- Department of Infectious Diseases, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Institute for Viral Hepatitis, The Second Affiliated Hospital, Chongqing Medical University, Chongqing, China
The antibody and B cell responses after inactivated SARS-CoV-2 vaccination have not been well documented in patients with autoimmune liver disease (AILD). Therefore, we conducted a prospective observational study that included AILD patients and healthy participants as controls between July 1, 2021, and September 30, 2021, at the Second Affiliated Hospital of Chongqing Medical University. All adverse events (AEs) after the COVID-19 vaccination were recorded and graded. Immunoglobulin (Ig)-G antibodies against the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein (anti-RBD-IgG) and neutralizicadng antibodies (NAbs) were tested following full-course vaccination (BBIBP-CorV or CoronaVac). In addition, SARS-CoV-2-specific B cells were detected by flow cytometry. In total, 76 AILD patients and 136 healthy controls (HCs) were included. All AEs were mild and self-limiting, and the incidences were similar between the AILD and HCs. The seropositivity rates of anti-RBD-IgG and NAbs in AILD were 97.4% (100% in HCs, p = 0.13) and 63.2% (84.6% in HCs, p < 0.001), respectively. The titers of anti-RBD-IgG and NAbs were significantly lower in AILD patients than those in HCs. After adjusting for confounders, immunosuppressive therapy was an independent risk factor for low-level anti-RBD-IgG (adjusted odds ratio [aOR]: 4.7; 95% confidence interval [CI], 1.5-15.2; p = 0.01) and a reduced probability of NAbs seropositivity (aOR, 3.0; 95% CI, 1.0-8.9; p = 0.04) in AILD patients. However, regardless of immunosuppressants, the SARS-CoV-2-specific memory B cells responses were comparable between the AILD and HC groups. Our results suggest that inactivated SARS-CoV-2 vaccines (BBIBP-CorV and CoronaVac) are safe, but their immunogenicity is compromised in patients with AILD. Moreover, immunosuppressants are significantly associated with poor antibody responses to the SARS-CoV-2 vaccines. These results could inform physicians and policymakers about decisions on screening the populations at higher risk of poor antibody responses to SARS-CoV-2 vaccines and providing additional vaccinations in patients with AILD.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has become a significant global public health threat. To date, more than 528 million people have been diagnosed with COVID-19, and more than 6 million deaths have been confirmed worldwide (1). Numerous studies have shown that people with comorbidities, including chronic liver disease, are highly vulnerable and have worse outcomes from COVID-19 than those without underlying liver disease (2–5). Therefore, liver societies have recommended vaccination against SARS-CoV-2 for patients with chronic liver diseases (6, 7). However, a series of case reports suggest that mRNA-based vaccines may induce autoimmune liver disease (8–16). This has caused concern among hepatologists as well as patients with autoimmune liver diseases (AILD) (17, 18).
AILD are chronic immune-mediated liver diseases that includ a wide range of disorders, such as autoimmune hepatitis (AIH), primary biliary cholangitis (PBC) and AIH-PBC overlap syndrome, which are frequently treated with either broad or targeted immunosuppressants. Moreover, some AILD patients usually have other autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus and Sjögren’s syndrome, which also require lifelong immunosuppressive drug therapy. Existing data have shown that immunosuppressants can significantly reduce the antibody response to COVID-19 mRNA vaccines or the Johnson & Johnson vaccine in liver transplant patients (19) and immune-mediated inflammatory disorders (20, 21).
In China, inactivated vaccines (BBIBP-CorV or CoronaVac) are widely used COVID-19 vaccines. Systematic evaluation of the safety and immunogenicity of these vaccines in people with AILD has been rare. Here, we aim to evaluate the safety and antibody responses after the whole-course COVID-19 vaccination and explore the association between immunosuppressants and antibody responses to inactivated SARS-CoV-2 vaccines in patients with AILD.
Patients and methods
Study design and participants
Between July 1, 2021, and September 30, 2021, we performed a prospective observational study at the Second Affiliated Hospital of Chongqing Medical University, China. We included participants aged older than 18 years, diagnosed with any of the prespecified immune-mediated liver disorders (AIH or AIH-PBC overlap syndrome) (22–24), no SARS-CoV-2 infection before receipt of the first vaccine dose (determined based on either a negative anti-SARS-CoV-2 IgM/IgG test or the absence of a positive polymerase chain reaction assay result for SARS-CoV-2, with no history of suspected clinical SARS-CoV-2 infection), completed whole-course COVID-19 vaccination (2 doses of BBIBP-CorV or CoronaVac vaccine), and were able to understand and complete questionnaires. Healthy participants were included as healthy controls (HCs). Participants with known pregnancy during study entry, those who did not complete the full course of vaccination, and those who provided incomplete vaccination information (including the date of first vaccine dose and complete vaccination and vaccine manufacturers) were excluded. Blood samples were collected for serological assays for SARS-CoV-2 at least 21 days after the whole-course vaccination from AILD patients and HCs.
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University and in accordance with the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants. This study has been registered at ClinicalTrials.gov (NCT05007665).
Variables and definitions
Clinical characteristics, including age, sex, body mass index (BMI), comorbidities, history of diseases and concomitant medications, of all patients were collected via a standardized questionnaire. The presence or absence of cirrhosis was confirmed using clinical or biochemical evidence, FibroScan, liver imaging (ultrasound, CT, or MRI) and endoscopy. The concomitant medications, especially types and doses of immunosuppression, were further confirmed through the prescribing information system in the hospital.
All adverse events (AEs) within 7 days and 30 days after COVID-19 vaccination were recorded and graded according to the National Medical Products Administration of China (version 2019). AEs related to vaccination were judged by investigators. Safety was evaluated by determining the overall incidence of AEs.
SARS-CoV-2 antibody test
The SARS-CoV-2 antibody against the spike protein receptor-binding domain (anti-RBD-IgG) was detected by indirect ELISA using the SARS-CoV-2 RBD antibody detection kit (Sino Biological, Beijing, China). The lower limit of quantification is 5.0 arbitrary units per mL (AU/mL). The neutralizing antibodies (NAbs) were detected by the competitive ELISA method using the SARS-CoV-2 neutralizing antibody detection kit (Sino Biological, Beijing, China). The details were described in our previous study (25).
SARS-CoV-2-specific B cells responses
Previous studies have described the selection of markers of memory B cells (MBCs) and their subsets (26, 27). For SARS-CoV-2-specific B cells detection, biotinylated SARS-CoV-2 spike RBD protein (Sino Biological, 40592-V08H2-B) was mixed with streptavidin BV421 (Biolegend, 405225) at a 4:1 molar ratio for one hour at 4°C to obtain the antigen probe. According to the manufacturer’s instructions, peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by Histopaque (Sigma–Aldrich, 10771) density gradient centrifugation. After washing with FACS buffer (PBS+2% FBS), PBMCs were stained for 30 minutes at 4°C using an antigen probe (1:33.3) and the following conjugated antibodies: anti-human CD3 (300430, Biolegend, 1:50), anti-human CD19 (302212, Biolegend, 1:50), anti-human CD21 (354918, Biolegend, 1:50), anti-human CD27 (356406, Biolegend, 1:50), anti-human IgG Fc (410722, Biolegend, 1:50), and anti-human IgM (314524, Biolegend, 1:50). After staining, the cells were rewashed and resuspended in 200 µl FACS buffer. Samples were then evaluated by flow cytometry (Beckman Coulter, CytoFLEX) and analyzed using FlowJo (Treestar, 10.0.7r2).
