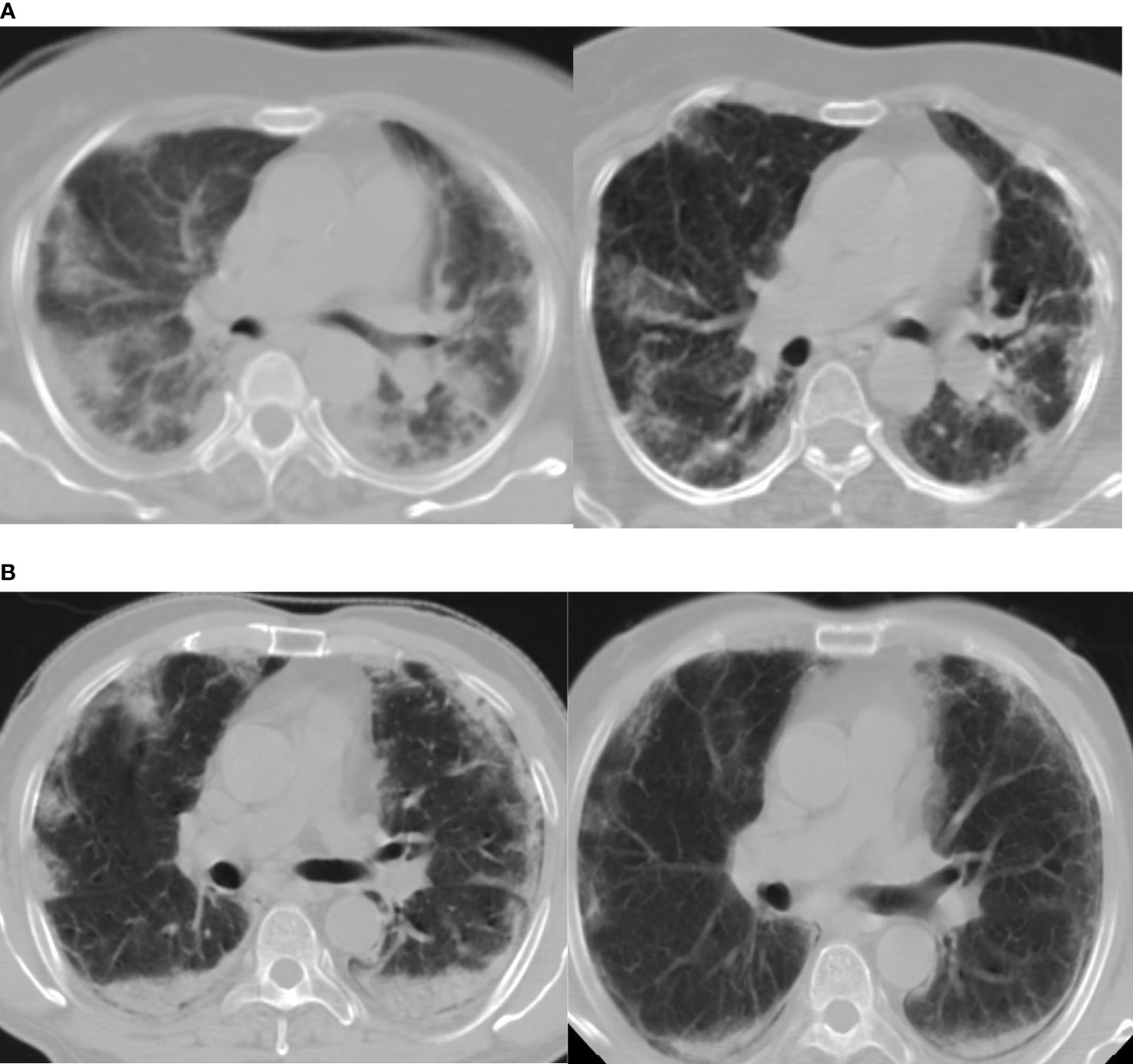
Figure 3 (A, B) Show marked radiological improvement on Day 7, compared to Day 1, of two different patients in the RT arm. Day 1(Left) D7(Right).
