- 1Department of Epidemiology and Health Statistics, School of Public Health, Wuhan University, Wuhan, China
- 2Population and Health Research Center, Wuhan University, Wuhan, China
Prenatal stress can affect pregnant women in an epigenetic way during the critical period of conception of their offspring. The study aims to investigate the relationship between peritraumatic distress, prenatal perceived stress, depression, and glucocorticoid receptor (NR3C1) DNA methylation among pregnant women who experienced COVID-19 lockdown in China. Study data were collected from 30 pregnant women in Wuhan and Huanggang, China. The Peritraumatic Distress Inventory was used to measure peritraumatic distress, the Edinburgh Postnatal Depression Scale was used to measure depressive symptoms, and the Perceived Stress Scale was used to measure perceived stress. DNA methylation in the exon 1F promoter region of NR3C1 gene from the venous blood mononuclear cell genome was characterized by bisulfite sequencing. Correlation and linear regression were used for data analysis. The mean level of peritraumatic distress, perceived stress, and depression was 6.30 (SD = 5.09), 6.50 (SD = 5.41), and 6.60 (SD = 4.85), respectively, with 23.33% of pregnant women being depressed. The mean NR3C1 methylation was 0.65 (SD = 0.22). Prenatal depression was positively correlated with the degree of methylation in venous blood from the mother (r = 0.59, p = 0.001), and depression predicted methylation of NR3C1 gene at the CpG 8 site (β = 0.05, p = 0.03). No association was found between peritraumatic distress as well as perceived stress and methylation of NR3C1. NR3C1 gene was susceptible to epigenetic modification of DNA methylation in the context of prenatal stress, and maternal depression was associated with increased NR3C1 methylation among women who experienced COVID-19 lockdown.
Introduction
In December 2019, the outbreak of coronavirus disease-2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) occurred in Wuhan, China (1–3). To slow down and finally end the spread of COVID-19, the Chinese government adopted a measure of lockdown in areas with outbreaks. Previous studies suggested that stress caused by negative events, such as COVID-19 lockdown, can affect vulnerable people, particularly pregnant women (4, 5). Data from several countries showed higher levels of maternal stress and more pronounced increases in depression, anxiety, and negative emotions during the COVID-19 pandemic (6–8). A multicenter study conducted in China also showed similar results (9).
Poor mental health status (e.g., prenatal stress and depression) during pregnancy has been reported to be associated with many negative health outcomes, including poor nutrition (10), suicidality (11) for mothers, preterm delivery (12), and impaired cognitive development (13) for offspring. One study conducted in Peru indicated that depressed pregnant women had a higher risk of suicidal ideation than non-depressed pregnant women (14). Previous research has shown that certain obstetric complications (15) and lower exclusive breastfeeding rates (16) were directly related to prenatal maternal stress, such as perceived stress and depression. Obstetric complications due to prenatal stress and depression include miscarriage (17), preeclampsia (18), congenital malformations (19), preterm birth (18), and low birth weight of the fetus (20). Thus, it is essential to pay more attention to the mental health status of pregnant women.
However, the underlying mechanisms by which prenatal poor mental health causes these negative health outcomes are still being debated. Disturbances in the activity of the hypothalamic–pituitary–adrenal (HPA) axis may be used as a theoretical basis for understanding the adverse outcomes. During pregnancy, women are exposed to various stressors that may be related to HPA activation. In studies of major depressive disorder, an increase in HPA axis activity was found in depressed patients with elevated basal cortisol (21, 22). The affinity of glucocorticoid receptors (GRs) for cortisol is relatively low and therefore is primarily responsive to continuous and greater levels of cortisol (23). The HPA axis can be activated throughout a lifetime by psychosocial stress (7). Sustained stressful conditions have chronic and ultimately damaging effects on the body’s stress regulation through the downregulation of GR by epigenetic mechanisms (24).
Recent studies have shown that the link between maternal prenatal mental health status and HPA axis function may be mediated by DNA methylation, a possible epigenetic mechanism that eventually leads to adverse outcomes for the mother (25) and infant (26). DNA methylation regulates gene expression but does not change the sequence of DNA. The methyl group is transferred to the C5 position of cytosine, forming 5-methylcytosine, which is the process of DNA methylation. DNA methylation within the C-phosphate-G (CpG) dinucleotide context is a pivotal factor in regulating gene expression. Previous studies have found that several DNA methylation changes may be related to the function of the fetal HPA axis (27), including the nuclear receptor subfamily 3, group C, member 1 (NR3C1) DNA methylation (28). GR production is encoded through NR3C1, which contributes to the inhibition of cortisol production. NR3C1 DNA methylation homeostasis maintains the normal function of the HPA axis negative feedback loop and protects the fetus from excessive cortisol exposure (29). Empirical studies showed that prenatal stress led to methylation of NR3C1 gene in both mother and fetus and that elevated methylation was associated with poor fetal outcomes and risk of disease in offspring’s adulthood (30, 31). Another study in the United States also found that maternal depression during pregnancy was associated with increased placental NR3C1 CpG 2 methylation and higher levels of infant lethargy, lower muscle tone, and poorer self-regulation (32).
Previous studies on the effects of stress on maternal DNA methylation mainly focused on the stress of war in war-torn African countries (30, 33) or the stress of discrimination among Latina mothers in the United States (34, 35). COVID-19 presents a new challenge to humanity as a completely new disease that causes stress unlike the above types of stress. With regard to the first COVID-19 wave pandemic, which resulted in a large number of infected individuals and deaths, considerable uncertainty remains about the characteristics of the virus and the infection. Against this background, the Chinese government took measures to lock down the city to stop the spread of SARS-CoV-2. During the lockdown period, the residents confined their activities to their households. The lack of an effective vaccine and treatment during the first COVID-19 wave also increased the public’s fear of infection. A study has shown that prenatal stress related to COVID-19 is associated with methylation of the gene in infants (36) and that it is possible that the COVID-19-related stress exposure during this special period may also exert effects on the NR3C1 DNA methylation in pregnant women.