Statistical analysis
Data are presented as the median (interquartile range, IQR) for continuous variables and proportions for categorical variables. Continuous variables were compared using Student’s t test for the variables of age and BMI. Categorical variables were compared using Fisher’s exact test or the chi-square test for sex, cirrhosis, comorbidities, immunosuppressants, and vaccine types. One-way analysis of variance (ANOVA) was used to compare the results of multiple groups, and Tukey’s correction was used to correct for comparisons between groups. Negative responses and relatively low antibody levels following SARS-CoV-2 vaccination were classified as poor antibody responses in previous studies (19, 28, 29). In this study, a poor antibody response was defined as an anti-RBD-IgG titer less than the median value (≤ 34.0 AU/ml) or negative for NAbs. Multiple logistic regression was used to explore the independent variables associated with poor antibody responses and presented as odds ratios (ORs) (95% confidence intervals, CIs) with adjustment for potential confounding factors. Statistical analysis was performed using EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA) and R (http://www.Rproject.org, the R Foundation). All statistical tests were two-sided, and p < 0.05 was considered statistically significant.
Results
Characteristics of participants
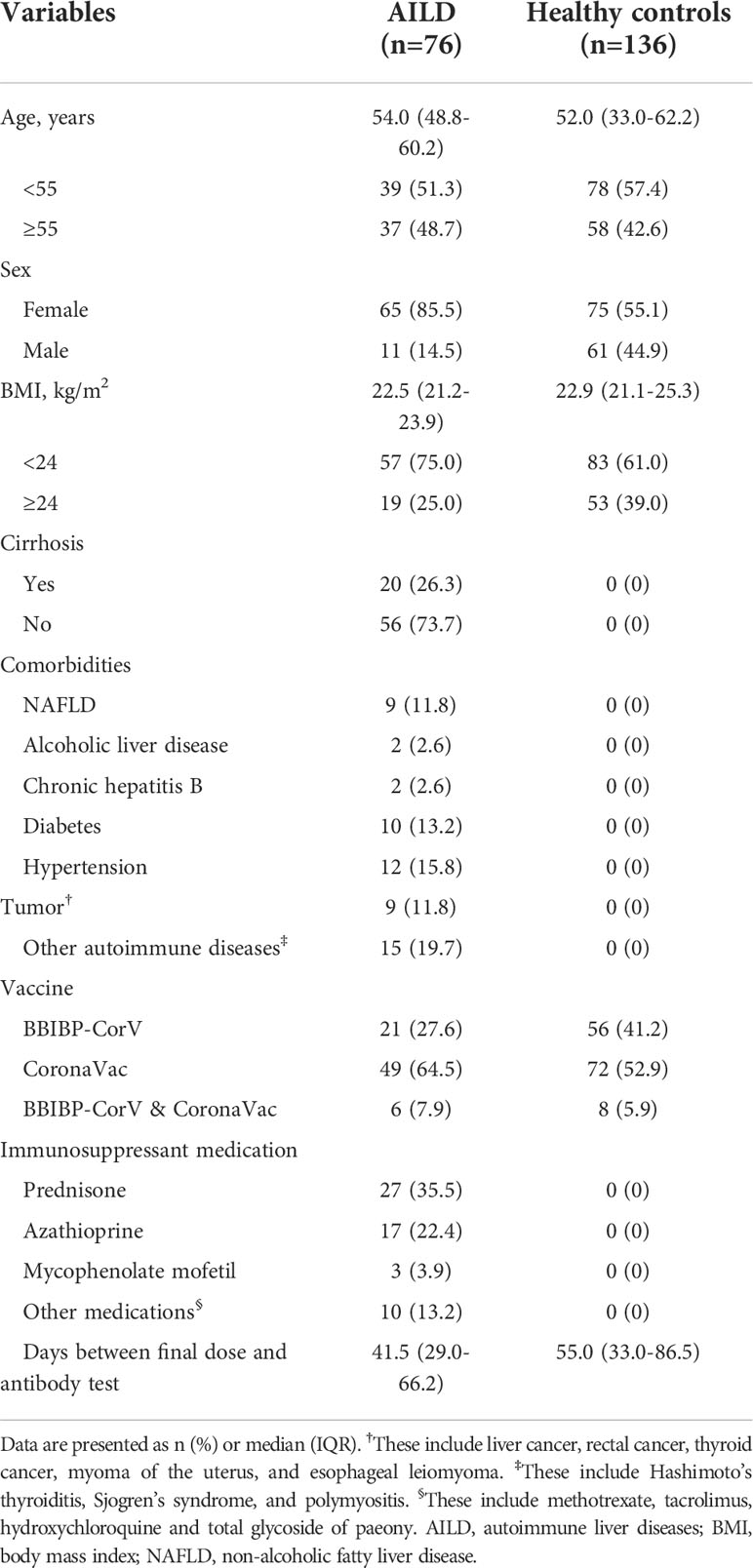
76 eligible AILD patients and 136 HCs were included in the study. The characteristics of the participants are shown in Table 1. Briefly, the median age was 54.0 years (IQR: 48.8-60.2 years) in AILD patients and 52.0 years (IQR: 33.0-62.2 years) in the HC group. The majority of participants were female (85.5% [65/76] in AILD patients and 55.1% [75/136] in HC). The median BMI and proportion of vaccine types in AILD patients and HCs were similar (22.5 kg/m2 [IQR: 21.2-23.9 kg/m2] vs. 22.9 kg/m2 [IQR: 21.1-25.3 kg/m2]). The median postvaccination time was 41.5 days (IQR: 29.0-66.2 days) and 55.0 (IQR: 33.0-86.5 days) for the AILD patients and HCs, respectively. Additionally, among these AILD patients, 20 (26.3%) had cirrhosis, and almost half (46.1%, 35/76) received one or more immunosuppressant medications.
