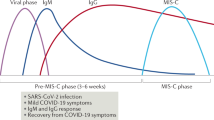
Abstract
Background
There has been a recent upsurge in the cases of Multisystem inflammatory syndrome in children (MIS-C) associated with Coronavirus disease (COVID-19). We performed a systematic review and meta-analysis on the demographic profile, clinical characteristics, complications, management, and prognosis of this emerging novel entity.
Methods
Using a predefined search strategy incorporating MeSH terms and keywords, all known literature databases were searched up till 10th July 2020. The review was done in accordance with PRISMA guidelines and registered in PROSPERO (CRD4202019757).
Results
Of the 862 identified publications, 18 studies comprising 833 patients were included for meta-analysis. The socio-demographic profile showed male predilection (p = 0.0085) with no significant racial predisposition. A higher incidence of gastrointestinal symptoms (603/715, 84.3%), myocarditis (191/309, 61.8%), left ventricular dysfunction (190/422, 45.0%), pericardial (135/436, 31.0%) and neurological symptoms (138/602, 22.9%) was reported. Serological evidence of SARS-CoV-2 had higher sensitivity compared to rtPCR (291/800, 36.4% vs 495/752, 65.8%; p < 0.001). Coronary artery anomaly (CAA) was reported in 117/681 in 9 publications (17.2%). A total of 13 (1.6%) fatalities were reported.
Conclusion
Clinicians need to be vigilant in identifying the constellation of these symptoms in children with clinical or epidemiologic SARS-CoV-2 infection. Early diagnosis and treatment lead to a favorable outcome.
Impact
Key message
-
This review analyses the demographic profile, clinical spectrum, management strategies, prognosis, and pathophysiology of MIS-C among children with SARS-CoV-2 infection.
-
The stark differences of MIS-C from Kawasaki disease with respect to demographics and clinical spectrum is addressed.
-
Over-reliance on rtPCR for diagnosis can miss the diagnosis of MIS-C.
New addition to existing literature
-
The first systematic review and meta-analysis of published literature on MIS-C associated with COVID-19.
Impact
-
The article will serve to spread awareness among the clinicians regarding this emerging novel entity, so that diagnosis can be made early and management can be initiated promptly.
Similar content being viewed by others
Introduction
Having acquired the status of the pandemic by the World Health Organisation, Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) has continued to wreak havoc throughout the globe, creating new records of casualties and death tolls almost daily. On the brighter side, children have reported a relatively lesser incidence of the disease. The first reported childhood infection with SARS-CoV-2 dates back to January 2020, in a family of six members with a history of travel to Wuhan, China.1 Currently, as the numbers show, children <18 years constitute only 1.7% of the cases in the USA2 and 2% of a large observational cohort in the UK.3,4 Kawasaki disease shot into the limelight yet again after a cluster of cases in the pediatric age group emerged across various parts of the world. Bergamo, a place in Italy, with a very high burden of COVID-19 cases reported a 30 fold rise in the incidence of this rare pediatric vasculitis syndrome.5,6 Since then, there has been a flurry of case reports, case series and observational studies over a short span of time in various parts of the world amidst this ever-growing pandemic, shedding light on yet another dimension of SARS-CoV-2, previously unheard of.
Kawasaki-COVID-19—The connection
Kawasaki disease (KD) represents a relatively uncommon but potentially severe pediatric vasculitis syndrome that affects multiple systems. Coronary artery abnormality (CAA) is considered to be one of the most dreaded complications. It was first described in 1967 by a Japanese pediatrician, Dr. Tomisaku Kawasaki, as acute vasculitis with mucocutaneous lymph node syndrome.7 Bacterial infections like Staphylococcus aureus, Streptococcus pyogenes and Yersinia pseudotuberculosis have been implicated in the etiopathogenesis of KD. However, the causal association could not be proved based on advanced modern molecular studies.8 A prospective study of KD in the Netherlands showed a mean annual incidence of 5.8/100,000 among children less than five years of age.9 Chang et al. in 2013 showed a higher rate of viral isolation in 226 patients of Kawasaki disease in which enterovirus was the most common followed by adenovirus, human rhinovirus, and coronavirus.10 The rapidly increasing reports of this multisystem inflammation with definite temporal association with SARS-COV-2 infection led researchers of United Kingdom Royal College of Pediatric and Child Health (UKRCPCH) to formulate an interim case definition, which was provisionally assigned the name of Pediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS).11 To facilitate early recognition of these cases, the World Health Organization (WHO) and the Centre for Disease Control and Prevention (CDC, United States) also came up with case definitions to track the disease early in its natural course and deploy measures for early effective management. See Supplement Appendix for case definitions.12,13
Need for the study
As the number of reports of multisystem inflammatory disease in children associated with COVID-19 keeps uprising to significant numbers, a systematic review with meta-analysis combining all the available literature of this disease entity was felt as a need to the hour to provide an in-depth understanding of the clinico-demographic profile, management strategies, and prognosis.
Methods
Research question
To analyze the demographic profile, clinical characteristics, management strategies, prognosis, and finally assess the hypothetical pathophysiological pathways of the multisystem inflammatory syndrome in children (MIS-C) less than 21 years of age temporally associated with SARS-CoV-2 infection.
Protocol and registration
This systematic review with meta-analysis was constructed in accordance with the framework of Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) (see PRISMA checklist in Supplementary Appendix). The protocol was registered under the International Prospective Register of Systematic Reviews (PROSPERO) (CRD4202019757).