In the present study, we aimed to investigate whether and to what extent prenatal peritraumatic distress, perceived stress, and depression in Chinese pregnant women during COVID-19 lockdown affect maternal NR3C1 DNA methylation. The findings of the study will provide valuable evidence for future effective intervention and prevention programs targeting poor mental health among pregnant women in the context of the COVID-19 pandemic or other public health emergencies.
Materials and methods
Participants and sampling
The study included 30 randomly selected participants from the Maternal and Child Health Cohort Study of 293 pregnant women (37). Participants in the study were pregnant women who were 18–49 years old, at their second and third trimesters of pregnancy, without a diagnosis of mental disorders and medical complications prior to and during pregnancy. All mothers who participated in this study tested negative for COVID-19. These participants were recruited from Huganggang Maternal and Child Health Hospital and Wuhan Zhenai Maternal Hospital from May to July 2020. Between late January and early April 2020, residents of both Wuhan and Huanggang experienced a lockdown period of approximately 2 months. Wuhan is the capital city of Hubei Province, with a population of 11.21 million and a per capita GDP of 145545 RMB. Huanggang City is adjacent to Wuhan with a population of 6.33 million per capita GDP of 36,685 RMB. Only participants who agreed to participate and signed the informed consent were enrolled, and the participation was voluntary and confidential. Pregnant women have the right to withdraw or quit at any time. The differences in key outcome variables between the two cities were compared, and no significant differences were found.
Data collection
The study data consisted of two parts, including a survey (maternal mental health survey) and blood samples from mothers. The survey was delivered through a paper–pencil approach. Participants were asked to complete the survey independently in a private room. The trained data collectors were prepared to provide help if needed. Upon completion, a data collector would double-check the questionnaire and make sure every part was completed.
Regarding the blood samples, venous blood samples from mothers were collected on the day of childbirth. Venous blood pretreated with EDTA was transferred to lyophilized tubes and then placed in a −80°C refrigerator. Upon completing the survey and collecting blood samples, participants would receive a reward of 30 RMB. The study was approved by Wuhan University Medical Ethics Committee.
Measures
Peritraumatic distress inventory
The maternal peritraumatic distress related to the pandemic was measured using the Peritraumatic Distress Inventory (PDI) (38). The scale consists of 13 items, and typical items include “I felt sadness and grief” and “I thought I might die”. Each item is measured using a 5-point Likert scale ranging from “0 = not at all true” to “4 = extremely true”. The total score of PDI was calculated with a higher score indicating severer peritraumatic distress in pregnant women. Cronbach’s alpha of PDI was 0.84 in the study.
Perceived stress
The maternal perceived stress was measured using the Perceived Stress Scale (PSS) (39). The scale consists of 10 items, and typical items include “In the last month, how often have you felt that you were unable to control the important things in your life?” and “In the last month, how often have you been able to control irritations in your life?”. Each item is measured using a 5-point Likert scale ranging from “0 = never” to “4 = very often”. The total score of PSS was calculated with a higher score indicating greater stress perceived by pregnant women. Cronbach’s alpha of PSS was 0.93 in the study.
Depression
Maternal depression was measured using the Edinburgh Postnatal Depression Scale (EPDS) (40). The scale consists of 10 items, and typical items include “I have been able to laugh and see the funny side of things” and “I have blamed myself unnecessarily when things went wrong”. Each item is measured using a 4-point Likert scale ranging from “0 = not at all” to “3 = very often”. The total score of EPDS was calculated with a higher score indicating a greater level of depressive symptoms. The EPDS score was categorized into depression and non-depression with a cutoff value of 10. Cronbach’s alpha of EPDS was 0.65 in the study.
Demographic variables
The demographic variables in the study included age (in years and categorized into ≤25, 26–30, and >30), city (Wuhan/Huanggang), residence (rural/urban), education (middle school or lower, high school, and college or higher), husband’s education (middle school or lower, high school, and college or higher), employment status (full-time job and part-time job/others), monthly income (in RMB, ≤2,000, 2,001–5,000, and >5,000), annual household income (in RMB, ≤10,000, 10,001–20,000, and >20,000), and family size (in the number of persons, ≤3, 4, and ≥5).
DNA extraction and bisulfite modification
Venous blood samples collected from 30 mothers were used to assess the methylation of the GR-1F promoter of the NR3C1 exon. Genomic DNA was extracted using the TIANamp Blood DNA Midi Kit (Tiangen, China). Quantification of purified DNA was carried out using an ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA). Samples of DNA (500 ng) were bisulfated using the QIAGEN DNA methylation kit (QIAGEN, Valencia, CA, USA) and stored at −80°C.
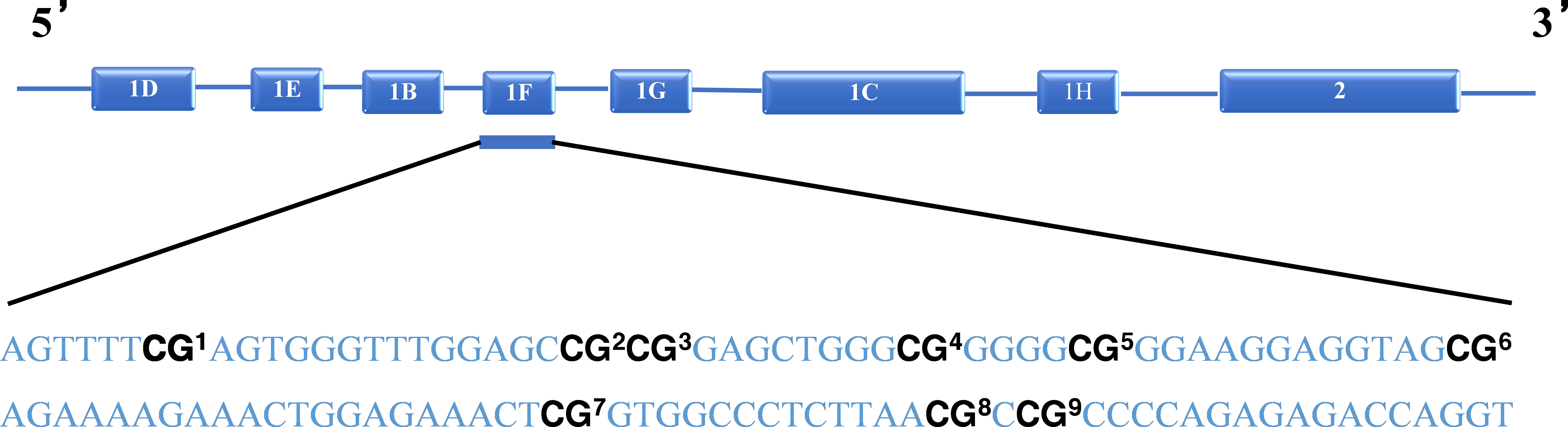
Cytosine methylation assessment
Nine C-phosphate-G (CpG) sites of the GR-1F promoter of NR3C1 were used as the target CpG site of methylation (Figure 1). The number of methylated clones per CpG site was converted to percentages, and the methylation percentages of the nine CpG sites were summed as a total methylation percentage. The primers for amplification were forward: 5′-GTTAAGGGGTAGAGCGAGT TTT-3′, and reverse: 5′-AAAACCGACCTAATCTCTCTAAAAC-3′. A bisulfite conversion control was used for each sequencing read for NR3C1. In order to call the methylation level of the samples, for all samples analyzed, the bisulfite conversion rate was >95%. A triplicate of all assays was carried out on the identical DNA template converted by bisulfite. In case any of the replicates varied by >10%, the assay was repeated for that sample.