COVID−19 vaccination safety
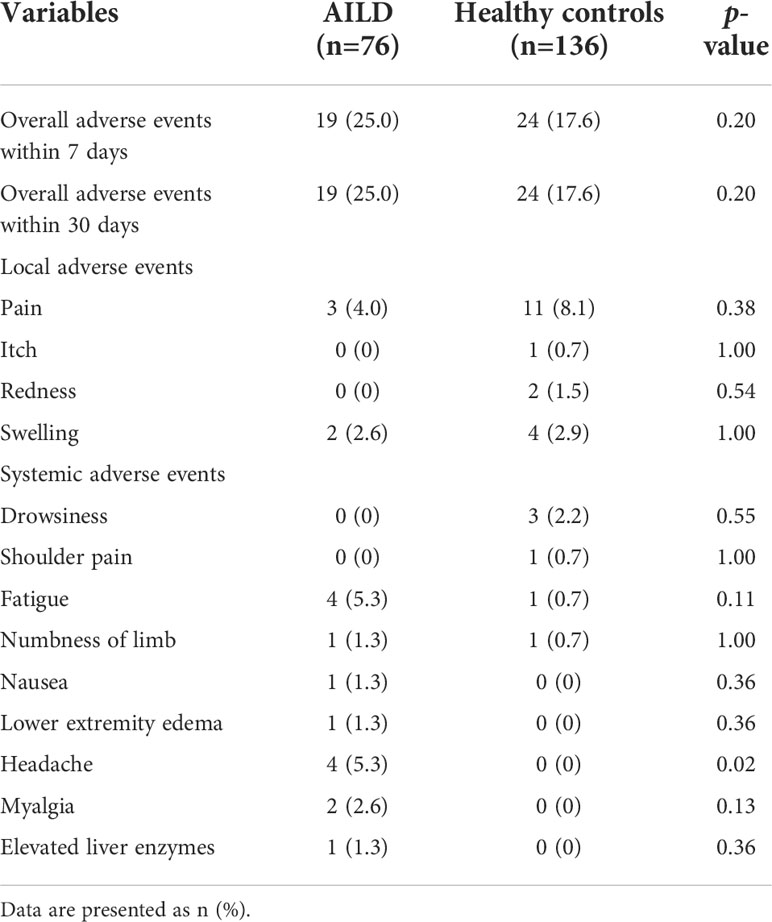
The overall incidence of AEs within 7 days (25.0% [19/76] vs 17.6% [24/136]) and 30 days (25.0% [19/76] vs 17.6% [24/136]) after COVID-19 vaccination was slightly higher in AILD patients than in the HC group (p = 0.20) (Table 2). All AEs were mild, and none of them had any serious AEs. The common AEs in AILD patients in local AEs were pain at the injection site (4.0%, 3/76); in systemic AEs were fatigue (5.3%, 4/76) and headache (5.3%, 4/76). The most common AE in HC was local pain at the injection site (8.1%, 11/136) (Table 2). Notably, one patient increased the serum gamma-glutamyl-transpeptidase (GGT) level from 20 U/L to 90 U/L (upper limit of normal: 45 U/L) after vaccination and returned to normal after continuing the original treatment strategy during the follow-up. Another patient’s antinuclear antibody (ANA) was positive at a titer of 1:320 before vaccination. It increased to 1:1000 after vaccination, but the liver function test, serum IgG, anti-liver-kidney microsomal, anti-smooth muscle, anti-mitochondrial antibodies, and anti-soluble liver antigen were normal, and the patient had no symptoms of discomfort.
Antibody responses after COVID-19 vaccination
The seropositivity for anti-RBD-IgG was 97.4% (74/76) in AILD patients, which was similar to that in the HC group (100%) (p = 0.13) (Figure 1A). However, anti-RBD-IgG levels were significantly lower in AILD patients than those in HCs (mean: 49.1 AU/mL vs 71.9 AU/mL, p = 0.02) (Figure 1B). Regarding NAbs, seropositivity (63.2% [48/76] vs 84.6% [115/136]) and antibody titers were both significantly lower in AILD patients than those in HCs (p < 0.001) (Figures 1D, E). Compared with controls, anti-RBD-IgG and NAbs levels seemed to decrease slightly over time after the second dose vaccination in AILD patients (Figures 1C, F).
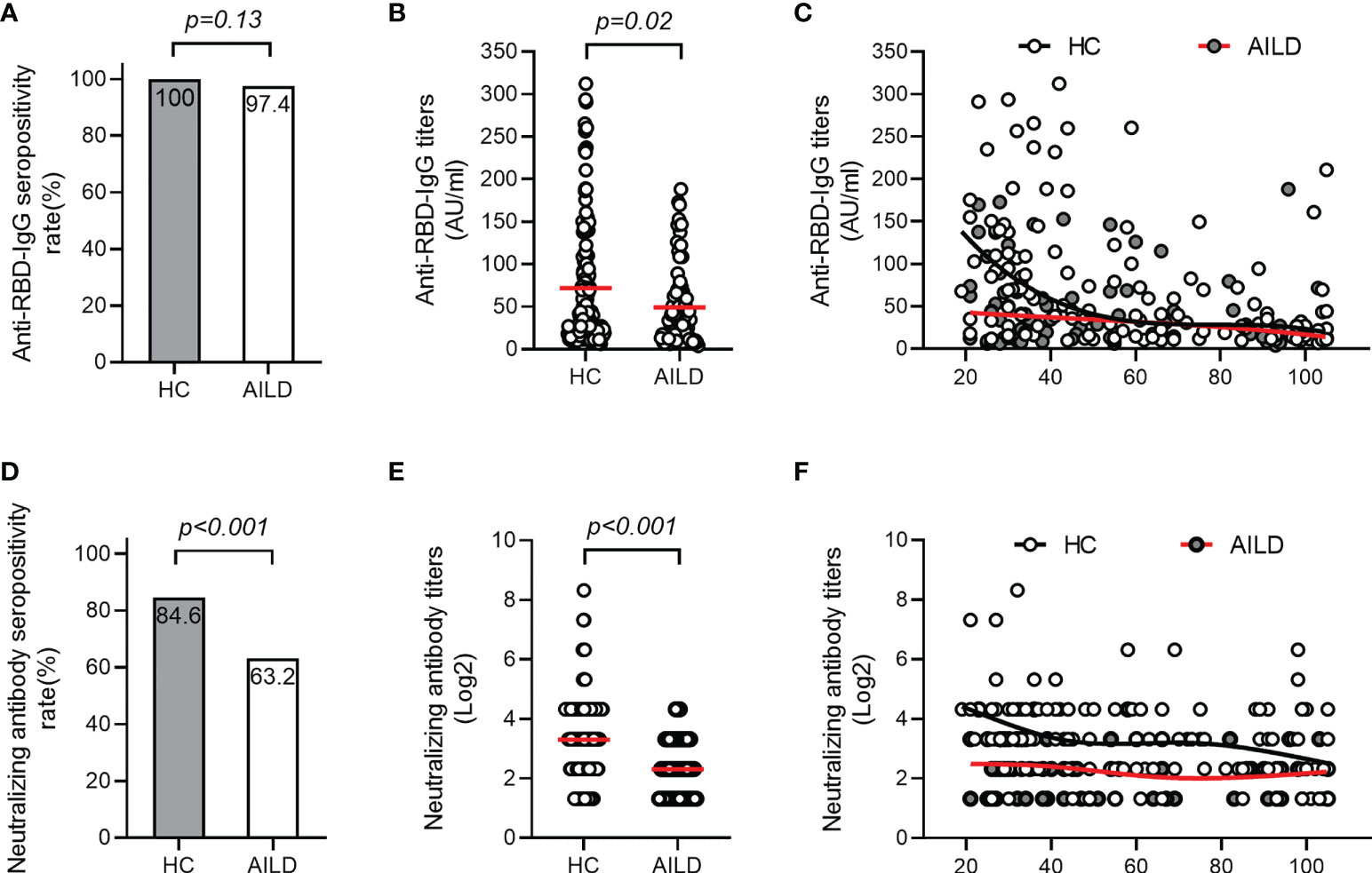
Figure 1 Antibody responses after COVID-19 vaccination in patients with AILD and healthy controls. The seropositivity rates and titers of anti-RBD-IgG (A, B) and NAbs (D, E) in patients with AILD and healthy controls. The distribution of anti-RBD-IgG (C) and NAbs (F) antibody titers over time in patients with AILD and healthy controls. AILD, autoimmune liver disease; anti-RBD-IgG, spike receptor-binding domain IgG antibody; NAbs, neutralizing antibodies.