The ratio of SpO2/FiO2 significantly decreased on the 3rd day in the LDRT arm which is similar to the other prospective studies but on days 7 and 14 of hospital stay, this significance was lost. A decrease in the level of biochemical markers of acute phase reactants (LDH, CRP, ferritin, and D-dimer) was seen in the RT arm and the non-RT arm. However, it failed to achieve clinical significance on comparison between the two arms.
The median duration of hospital stay is 16.8 days for the RT arm and 28 days in the non-RT arm. Although there was a trend towards early improvement, the difference did not show any statistical difference between the two arms after the third day. Lymphopenia was not seen in both arms suggesting no added deterioration with LDRT (27).
An interim analysis of our study failed to have any statistical improvement in either primary or secondary endpoints although the third day FiO2 was significantly better in the RT arm. Analyzing the two arms NEWS-2 score shows the RT arm continues to improve whereas the non-RT arm plateaus indicating that if more patients are added by virtue of completing the study might be able to reach statistical significance. Therefore, our results are not in complete agreement with the initial experiences reported from the non-randomized single-arm studies, which observed marked clinical, biochemical and radiographic improvement after LDRT for COVID-19 pneumonia.
Limitations
The major limitations of this study include 1) Small sample size as it was extremely difficult to obtain proper consent of the patients and to judiciously select the moderately severe COVID-19 pneumonia patients. Differences were seen only for SpO2/FiO2 and LDH and only at day 3 while other clinical radiological and biochemical parameters failed to attain statistical significance due to very small size at the time of interim analysis. 2) Radiologist who prepared the 25 points CT severity score was blinded; however the treating physician and the team of radiation oncology were not blinded for this study. 3) The course of COVID-19 pneumonia and its clinical, biochemical, and radiological manifestations are itself heterogeneous and may vary between a wide range of spectrum. Similarly, patients with COVID-9 pneumonia respond differently to the standard protocol of the treatment. Hence it is expected that LDRT will not produce a similar response to all patients in the cohort, although we meticulously tried to include moderately affected patients.
Conclusion
LDRT seems to have the potential to prevent moderate COVID-19 pneumonia from deteriorating to severe category. However, further randomized clinical trial with a greater number of such patients is warranted to establish the definitive role of LDRT in the management of COVID-19 pneumonia.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the All India Institute of Medical Sciences, Patna, India. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
PS: Conceptualization, data curation, funding acquisition, investigation, project administration, writing - review and editing. AM: Conceptualization, data curation, investigation, writing - review and editing. DS: Conceptualization, data curation, investigation, software, writing - review and editing. SK: Conceptualization, formal analysis, validation, writing - review and editing. AK: Conceptualization, formal analysis, validation, writing - review and editing. AR: Investion, resources. RR: Investigation, resources. MV: Investigation, resources. DKR: Conceptualization, investigation. DB: Conceptualization, investigation. AS: Software validation. ASi: Investigation, software. RS: Investigation, resources. ASa: Investigation, resources. AT: Investigation, resources. AKB: Investigation, supervision, SC: Investigation, supervision. RK: Investigation, resources, PK: Investigation, resources, BM: Investigation, resources. AR: Statistical analysis. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
Intramural funding with no I- 27/621 was provided from All India Institute of Medical Sciences Patna.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA (2020) 323(13):1239–42. doi: 10.1001/JAMA.2020.2648
2. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in Critically Ill Patients in the Seattle Region — Case Series. N Engl J Med (2020) 382(21):2012–22. doi: 10.1056/NEJMOA2004500
3. de Souza R, Mhatre S, Qayyumi B, Chitkara G, Madke T, Joshi M, et al. Clinical Course and Outcome of Patients With COVID-19 in Mumbai City: An Observational Study. BMJ Open (2021) 11(5):e042943. doi: 10.1136/BMJOPEN-2020-042943
4. Gray WK, Navaratnam AV, Day J, Babu P, Mackinnon S, Adelaja I, et al. Variability in COVID-19 in-Hospital Mortality Rates Between National Health Service Trusts and Regions in England: A National Observational Study for the Getting It Right First Time Programme. EClinicalMedicine (2021) 35:100859. doi: 10.1016/J.ECLINM.2021.100859
5. WHO Recommends Against the Use of Remdesivir in COVID-19 Patients. Available at: https://www.who.int/news-room/feature-stories/detail/who-recommends-against-the-use-of-remdesivir-in-covid-19-patients (Accessed October 16, 2021).
6. WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for Covid-19 — Interim WHO Solidarity Trial Results. N Engl J Med (2020) 384(6):497–511. doi: 10.1056/NEJMOA2023184
7. Myrstad M, Ihle-Hansen H, Tveita AA, Andersen EL, Nygård S, Tveit A, et al. National Early Warning Score 2 (NEWS2) on Admission Predicts Severe Disease and in-Hospital Mortality From Covid-19 - a Prospective Cohort Study. Scand J Trauma Resusc Emergency Med (2020) 28(1):66. doi: 10.1186/s13049-020-00764-3
8. Carr E, Bendayan R, Bean D, Stammers M, Wang W, Zhang H, et al. Evaluation and Improvement of the National Early Warning Score (NEWS2) for COVID-19: A Multi-Hospital Study. BMC Med (2021) 19:23. doi: 10.1186/s12916-020-01893-3
9. National Cancer Institute (US), Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events:(CTCAE). V5. Cancer Therapy Evaluation Program (2017) Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (Accessed October16, 2021).
10. Available at: https://www.mohfw.gov.in/pdf/UpdatedDetailedClinicalManagementProtocolforCOVID19adultsdated24052021.pdf (Accessed on 28 April 2021).
11. Available at: https://aiimspatna.org/advertisement/Covid_SOP_AIIMSP_version_2_01.pdf.
12. Fried DC. Die Röntgenbestrahlung Akuter und Subakuter Eitriger Entzündungen. Acta Radiologica (2016) 9(2):109–16. doi: 10.1177/028418512800900201
13. Scott WR. X-Ray Therapy in the Treatment of Acute Pneumonial. Radiology (1939) 33(3):331–49. doi: 10.1148/33.3.331
14. Calabrese EJ, Dhawan G. How Radiotherapy Was Historically Used To Treat Pneumonia: Could It Be Useful Today? Yale J Biol Med (2013) 86(4):555.
15. Ameri A, Rahnama N, Bozorgmehr R, Mokhtari M, Farahbakhsh M, Nabavi M, et al. Low-Dose Whole-Lung Irradiation for COVID-19 Pneumonia: Short Course Results. Int J Radiat Oncol Biol Phys (2020) 108(5):1134. doi: 10.1016/J.IJROBP.2020.07.026
16. Sanmamed N, Alcantara P, Cerezo E, Gaztañaga M, Cabello N, Gómez S, et al. Low-Dose Radiation Therapy in the Management of Coronavirus Disease 2019 (COVID-19) Pneumonia (LOWRAD-Cov19): Preliminary Report. Int J Radiat Oncol Biol Phys (2021) 109(4):880–5. doi: 10.1016/J.IJROBP.2020.11.049
17. Hess CB, Buchwald ZS, Stokes W, Nasti TH, Switchenko JM, Weinberg BD, et al. Low-Dose Whole-Lung Radiation for COVID-19 Pneumonia: Planned Day 7 Interim Analysis of a Registered Clinical Trial. Cancer (2020) 126(23):5109–13. doi: 10.1002/CNCR.33130
18. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult Haemophagocytic Syndrome. Lancet (2014) 383:1503–16. doi: 10.1016/S0140-6736(13)61048-X. Elsevier B.V.
19. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet (2020) 395(10229):1033–4. doi: 10.1016/S0140-6736(20)30628-0
20. Lara PC, Burgos J, Macias D. Low Dose Lung Radiotherapy for COVID-19 Pneumonia. The Rationale for a Cost-Effective Anti-Inflammatory Treatment. Clin Transl Radiat Oncol (2020) 23:27–9. doi: 10.1016/j.ctro.2020.04.006
21. Sharma DN, Guleria R, Wig N, Mohan A, Rath G, Subramani V, et al. Low Dose Radiation Therapy for COVID-19 Pneumonia: A Pilot Study. Br J Radiol (2021) 94(1126):20210187. doi: 10.1259/bjr.20210187
22. Ganesan G, Ponniah S, Sundaram V, Marimuthu PK, Pitchaikannu V, Chandrasekaran M, et al. Whole Lung Irradiation as a Novel Treatment for COVID-19: Final Results of the Prospective Randomized Trial (WINCOVID Trial). Radiother Oncol (2021) 167:133–42. doi: 10.1016/j.radonc.2021.12.024
23. Papachristofilou A, Finazzi T, Blum A, Zehnder T, Zellweger N, Lustenberger J, et al. Low Dose Radiation Therapy for Severe COVID-19 Pneumonia: A Randomized Double-Blind Study. Int J Radiat Oncol Biol Phys (2021) 110(5):1274e1282. doi: 10.1016/j.ijrobp.2021.04.029
24. García-Hernández T, Romero-Expósito M, Sánchez-Nieto B. Low Dose Radiation Therapy for COVID-19: Effective Dose and Estimation of Cancer Risk. Radiother Oncol (2020) 153:289–95. doi: 10.1016/j.radonc.2020.09.051
25. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of Ischemic Heart Disease in Women After Radiotherapy for Breast Cancer. N Engl J Med (2013) 368:987–98. doi: 10.1056/NEJMoa1209825
26. Taylor C, Correa C, Duane FK, Aznar MC, Anderson SJ, Bergh J, et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence From Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J Clin Oncol (2017) 35:1641–9. doi: 10.1200/JCO.2016.72.0722
Keywords: COVID-19, low dose radiation, CT severity score, NEWS 2, COVID pneumonia
Citation: Singh P, Mandal A, Singh D, Kumar S, Kumar A, Rakesh A, Ranjan R, Verma M, Rai DK, Bhushan D, Shankar A, Sinha A, Saini R, Saha A, Thovarayi A, Baral AK, Chauhan S, Kumar R, Kakoty P, Modak B and Ranjan A (2022) Interim Analysis of Impact of Adding Low Dose Pulmonary Radiotherapy to Moderate COVID-19 Pneumonia Patients: IMpaCt-RT Study. Front. Oncol. 12:822902. doi: 10.3389/fonc.2022.822902
Received: 26 November 2021; Accepted: 22 February 2022;
Published: 29 March 2022.
Edited by:
Alessio Bruni, University Hospital of Modena, ItalyReviewed by:
Kurtuluş Aksu, University of Health Sciences Atatatürk Chest Diseases and Thoracic Surgery Training and Research Hospital, TurkeyElisabeth Cardis, Instituto Salud Global Barcelona (ISGlobal), Spain
Copyright © 2022 Singh, Mandal, Singh, Kumar, Kumar, Rakesh, Ranjan, Verma, Rai, Bhushan, Shankar, Sinha, Saini, Saha, Thovarayi, Baral, Chauhan, Kumar, Kakoty, Modak and Ranjan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pritanjali Singh, drpritanjalis@gmail.com
 Pritanjali Singh
Pritanjali Singh Avik Mandal
Avik Mandal Dharmendra Singh
Dharmendra Singh Subhash Kumar2
Subhash Kumar2 Amarjeet Kumar
Amarjeet Kumar Amrita Rakesh
Amrita Rakesh Rakesh Ranjan
Rakesh Ranjan Abhishek Shankar
Abhishek Shankar Arkaprava Sinha
Arkaprava Sinha Rohit Saini
Rohit Saini Arijit Saha
Arijit Saha Ashwin Thovarayi
Ashwin Thovarayi Rajhans Kumar
Rajhans Kumar Priya Kakoty
Priya Kakoty Bithika Modak
Bithika Modak