Statistical analysis
Descriptive analyses (e.g., frequency, percentage, mean, and standard deviation) were used to describe the sample characteristics. Student’s t-test and ANOVA were used to compare the methylation levels across demographic variables and different levels of depression. Correlation analysis was used to associate the peritraumatic distress, perceived stress, depression, and NR3C1 gene methylation. Mixed-effects modeling analysis was used to investigate the association between peritraumatic distress, perceived stress, depression, and methylation in different CpG sites when controlling for covariates and including sampling location (Wuhan and Huanggang) as a random effect. The false discovery rate (FDR) method was performed to adjust the multiple testing. All statistical analyses were performed using the IBM SPSS version 25.0 (IBM Corp, New York, USA).
Results
Characteristics of the study sample
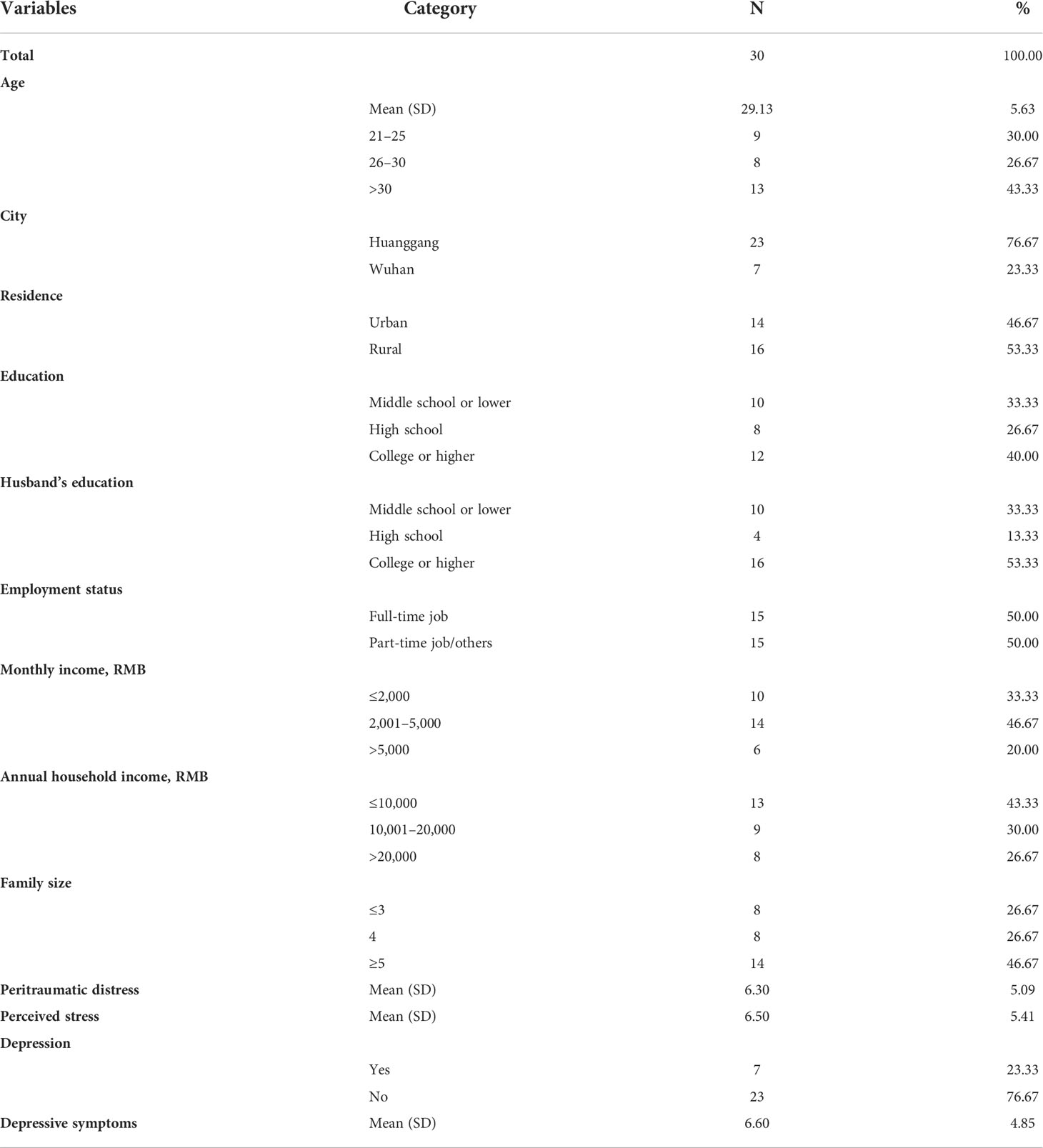
Results in Table 1 show that the mean age of the study sample was 29.13 years (SD = 5.63). In total, 76.67% of participants came from Huanggang City. More than half of the participants (53.33%) came from rural areas, 40.00% had a college education or higher, 53.33% of the pregnant women’s husbands had a college education or higher, and 50% had a full-time job. Approximately 80% of participants earned less than 5,000 RMB (equivalent to $770) per month, only 26.67% had an annual household income >20,000 RMB (equivalent to $3,090), and 26.67% had a family size of ≤3 and 46.67% with ≥5. The mean peritraumatic distress of mothers was 6.30 (SD = 5.09). The mean perceived stress was 6.50 (SD = 5.41). The mean depression of mothers was 6.60 (SD = 4.85), and a total of 23.33% were depressed.