Effect of immunosuppressants on antibody responses
Analysis of clinical features showed no significant correlations between poor antibody responses and parameters such as age, sex, BMI, cirrhosis, and comorbidities (all p > 0.05) (Table 3). However, low-level antibodies were significantly related to the types of vaccine (p = 0.01) (Table 3, Supplementary Figure 1A) and the use of immunosuppressants (p = 0.04) (Table 3; Supplementary Figure 1C), and these results were consistent with univariate analysis (Supplementary Table 1). Furthermore, after adjusting for potential confounding factors (age, BMI, sex, cirrhosis, comorbidities, types of vaccine, and days between final dose and antibody test) in multiple logistic regression analysis, the use of immunosuppressants remained significantly related to low-level antibody levels (Figure 2). Compared with patients with no immunosuppressive medication, the crude odds ratio (OR) of low-level antibody response risk among patients who used immunosuppressants was 3.3 (95% CI, 1.3-8.5; p = 0.01), and their adjusted OR (aOR) increased to 4.9 (95% CI, 1.5-15.6; p = 0.01). Notably, the risk trend does not seem to increase with the number of immunosuppressants. The aORs of the use of one and more immunosuppressive medications were 5.0 (95% CI, 1.1-23.1; p = 0.04) and 4.9 (95% CI, 1.1-19.2; p = 0.03), respectively (Figure 2).
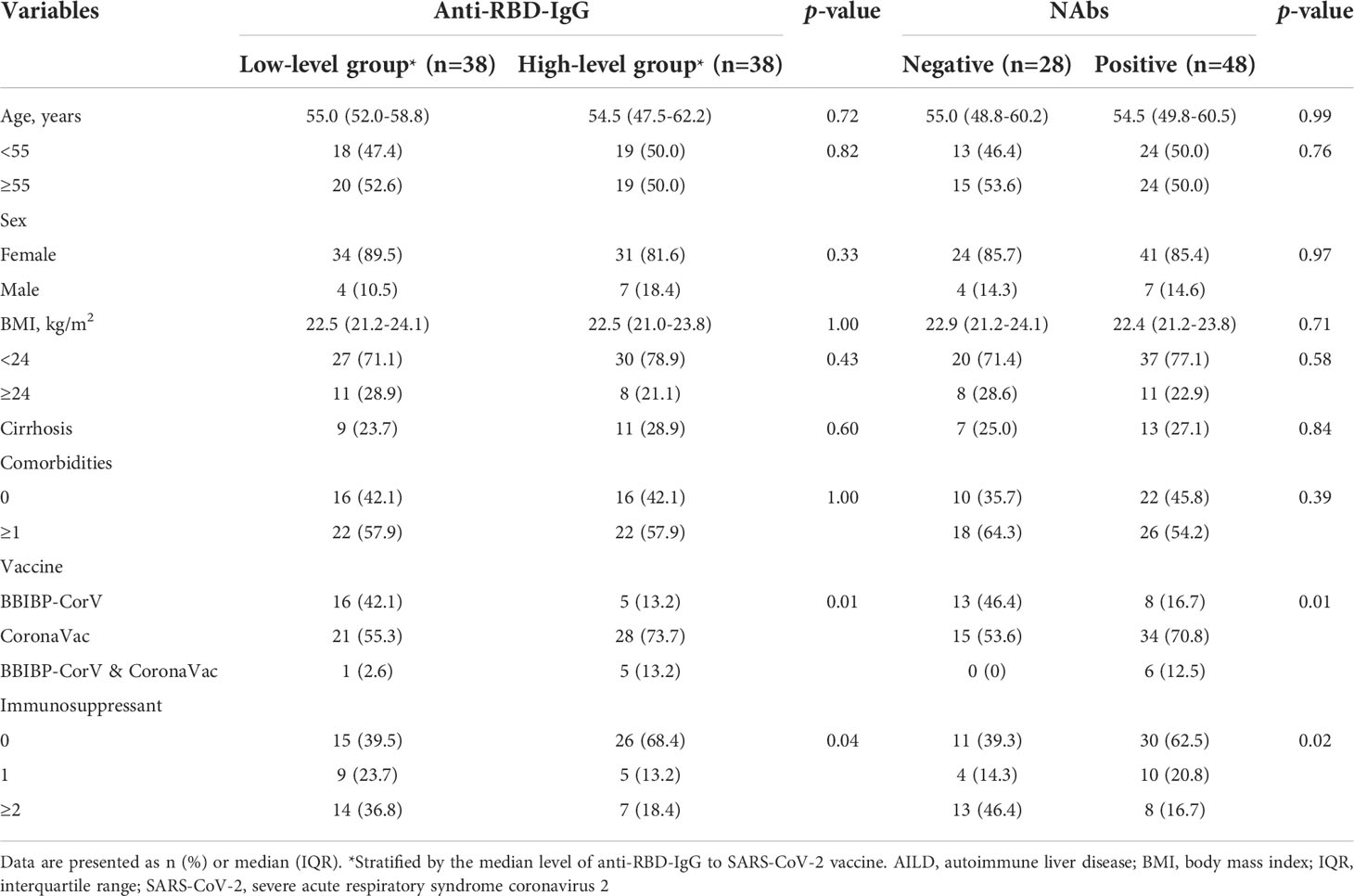
Table 3 Distribution of clinical characteristics by serum antibody titers to SARS-CoV-2 vaccine in patients with AILD.
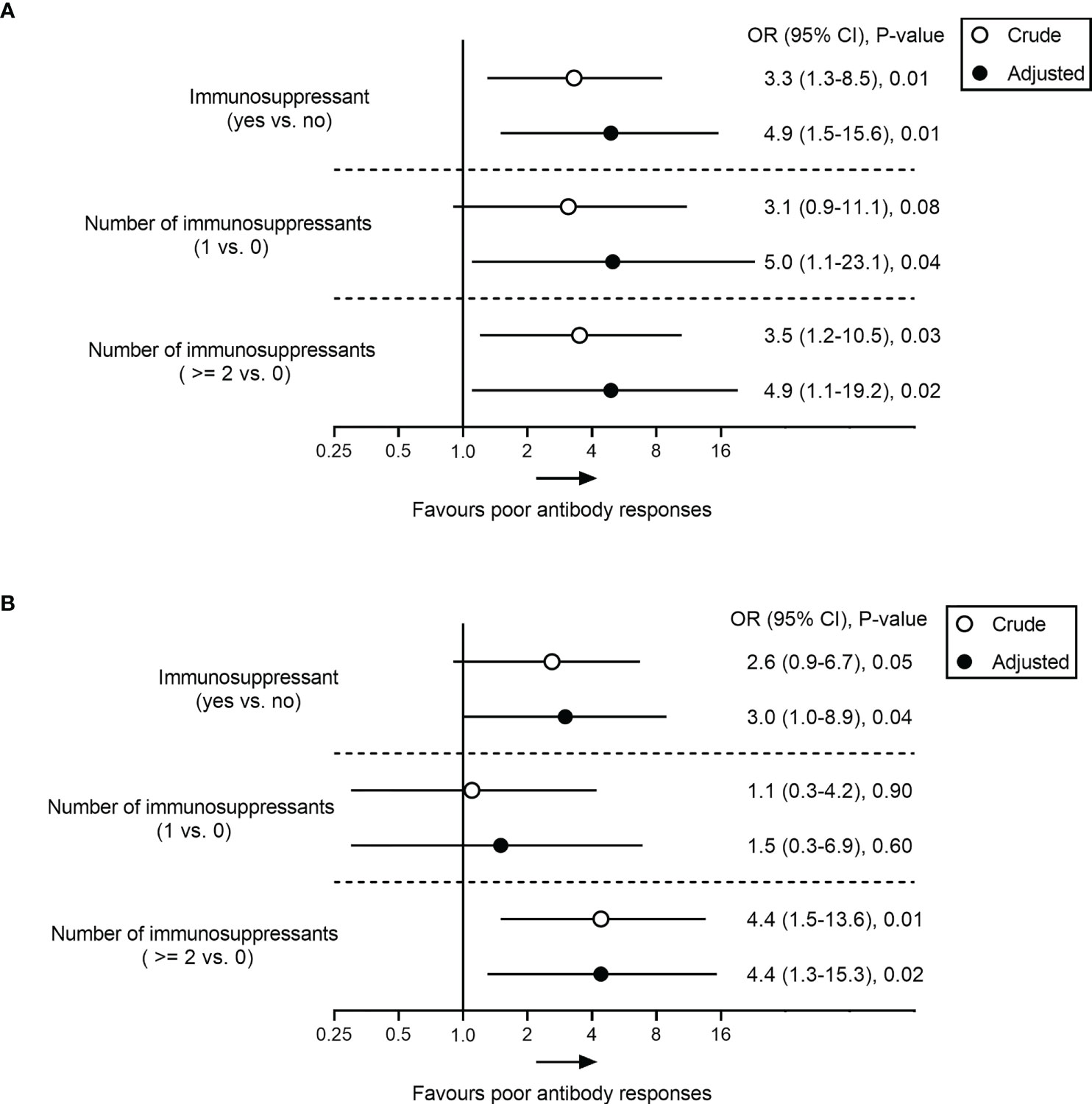
Figure 2 Association analysis between immunosuppressant and poor antibody responses to SARS-CoV-2 vaccine in patients with AILD. Odds ratio (95% CI) of poor response to RBD-IgG (A) and NAbs (B) in immunosuppressant-treated AILD patients in crude (unadjusted) and adjusted models compared with healthy controls. The adjusted model was adjusted age, BMI, sex, cirrhosis, comorbidities, types of vaccine, and days between final dose and antibody test. AILD, autoimmune liver diseases; BMI, body mass index; CI, confidence interval; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Similar results were also observed for NAbs responses in the AILD patients. Negative NAbs were significantly associated with the types of vaccine (p = 0.01) (Supplementary Figure 1B) and immunosuppressants (p = 0.02) (Supplementary Figure 1D), except for age, sex, BMI, cirrhosis, and comorbidities (Table 3) (Supplementary Table 1). After adjusting for confounding factors, immunosuppressants were associated with a reduced probability of NAbs seropositivity (aOR, 3.0; 95% CI, 1.0-8.9; p = 0.04), especially when ≥2 immunosuppressive medications were used (aOR, 4.4; 95% CI, 1.3-15.3; p = 0.02) (Figure 2).
Concisely, the results suggested that immunosuppressive therapy was an independent risk factor for poor antibody responses to COVID-19 vaccination in patients with AILD.
Specific B cells responses after COVID-19 vaccination
The mean frequencies of B cells (CD3-CD19+) in AILD patients with and without immunosuppressive therapy were 9.42% and 8.63%, respectively, with no statistically significant difference compared with those in HCs (8.82%). The frequency of total MBCs (CD3-CD19+CD27+) was significantly higher in HC group than in patients with AILD who were not treated with immunosuppressants (37.6% vs 29.7%, p = 0.01), while the frequency in patients treated with immunosuppressants was at an intermediate level (Supplementary Figure 2). To further investigate the humoral immune response to the SARS-CoV-2 vaccine, the frequency and phenotype of specific B cells were also detected. As expected, the percentage of specific B cells was very low in the peripheral blood of AILD patients and the HC group. No significant difference was found in the frequency of RBD-specific B cells (CD3-CD19+RBD+) and IgG RBD-specific memory B cells (IgG+CD3-CD19+RBD+CD27+) between the AILD and HC groups, regardless of immunosuppressants (Figures 3A, B). However, the frequency of IgM RBD-specific MBCs (IgM+CD3-CD19+RBD+CD27+) was significantly lower in AILD patients with (17.2% vs 25.3%, adjusted p < 0.01) or without immunosuppressants (19.4% vs 25.3%, adjusted p = 0.03) than in the HC group (Figure 3C). To better understand the functional phenotype of RBD-specific MBCs, we further compared RBD-specific resting MBCs (CD3-CD19+RBD+CD21+CD27+), RBD-specific activated MBCs (CD3-CD19+RBD+CD21-CD27+), RBD-specific atypical MBCs (CD3-D19+RBD+CD21-CD27-), and RBD-specific intermediate MBCs (CD3-CD19+RBD+CD21+CD27-) between AILD patients and HC groups. Compared with HCs, AILD patients without immunosuppressants had a lower frequency of RBD-specific activated MBCs (13.0% vs 16.9%, adjusted p = 0.03) and a higher frequency of RBD-specific intermediate MBCs (47.1% vs 39.9%, adjusted p = 0.02), but not in patients with immunosuppressants. Moreover, there was no significant difference in RBD-specific resting MBCs and RBD-specific atypical MBCs between AILD patients and the HC groups (Figures 3D–G). These results indicate that patients with AILD may develop humoral immunity as robust as in a healthy population when receiving a booster dose or against SARS-CoV-2 infection despite ongoing immunosuppression.