Differences in NR3C1 DNA methylation across demographic groups
Results in Table 2 show that among the total sample, the mean NR3C1 gene methylation was 0.65 (SD = 0.22). There were no significant differences in NR3C1 DNA methylation across demographic groups.
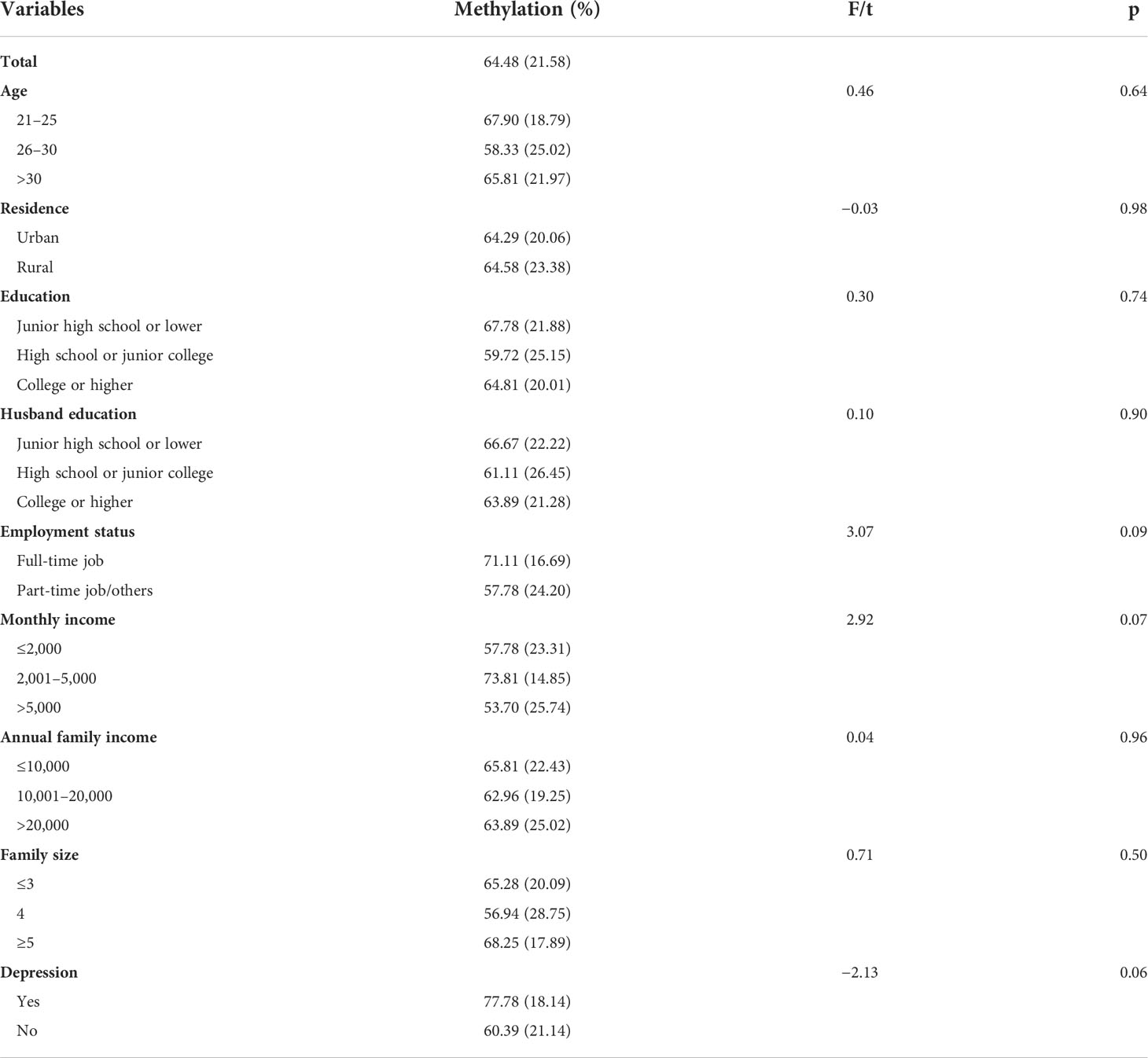
Table 2 Levels of NR3C1 methylation among pregnant women, overall and by demographic groups, and levels of depression.
Associations between NR3C1 DNA methylation and peritraumatic distress, perceived stress, and depression
Results in the lower panel of Table 2 show that pregnant women who were depressed had a higher NR3C1 DNA methylation level than women who were not depressed with a marginal significance level (t = −2.13, p = 0.06).
Results in Figures 2A, B show that NR3C1 methylation was not significantly correlated with peritraumatic distress (r = −0.09, p = 0.67) and perceived stress (r = 0.12, p = 0.54). Results in Figure 2C show that NR3C1 methylation was significantly correlated with depression (r = 0.59, p = 0.001).
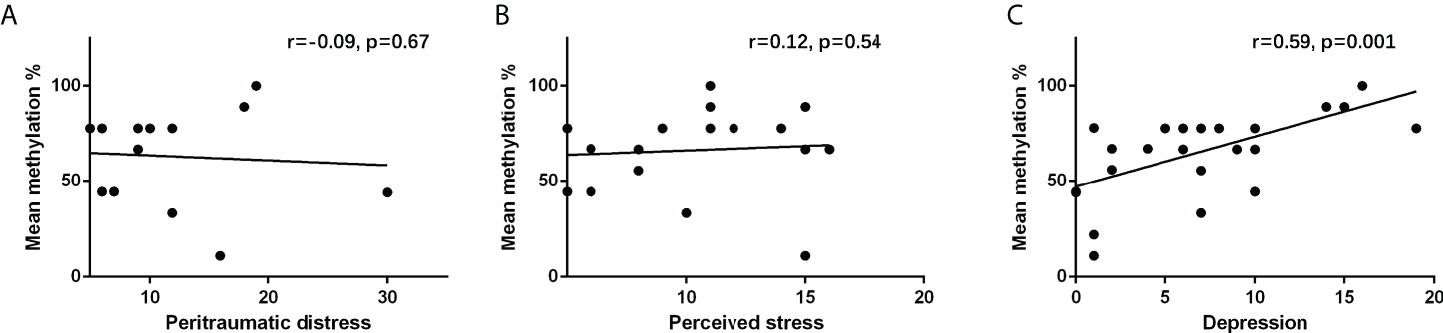
Figure 2 Correlation between (A) peritraumatic distress and NR3C1 gene methylation, (B) perceived stress and NR3C1 gene methylation, and (C) depression and NR3C1 gene methylation.
Results in Table 3 show that none of them were associated with peritraumatic distress or perceived stress among nine CpG sites in NR3C1 DNA promotor. Still, one of them (CpG 8) was found to be significantly associated with depression (β = 0.05 p = 0.03, R2 = 0.21).
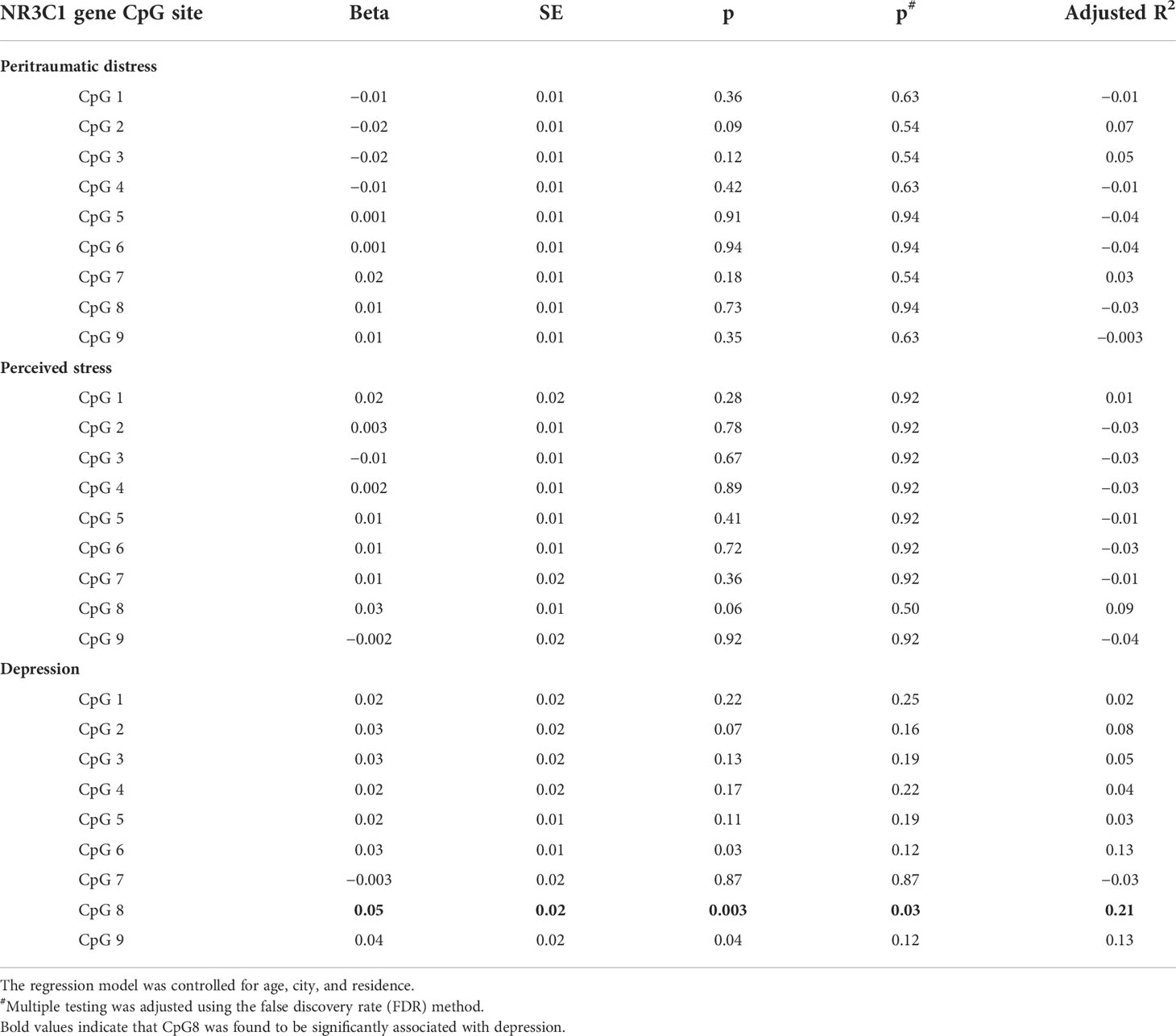
Table 3 Effects of peritraumatic distress, perceived stress and depression on NR3C1 gene CpG sites methylation among pregnant women.
Discussion
To the best of our knowledge, this is the first study to investigate the relationship between prenatal stress, depression, and methylation of NR3C1 gene in venous blood of pregnant Chinese women who experienced COVID-19 lockdown. The results of this study add new data to deepen our understanding of the role of maternal mental health in maternal gene methylation changes during infectious disease epidemics.