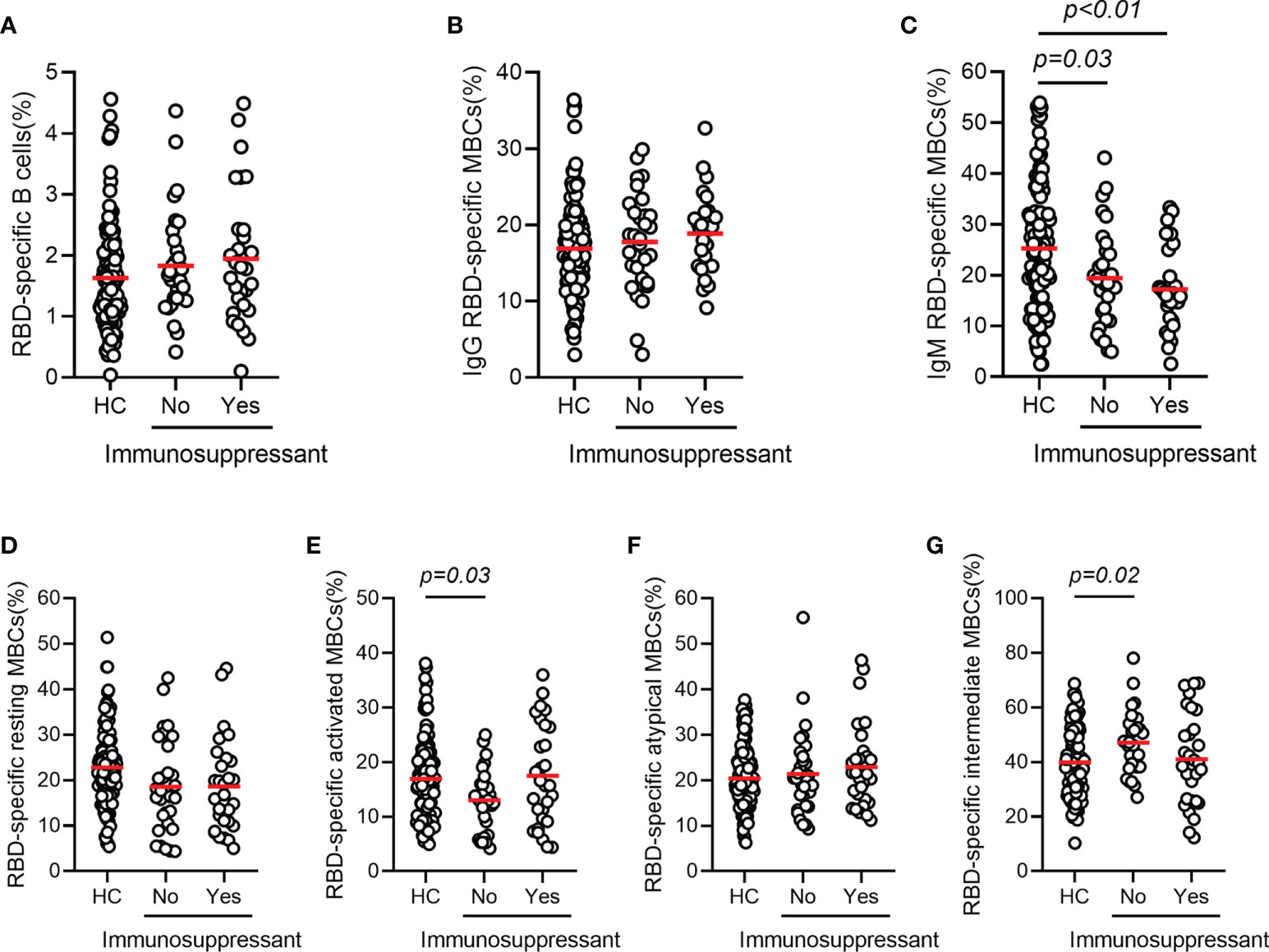
Figure 3 RBD-specific B cell responses after COVID-19 vaccination in patients with AILD and healthy controls. Frequency of RBD-specific B cells (A), IgG RBD-specific MBCs (B), IgM RBD-specific MBCs (C), RBD-specific resting MBCs (D), RBD-specific activated MBCs (E), RBD-specific atypical MBCs (F) and RBD-specific intermediate MBCs (G) in patients with AILD and healthy controls. AILD, autoimmune liver disease; MBCs, memory B cells; RBD, receptor-binding domain.
Discussion
AILD is a chronic disease characterized by immune-mediated disorders. Safety and immunogenicity have been of concern in patients with AILD since vaccination against COVID-19. In this prospective observational study, we found that inactivated SARS-CoV-2 vaccines achieved a favorable safety profile, but their immunogenicity is compromised in patients with AILD. The use of immunosuppressants had an estimated 3- to 5-fold increased risk of poor antibody responses to the SARS-CoV-2 vaccine. Moreover, the specific MBCs responses were comparable between patients in the AILD and HC groups despite ongoing immunosuppression.
Similar to the general population and other chronic liver diseases, such as NAFLD, liver transplantation, chronic hepatitis B, and liver cirrhosis, adverse events related to the COVID-19 vaccine in patients with AILD were mild and self-resolved within a few days after vaccination (19, 30–32). However, after the whole-course vaccination, one patient experienced an increased serum GGT level, and another experienced a sharply increased ANA titer. Because they refused liver biopsy, it is unclear whether this phenomenon reflects the fluctuation of the disease itself or whether the vaccine induces new-onset autoimmune diseases. Fortunately, we did not observe any evidence of clinical deterioration in neither patients during follow-up over 6 months. Herein, we believe that the COVID-19 inactivated vaccine is safe in patients with AILD.