Some studies reported that COVID-19 lockdown causes elevated stress and depression in pregnant women (5–8), but we did not find elevated levels of maternal stress and depression as compared to foreign studies (7, 41–43) as well as studies conducted in China before the COVID-19 pandemic (44, 45). These findings suggest that the citywide lockdown did not significantly increase prenatal stress and depression. A possible explanation may include that Hubei Province and Wuhan City received great social, emotional, and instrumental support nationwide, improving their capacity against the COVID-19-related stress (46, 47). In addition, in the context of traditional Chinese values, pregnant women in China receive more care and social support from their families and society, thus, they can better cope with stress (48). The study’s findings indicated that prenatal peritraumatic distress or perceived stress was not significantly associated with maternal NR3C1 gene methylation. This is inconsistent with a previous study conducted in the Democratic Republic of Congo that showed a significant association between mean methylation and peritraumatic distress or mundane stress (30). Furthermore, a study conducted in the United States showed a significantly strong association between everyday discrimination and NR3C1 gene methylation (35). Meanwhile, many studies conducted in Africa also found that prenatal war stress was associated with NR3C1 gene methylation (30, 33). It is likely that the difference may be attributable to the type and level of stress experienced by the women in this study. The pregnant Chinese women in this study experienced stress during the COVID-19 lockdown period, but not as much as the stress of war in Africa or the stress of discrimination experienced by Latina women in the United States. Although the effect of peritraumatic distress and perceived stress on DNA methylation was insignificant, as a prenatal stressor, maternal stress, if not being coped with appropriately, may subsequently lead to a range of adverse health effects on both the mothers and their fetus.
Compared with prenatal stress, maternal prenatal depression showed a different map. The findings showed that pregnant women who were depressed had significantly higher levels of NR3C1 gene methylation. There may be a dose–response effect, with higher levels of methylation in pregnant women with more severe depressive symptoms. However, a recent study conducted among 163 mother–infant dyads in the United States reported that methylation of NR3C1 gene was not associated with depressive symptoms (49). The sample used in the US study was cord blood, whereas venous blood from the mother was used in our study. The difference in tissues may have contributed to the inconsistent results. Further, our study demonstrated a correlation between prenatal depression exposure and DNA methylation at a specific CpG 8 site of NR3C1 gene. No associations between other CpG sites and methylation levels were found. The analytic sites for NR3C1 gene in the US study were in the CpG island shore region, whereas our analytic site was in the exon 1F promoter region of NR3C1 gene. The difference in detection sites may affect the results.
The relationship between NR3C1 methylation and depression has been validated in studies of mothers and their infants (29, 32), with children of mothers with depression having higher DNA methylation of NR3C1 gene than children of mothers without depression. While most studies have focused on the relationship between maternal prenatal mental health and methylation of NR3C1 gene in the offspring, the present study provided an alternative approach to investigate the relationship between prenatal depression and maternal NR3C1 methylation. The methylation changes in the offspring have resulted from persistent elevations in cortisol caused by dysfunction of the mother’s HPA axis, which may be attributable to the maternal gene methylation.
In the present study, we also examined whether sociodemographic factors were associated with DNA methylation of NR3C1, but we found that maternal NR3C1 DNA methylation was not associated with age, residence, education, husband’s education, employment status, monthly income, annual family income, or family size. The results may be attributed to the relatively homogenous sample in gene levels, although they were differed in sociodemographic factors.
There are several limitations to this study. Firstly, the study was conducted among 30 pregnant women, which is a limited sample size. Although many studies on methylation have relatively similar sample sizes (50–52), a larger sample size will be more convincing to interpret the results. Despite the limited sample size, the range of reported depressive symptom scores was broad. Some mothers scored very low on depression, suggesting that the sample was not exclusive to highly depressed mothers. Secondly, peritraumatic distress, perceived stress, and depression were measured using self-reported scales, so recall bias may exist. Finally, our study examined only nine CpG sites across CpG islands in the NR3C1 exon. The possibility that prenatal depression may have differential effects on different CpG sites could be optimized by using genome-wide bisulfite sequencing in future studies.
The results of this study showed for the first time that prenatal maternal depression was associated with the methylation of NR3C1 gene among pregnant women in China. Further studies in a longitudinal cohort are needed to understand the subsequent effects of prenatal mental health of pregnant women who experienced COVID-19 lockdown on maternal and fetal outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee at Wuhan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HY had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: LW, HY, and BY. Acquisition, analysis, or interpretation of data: XY, MZ, JL, DL, and XL. Drafting of the manuscript: LW and BY. Critical revision of the manuscript for important intellectual content: HY and BY. Statistical analysis: LW. Administrative, technical, or material support: LW and XY. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank all participants in this study for sharing their life experiences and perceptions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mo Q, Chen X, Yu B, Ma Z. Levels of economic growth and cross-province spread of the covid-19 in china. J Epidemiol Community Health (2021) 75(9):824–8. doi: 10.1136/jech-2020-214169
2. Zhang Y, Yu B, Chen X, Rich S, Mo Q, Yan H. Dynamics of the coronavirus disease 2019 (covid-19) epidemic in wuhan city, hubei province and china: A second derivative analysis of the cumulative daily diagnosed cases during the first 85 days. Glob Health J (2021) 5(1):4–11. doi: 10.1016/j.glohj.2021.02.001
3. Chen X, Yu B. First two months of the 2019 coronavirus disease (covid-19) epidemic in china: Real-time surveillance and evaluation with a second derivative model. Glob Health Res Policy (2020) 5:7. doi: 10.1186/s41256-020-00137-4
4. Babenko O, Kovalchuk I, Metz GAS. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev (2015) 48:70–91. doi: 10.1016/j.neubiorev.2014.11.013
5. Meraya AM, Syed MH, Yasmeen A, Mubaraki AA, Kariry HD, Maabouj W, et al. Covid-19 related psychological distress and fears among mothers and pregnant women in saudi arabia. PloS One (2021) 16(8):e0256597. doi: 10.1371/journal.pone.0256597
6. López-Morales H, Del Valle MV, Canet-Juric L, Andrés ML, Galli JI, Poó F, et al. Mental health of pregnant women during the covid-19 pandemic: A longitudinal study. Psychiatry Res (2021) 295:113567. doi: 10.1016/j.psychres.2020.113567
7. Stepowicz A, Wencka B, Bieńkiewicz J, Horzelski W, Grzesiak M. Stress and anxiety levels in pregnant and post-partum women during the covid-19 pandemic. Int J Environ Res Public Health (2020) 17(24):9450. doi: 10.3390/ijerph17249450
8. Ayaz R, Hocaoğlu M, Günay T, Yardımcı OD, Turgut A, Karateke A. Anxiety and depression symptoms in the same pregnant women before and during the covid-19 pandemic. J Perinat Med (2020) 48(9):965–70. doi: 10.1515/jpm-2020-0380
9. Wu Y, Zhang C, Liu H, Duan C, Li C, Fan J, et al. Perinatal depressive and anxiety symptoms of pregnant women during the coronavirus disease 2019 outbreak in china. Am J Obstet Gynecol (2020) 223(2):240. doi: 10.1016/j.ajog.2020.05.009
10. Avalos LA, Caan B, Nance N, Zhu Y, Li D-K, Quesenberry C, et al. Prenatal depression and diet quality during pregnancy. J Acad Nutr Diet (2020) 120(6):972–84. doi: 10.1016/j.jand.2019.12.011
11. Mauri M, Oppo A, Borri C, Banti S. Suicidality in the perinatal period: comparison of two self-report instruments. Results Pnd-Rescu Arch Womens Ment Health (2012) 15(1):39–47. doi: 10.1007/s00737-011-0246-y
12. Scheinost D, Kwon SH, Lacadie C, Sze G, Sinha R, Constable RT, et al. Prenatal stress alters amygdala functional connectivity in preterm neonates. NeuroImage Clin (2016) 12:381–8. doi: 10.1016/j.nicl.2016.08.010
13. Bergman K, Sarkar P, Glover V, O’Connor TG. Maternal prenatal cortisol and infant cognitive development: Moderation by infant-mother attachment. Biol Psychiatry (2010) 67(11):1026–32. doi: 10.1016/j.biopsych.2010.01.002
14. Gelaye B, Addae G, Neway B, Larrabure-Torrealva GT, Qiu C, Stoner L, et al. Poor sleep quality, antepartum depression and suicidal ideation among pregnant women. J Affect Disord (2017) 209:195–200. doi: 10.1016/j.jad.2016.11.020
15. Goletzke J, Kocalevent RD, Hansen G, Rose M, Becher H, Hecher K, et al. Prenatal stress perception and coping strategies: Insights from a longitudinal prospective pregnancy cohort. J psychosomatic Res (2017) 102:8–14. doi: 10.1016/j.jpsychores.2017.09.002
16. Tuthill EL, Pellowski JA, Young SL, Butler LM. Perinatal depression among hiv-infected women in kwazulu-natal south africa: Prenatal depression predicts lower rates of exclusive breastfeeding. AIDS Behav (2017) 21(6):1691–8. doi: 10.1007/s10461-016-1557-9
17. Geisler M, Meaney S, Waterstone J, O’Donoghue K. Stress and the impact on the outcome of medically assisted reproduction. Eur J Obstet Gynecol Reprod Biol (2020) 248:187–92. doi: 10.1016/j.ejogrb.2020.03.006
18. Traylor CS, Johnson JD, Kimmel MC, Manuck TA. Effects of psychological stress on adverse pregnancy outcomes and nonpharmacologic approaches for reduction: An expert review. Am J Obstet Gynecol MFM (2020) 2(4):100229. doi: 10.1016/j.ajogmf.2020.100229
19. Nimby GT, Lundberg L, Sveger T, McNeil TF. Maternal distress and congenital malformations: do mothers of malformed fetuses have more problems? J Psychiatr Res (1999) 33(4):291–301. doi: 10.1016/S0022-3956(99)00004-7
20. Ding X, Liang M, Wu Y, Zhao T, Qu G, Zhang J, et al. The impact of prenatal stressful life events on adverse birth outcomes: a systematic review and meta-analysis. J Affect Disord (2021) 287:406–16. doi: 10.1016/j.jad.2021.03.083
21. Murphy BE. Steroids and depression. J Steroid Biochem Mol Biol (1991) 38(5):537–59. doi: 10.1016/0960-0760(91)90312-S
22. Banki CM, Karmacsi L, Bissette G, Nemeroff CB. Csf corticotropin-releasing hormone and somatostatin in major depression: response to antidepressant treatment and relapse. Eur Neuropsychopharmacol (1992) 2(2):107–13. doi: 10.1016/0924-977X(92)90019-5
23. Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM, et al. Hpa axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry (2017) 22(4):527–36. doi: 10.1038/mp.2016.120
24. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol (2016) 6(2):603–21. doi: 10.1002/cphy.c150015
25. Lim JH, Kang Y-J, Bak HJ, Kim MS, Lee HJ, Kwak DW, et al. Epigenome-wide dna methylation profiling of preeclamptic placenta according to severe features. Clin Epigenet (2020) 12(1):128. doi: 10.1186/s13148-020-00918-1
26. Palma-Gudiel H, Córdova-Palomera A, Eixarch E, Deuschle M, Fañanás L. Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: A meta-analysis. Epigenetics (2015) 10(10):893–902. doi: 10.1080/15592294.2015.1088630
27. Sosnowski DW, Booth C, York TP, Amstadter AB, Kliewer W. Maternal prenatal stress and infant dna methylation: A systematic review. Dev Psychobiol (2018) 60(2):127–39. doi: 10.1002/dev.21604