Among the AILD patients, the COVID-19 inactivated vaccine showed an efficient antibody response of anti-RBD-IgG (97.4%). This was similar in a multicenter study of NAFLD patients in China (95.5%) but much higher than the reported seropositivity of SARS-CoV-2 RBD-specific antibodies in patients with chronic hepatitis B virus infection who were also vaccinated with inactivated COVID-19 vaccines (87.25%) (31, 32). This can be attributed to the large percentage of patients in our study were female (85.5% vs 27.5%). Xiang et al. found that female patients exhibited higher seropositivity for SARS-CoV-2 RBD-specific antibodies than males with chronic hepatitis B (95.1% vs 84.3%) 32. A similar finding that female vaccine recipients showed more robust antibody responses to COVID-19 vaccination was also reported in a clinical trial in Turkey (30). Similar to chronic hepatitis B, the NAbs seropositivity was lower than that of anti-RBD-IgG in patients with AILD. We found that immunosuppressants have an estimated 3- to 5-fold increased risk of poor antibody responses to the SARS-CoV-2 vaccine. In a nationwide multicenter prospective cohort study of 125 patients with multiple sclerosis, Bsteh et al. reported that immunosuppressive therapy could significantly reduce the probability of NAbs seropositivity after symptomatic COVID-19 (OR, 0.51; 95% CI, 0.17-0.82) (33). This finding might partly explain why the seropositivity and titer of NAbs in AILD patients are lower than those in HCs. However, a cohort study in patients with immune-mediated inflammatory disorders on immunosuppressants showed that only certain specific immunosuppressants attenuated the humoral responses after SARS-CoV-2 mRNA vaccine (21). More studies are needed to compare the effects of multiple types of immunosuppressants on different types of SARS-CoV-2 vaccines.
This study found that the RBD-specific MBCs responses were comparable between patients with AILD and HCs despite ongoing immunosuppression. It’s consistent with Kirchner et al. in a small study showing that patients with AIH receiving immunosuppressive therapy still developed strong humoral and cellular immunity to SARS-CoV-2 (34). Given the role of MBCs, it is speculated that patients with AILD may develop humoral immunity as robust as those in a healthy population when receiving a booster dose or against SARS-CoV-2 infection.
There are several main limitations in this study. First, a lack of longitudinal serial antibody testing limits the possibility of measuring a change in antibody levels individually. Second, the relatively small sample size of the study and the lack of subgroup analyses, such as strength of immunosuppression and antibody response, may reduce the reliability and limit the generalizability of the findings. Third, the antibody response is only part of the immunogenicity of the COVID-19 vaccine, so there is a need to explore the T-cell response. However, given the unprecedented nature of the COVID-19 pandemic and the low prevalence of AILD, we believe that our study offers valuable insights into the management of these patients to clinicians.
In conclusion, the COVID-19 inactivated SARS-CoV-2 vaccines (BBIBP-CorV and CoronaVac) are safe, but their immunogenicity is compromised in patients with AILD. In addition, immunosuppressants are significantly associated with poor antibody responses to the SARS-CoV-2 vaccines. These results could inform physicians and policymakers about decisions on screening the populations at higher risk of poor antibody responses to SARS-CoV-2 vaccines and providing additional vaccinations in patients with AILD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by ethics committee of the Second Affiliated Hospital of Chongqing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
The authors DC, DZ, and HR contributed to the conception and design and critical revision of important intellectual content. Data collection was performed by YW, LA, MK, ZC, MC, MP, NL, and PH. Statistical analysis was performed by HL and YW. The first draft of the manuscript was written by HL. All authors approved the final version and agreed to be accountable for all aspects of the work.
Funding
This work is supported by the National Science and Technology Major Project of China (2017ZX10202203-007, 2017ZX10202203-008, 2018ZX10302206-003) and a pilot project of clinical cooperation between traditional Chinese and western medicine for significant and complicated diseases of the National Administration of Traditional Chinese Medicine: hepatic fibrosis.
Acknowledgments
We also acknowledge the support of the Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University, the National Natural Science Foundation of China (81772198,81902068), and the Natural Science Foundation of Chongqing, China (cstc2020jcyj-msxmX0389).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.988004/full#supplementary-material
Supplementary Figure 1 | Antibody response rates after COVID-19 vaccination. The seropositivity rates (A-B) and titers (C-D) of anti-RBD-IgG and NAbs among the different types of vaccines (BBIBP-CorV, CoronaVac and BBIBP-CorV & CoronaVac) or different groups of immunosuppressive medications (0, 1, ≥2). AILD, autoimmune liver diseases; anti-RBD-IgG, spike receptor-binding domain IgG antibody; NAbs, neutralizing antibodies.
Supplementary Figure 2 | The frequencies of total B cells and MBCs in AILD patients with and without immunosuppressive therapy. The frequencies of B cells (CD3-CD19+) (A) and MBCs (CD3-CD19+CD27+) in AILD patients after COVID-19 vaccination. AILD, autoimmune liver diseases; COVID-19, coronavirus disease 2019; HCs, healthy controls; MBCs, memory B cells.
Supplementary Table 1 | Univariate analysis of factors associated with poor antibody responses to SARS-CoV-2 vaccine in patients with AILD.
Abbreviations
AEs, adverse events; AIH, autoimmune hepatitis; AILD, autoimmune liver diseases; ANA, antinuclear antibody; BMI, body mass index; CI, confidence interval; COVID-19, coronavirus disease 2019; GGT, gamma-glutamyl-transpeptidase; HCs, healthy controls; IgG, immunoglobulin G; IQR, interquartile range; MBCs, memory B cells; NAbs, neutralizing antibodies; NAFLD, non-alcoholic fatty liver disease; OR, odds ratio; PBC, primary biliary cholangitis; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
References
1. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis (2020) 20(5):533–4. doi: 10.1016/S1473-3099(20)30120-1
2. Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol (2021) 74(3):567–77. doi: 10.1016/j.jhep.2020.09.024
3. Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; the APCOLIS study (APASL COVID-19 liver injury spectrum study). Hepatol Int (2020) 14(5):690–700. doi: 10.1007/s12072-020-10072-8
4. Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: A systematic review and meta-analysis. Hepatol Int (2020) 14(5):612–20. doi: 10.1007/s12072-020-10078-2
5. Marjot T, Webb GJ, ASt B, Moon AM, Stamataki Z, Wong VW, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol (2021) 18(5):348–64. doi: 10.1038/s41575-021-00426-4
6. Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol (2021) 74(4):944–51. doi: 10.1016/j.jhep.2021.01.032
7. Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, et al. American Association for the study of liver diseases expert panel consensus statement: Vaccines to prevent coronavirus disease 2019 infection in patients with liver disease. Hepatology (2021) 74(2):1049–64. doi: 10.1002/hep.31751
8. Clayton-Chubb D, Schneider D, Freeman E, Kemp W, Roberts SK. Autoimmune hepatitis developing after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. J Hepatol (2021) 75(5):1249–50. doi: 10.1016/j.jhep.2021.06.014
9. Londono MC, Gratacos-Gines J, Saez-Penataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination - still casualty? J Hepatol (2021) 75(5):1248–9. doi: 10.1016/j.jhep.2021.06.004
10. Garrido I, Lopes S, Simoes MS, Liberal R, Lopes J, Carneiro F, et al. Autoimmune hepatitis after COVID-19 vaccine - more than a coincidence. J Autoimmun (2021) 125:102741. doi: 10.1016/j.jaut.2021.102741
11. Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J Hepatol (2021) 75(1):222–4. doi: 10.1016/j.jhep.2021.04.003
12. Bril F. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: One or even several swallows do not make a summer. J Hepatol (2021) 75(5):1256–7. doi: 10.1016/j.jhep.2021.08.001
13. Lodato F, Larocca A, D'Errico A, Cennamo V. An unusual case of acute cholestatic hepatitis after m-RNABNT162b2 (Comirnaty) SARS-CoV-2 vaccine: Coincidence, autoimmunity or drug-related liver injury. J Hepatol (2021) 75(5):1254–6. doi: 10.1016/j.jhep.2021.07.005
14. McShane C, Kiat C, Rigby J, Crosbie Ó. The mRNA COVID-19 vaccine - a rare trigger of autoimmune hepatitis? J Hepatol (2021) 75(5):1252–4. doi: 10.1016/j.jhep.2021.06.044
15. Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel) (2021) 9(5):435. doi: 10.3390/vaccines9050435
16. Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sánchez Valadez TI, Jara LJ. Two cases of graves' disease following SARS-CoV-2 vaccination: An Autoimmune/Inflammatory syndrome induced by adjuvants. Thyroid Off J Am Thyroid Assoc (2021) 31(9):1436–9. doi: 10.1089/thy.2021.0142
17. Ferri C, Ursini F, Gragnani L, Raimondo V, Giuggioli D, Foti R, et al. Impaired immunogenicity to COVID-19 vaccines in autoimmune systemic diseases. High prevalence non-response different patients' subgroups. J Autoimmun (2021) 125:102744. doi: 10.1016/j.jaut.2021.102744
18. Tzioufas AG, Bakasis AD, Goules AV, Bitzogli K, Cinoku II, Chatzis LG, et al. A prospective multicenter study assessing humoral immunogenicity and safety of the mRNA SARS-CoV-2 vaccines in Greek patients with systemic autoimmune and autoinflammatory rheumatic diseases. J Autoimmun (2021) 125:102743. doi: 10.1016/j.jaut.2021.102743
19. Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol (2021) 75(6):1434–9. doi: 10.1016/j.jhep.2021.08.008
20. Sieiro Santos C, Calleja Antolin S, Moriano Morales C, Garcia Herrero J, Diez Alvarez E, Ramos Ortega F, et al. Immune responses to mRNA vaccines against SARS-CoV-2 in patients with immune-mediated inflammatory rheumatic diseases. RMD Open (2022) 8(1):e001898. doi: 10.1136/rmdopen-2021-001898
21. Wieske L, van Dam KPJ, Steenhuis M, Stalman EW, Kummer LYL, van Kempen ZLE, et al. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol (2022) 4(5):e338–50. doi: 10.1016/S2665-9913(22)00034-0
22. Liver EAftSot. EASL clinical practice guidelines: Autoimmune hepatitis. J Hepatol (2015) 63(4):971–1004. doi: 10.1016/j.jhep.2015.06.030
23. Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American association for the study of liver diseases. Hepatology (2020) 72(2):671–722. doi: 10.1002/hep.31065
24. Liver EAftSot. EASL clinical practice guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol (2017) 67(1):145–72. doi: 10.1016/j.jhep.2017.03.022
25. He T, Zhou Y, Xu P, Ling N, Chen M, Huang T, et al. Safety and antibody response to inactivated COVID-19 vaccine in patients with chronic hepatitis b virus infection. Liver Int Off J Int Assoc Study Liver (2022) 42(6):1287–96. doi: 10.1111/liv.15173
26. Long QX, Jia YJ, Wang X, Deng HJ, Cao XX, Yuan J, et al. Immune memory in convalescent patients with asymptomatic or mild COVID-19. Cell Discov (2021) 7(1):18. doi: 10.1038/s41421-021-00250-9
27. Ogega CO, Skinner NE, Blair PW, Park HS, Littlefield K, Ganesan A, et al. Durable SARS-CoV-2 b cell immunity after mild or severe disease. J Clin Invest (2021) 131(7):e145516. doi: 10.1172/JCI145516
28. Mazzola A, Todesco E, Drouin S, Hazan F, Marot S, Thabut D, et al. Poor antibody response after two doses of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine in transplant recipients. Clin Infect Dis an Off Publ Infect Dis Soc America (2022) 74(6):1093–6. doi: 10.1093/cid/ciab580
29. Connolly CM, Chiang TP, Teles M, Frey S, Alejo JL, Massie A, et al. Factors associated with poor antibody response to third-dose SARS-CoV-2 vaccination in patients with rheumatic and musculoskeletal diseases. Lancet Rheumatol (2022) 4(6):e382–4. doi: 10.1016/S2665-9913(22)00065-0
30. Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet (London England) (2021) 398(10296):213–22. doi: 10.1016/S0140-6736(21)01429-X
31. Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): A multicenter study. J Hepatol (2021) 75(2):439–41. doi: 10.1016/j.jhep.2021.04.026
32. Xiang T, Liang B, Wang H, Quan X, He S, Zhou H, et al. Safety and immunogenicity of a SARS-CoV-2 inactivated vaccine in patients with chronic hepatitis b virus infection. Cell Mol Immunol (2021) 18(12):2679–81. doi: 10.1038/s41423-021-00795-5
33. Bsteh G, Durauer S, Assar H, Hegen H, Heschl B, Leutmezer F, et al. Humoral immune response after COVID-19 in multiple sclerosis: A nation-wide Austrian study. Mult Scler (2021) 27(14):2209–18. doi: 10.1177/13524585211049391
Keywords: severe acute respiratory syndrome coronavirus 2, coronavirus disease 2019, vaccine, immunogenicity, safety, autoimmune liver diseases
Citation: Li H, Wang Y, Ao L, Ke M, Chen Z, Chen M, Peng M, Ling N, Hu P, Cai D, Zhang D and Ren H (2022) Association between immunosuppressants and poor antibody responses to SARS-CoV-2 vaccines in patients with autoimmune liver diseases. Front. Immunol. 13:988004. doi: 10.3389/fimmu.2022.988004
Received: 06 July 2022; Accepted: 20 September 2022;
Published: 05 October 2022.
Edited by:
Teodor Doru Brumeanu, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Fikri Y. Avci, School of Medicine, Emory University, United StatesYufeng Gao, First Affiliated Hospital of Anhui Medical University, China
Copyright © 2022 Li, Wang, Ao, Ke, Chen, Chen, Peng, Ling, Hu, Cai, Zhang and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Ren, renhong0531@cqmu.edu.cn; Dachuan Cai, cqmucdc@cqmu.edu.cn; Dazhi Zhang, dzhzhang@yahoo.com
†These authors have contributed equally to this work and share first authorship
 Hu Li
Hu Li Yuting Wang
Yuting Wang Ling Ao
Ling Ao Mingxia Ke
Mingxia Ke Zhiwei Chen
Zhiwei Chen Min Chen
Min Chen Mingli Peng
Mingli Peng Peng Hu
Peng Hu Dachuan Cai
Dachuan Cai Hong Ren
Hong Ren