28. Capron LE, Ramchandani PG, Glover V. Maternal prenatal stress and placental gene expression of nr3c1 and hsd11b2: The effects of maternal ethnicity. Psychoneuroendocrinology (2018) 87:166–72. doi: 10.1016/j.psyneuen.2017.10.019
29. Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (Nr3c1) and infant cortisol stress responses. Epigenetics (2008) 3(2):97–106. doi: 10.4161/epi.3.2.6034
30. Kertes DA, Kamin HS, Hughes DA, Rodney NC, Bhatt S, Mulligan CJ. Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-Pituitary-Adrenocortical system in mothers and newborns in the democratic republic of Congo. Child Dev (2016) 87(1):61–72. doi: 10.1111/cdev.12487
31. Nagarajan S, Seddighzadeh B, Baccarelli A, Wise LA, Williams M, Shields AE. Adverse maternal exposures, methylation of glucocorticoid-related genes and perinatal outcomes: A systematic review. Epigenomics (2016) 8(7):925–44. doi: 10.2217/epi.16.9
32. Conradt E, Lester BM, Appleton AA, Armstrong DA, Marsit CJ. The roles of DNA methylation of Nr3c1 and 11β-Hsd2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics (2013) 8(12):1321–9. doi: 10.4161/epi.26634
33. Rodney NC, Mulligan CJ. A biocultural study of the effects of maternal stress on mother and newborn health in the democratic republic of Congo. Am J Phys Anthropol (2014) 155(2):200–9. doi: 10.1002/ajpa.22568
34. Sluiter F, Incollingo Rodriguez AC, Nephew BC, Cali R, Murgatroyd C, Santos HP. Pregnancy associated epigenetic markers of inflammation predict depression and anxiety symptoms in response to discrimination. Neurobiol Stress (2020) 13:100273. doi: 10.1016/j.ynstr.2020.100273
35. Santos HP, Adynski H, Harris R, Bhattacharya A, Incollingo Rodriguez AC, Cali R, et al. Biopsychosocial correlates of psychological distress in latina mothers. J Affect Disord (2021) 282:617–26. doi: 10.1016/j.jad.2020.12.193
36. Provenzi L, Mambretti F, Villa M, Grumi S, Citterio A, Bertazzoli E, et al. Hidden pandemic: Covid-19-Related stress, Slc6a4 methylation, and infants’ temperament at 3 months. Sci Rep (2021) 11(1):15658. doi: 10.1038/s41598-021-95053-z
37. Yin xiaohong WL, Qi J, Menglan G, hong Y. Research on the psychological stress and its associated factors of pregnant women during the epidemics of emerging infectious disease. J Pub Health Prev Med (in Chinese) (2021) 32(4):4. doi: 10.3969/j.issn.1006-2483.2020.04.001
38. Brunet A, Weiss DS, Metzler TJ, Best SR, Neylan TC, Rogers C, et al. The peritraumatic distress inventory: A proposed measure of ptsd criterion A2. Am J Psychiatry (2001) 158(9):1480–5. doi: 10.1176/appi.ajp.158.9.1480
39. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav (1983) 24(4):385–96. doi: 10.2307/2136404
40. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry (1987) 150(6):782–6. doi: 10.1192/bjp.150.6.782
41. Boudou M, Séjourné N, Chabrol H. Childbirth pain, perinatal dissociation and perinatal distress as predictors of posttraumatic stress symptoms. Gynecol Obstet Fertil (2007) 35(11):1136–42. doi: 10.1016/j.gyobfe.2007.09.014
42. Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the covid-19 pandemic. J Affect Disord (2020) 277:5–13. doi: 10.1016/j.jad.2020.07.126
43. Durankuş F, Aksu E. Effects of the covid-19 pandemic on anxiety and depressive symptoms in pregnant women: A preliminary study. J Matern Fetal Neona (2022) 35(2):205–11. doi: 10.1080/14767058.2020.1763946
44. Zhou H, Li W, Ren Y. Poor sleep quality of third trimester exacerbates the risk of experiencing postnatal depression. Psychol Health Med (2020) 25(2):229–38. doi: 10.1080/13548506.2018.1549738
45. Yang Y, Mao J, Ye Z, Zeng X, Zhao H, Liu Y, et al. Determinants of sleep quality among pregnant women in China: A cross-sectional survey. J Matern Fetal Neona (2018) 31(22):2980–5. doi: 10.1080/14767058.2017.1359831
46. Chen X, Zou Y, Gao H. Role of neighborhood social support in stress coping and psychological wellbeing during the covid-19 pandemic: Evidence from hubei, China. Health Place (2021) 69:102532. doi: 10.1016/j.healthplace.2021.102532
47. Xiao H, Zhang Y, Kong D, Li S, Yang N. Social capital and sleep quality in individuals who self-isolated for 14 days during the coronavirus disease 2019 (Covid-19) outbreak in January 2020 in China. Med Sci Monit (2020) 26:e923921. doi: 10.12659/MSM.923921
48. Tang X, Lu Z, Hu D, Zhong X. Influencing factors for prenatal stress, anxiety and depression in early pregnancy among women in chongqing, China. J Affect Disord (2019) 253:292–302. doi: 10.1016/j.jad.2019.05.003
49. Dereix AE, Ledyard R, Redhunt AM, Bloomquist TR, Brennan KJ, Baccarelli AA, et al. Maternal anxiety and depression in pregnancy and DNA methylation of the glucocorticoid receptor gene. Epigenomics (2021) 21:1701–9. doi: 10.2217/epi-2020-0022
50. van Dongen J, Ehli EA, Slieker RC, Bartels M, Weber ZM, Davies GE, et al. Epigenetic variation in monozygotic twins: A genome-wide analysis of DNA methylation in buccal cells. Genes (2014) 5(2):347–65. doi: 10.3390/genes5020347
51. Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, et al. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl Psychiatry (2011) 1:e21. doi: 10.1038/tp.2011.21
Keywords: pregnant women, perceived stress, peritraumatic distress, depression, DNA methylation, glucocorticoid receptor, NR3C1
Citation: Wei L, Ying X, Zhai M, Li J, Liu D, Liu X, Yu B and Yan H (2022) The association between peritraumatic distress, perceived stress, depression in pregnancy, and NR3C1 DNA methylation among Chinese pregnant women who experienced COVID-19 lockdown. Front. Immunol. 13:966522. doi: 10.3389/fimmu.2022.966522
Received: 11 June 2022; Accepted: 04 August 2022;
Published: 25 August 2022.
Edited by:
Hai Huang, Feinstein Institute for Medical Research, United StatesCopyright © 2022 Wei, Ying, Zhai, Li, Liu, Liu, Yu and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Yan, yanhmjxr@whu.edu.cn; Bin Yu, binyu1029@whu.edu.cn
 Liqing Wei
Liqing Wei Xiaohong Ying
Xiaohong Ying Mengxi Zhai1
Mengxi Zhai1 Bin Yu
Bin Yu Hong Yan
Hong Yan