Data sources and searches
We searched the database platforms of MEDLINE, WHO COVID-19 database, LitCOVID, Google Scholar, Science direct, Web of Sciences, ProQuest, Springer library, Wiley online library, Taylor and Francis online, preprints from medRxiv and bioRxiv using the search terms “COVID 19”, “SARSCOV2”, “Multisystem inflammatory syndrome in children”, “MIS-C”, “Pediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2” and “PMIS-TS” for all the articles on 26th June 2020, which was subsequently updated with periodical searches till 10th July 2020. The search was performed by two independent reviewers using appropriately chosen relevant Medical subject headings (MeSH) terms and keywords. The complete list of search terms is provided in the Supplementary Appendix. Shadow search of the reference lists was done manually by independent reviewers at multiple points of time till 10th July 2020 to avoid missing key articles. The search was restricted to articles in the English language.
Study selection based on inclusion and exclusion criteria
The identified articles were reviewed critically based on title, date, place, sample size, authors, and the type of study by two independent reviewers (Dhar D and Samim M). All the abstracts were screened and stratified based on their type and sample size. The following inclusion criteria were taken into account for quantitative analysis of data: (1) case series with 10 or more sample size, (2) cohort studies and (3) retrospective observational studies, and (4) research letter or correspondence articles having data on patient characteristics. The articles which were excluded from quantitative analysis comprised case reports, viewpoints, perspectives, letter to the editors, case series with <10 patients, correspondence articles lacking patient data, and articles focusing only on pathogenesis. The two reviewers independently analyzed the included publications by full-text reading and screening of Supplementary Appendices. In case of discrepancy, a unanimous decision was attained through web-based conference discussions. The inter-rater reliability assessment of the included articles was guided by kappa statistics.14 However, owing to the relative novelty of the disease entity, the excluded articles were considered in the qualitative assessment to provide valuable insights into the depths of our clinical problem.
Data extraction and quality assessment
Data extraction was performed by two reviewers, independently, from the studies included for quantitative analysis. The extracted data were segregated into demographic parameters which included sample size, sex distribution, median age, racial distribution as defined in each study; clinical parameters comprising gastrointestinal symptoms, neurological symptoms, pericardial disease, myocarditis and left ventricular dysfunction (defined by ejection fraction <50%); parameters on complications comprising requirement of vasopressor support, invasive ventilatory support, the incidence of coronary artery abnormalities and mortality; management strategies which included use of Intravenous immunoglobulin, corticosteroids and need for second-line immunomodulators; and finally, COVID-19 status defined based on rtPCR and serological evidence (IgG, IgM, and IgA).
Risk of bias
Two reviewers assessed the robustness of each of these studies by using the National Institute of Health quality assessment tool for case series and observational cohort studies. Each study was evaluated for underlying potential flaws in methodology including sources of bias like patient selection, attrition, detection, confounding factor, and causality association. Any discrepancy was sorted by group discussion between reviewers and further confirmed by a third independent reviewer. The studies were then assigned “good”, “fair”, and “poor” status based on the responses. In view of the relative scarcity of data, it was unanimously decided by the board of authors to maintain a low threshold of inclusion for a study.
Data synthesis and statistics
The extracted data were tabulated in Microsoft Office Excel spreadsheet based on defined variables. Categorical variables were represented with percentages. Median value with interquartile range was used to designate continuous variables. The included publications were subjected to heterogeneity assessment. Pooled estimates with 95% confidence intervals were calculated using random-effects (REML) model after obtaining the effect sizes for each parameter. Cochrane’s Q and I2 statistics were implemented to estimate the heterogeneity quantitatively, based on the method described by Higgins et al.15 I2 values of 25, 50, and 75% were tentatively assigned an as low, moderate, and high degree of heterogeneity that is not attributable to chance alone. Negative values of I2 were considered equivalent to zero. p-values for each of the I2 data were calculated by using χ2 test with (n − 1) degrees of freedom (n = number of studies) and graphically depicted using Forest plot (Fig. 1). To look for Publication bias, funnel plot was constructed. Asymmetry of Funnel plot with meta-bias analysis was carried out by applying Egger’s regression test.16 The Begg and Mazumdar’s nonparametric rank correlation test was applied to assess the correlation between the ranks of effect sizes and the ranks of their variances.17 The heterogeneity and publication bias was computed using STATA - MP version 16.0. For descriptive analysis, the results were then plotted in the component bar diagram (refer to Fig. 2). All the variables could not be defined in every included study due to the unavailability of data. Finally, the individual independent groups were subjected to one-way ANOVA with the Bonferroni criterion.18 The obtained p-value of <0.05 was considered statistically significant. Box and whisker plot was designed using the estimated median with their interquartile range (refer to Fig. 2). The statistical analysis was done using a custom code written in MATLAB. In-depth analysis of any association of the independent variables concerning the severity parameters like Kawasaki disease shock syndrome (KDSS), the requirement of vasopressor support, and left ventricular dysfunction with low ejection fraction cardiac failure could not be carried out due to dearth of patient-specific information in the included publications.
a Coronary artery abnormalities in MIS-C across the studies represented by Forest plot. b Myocarditis in MIS-C across the studies represented by Forest plot. c Gastrointestinal symptoms in MIS-C across the studies represented by Forest plot. d Left ventricular dysfunction in MIS-C across the studies represented by Forest plot. e pericardial disease in MIS-C across the studies represented by Forest plot. f Need for invasive ventilator support in MIS-C across the studies represented by Forest plot. g Neurological symptoms in MIS-C across the studies represented by Forest plot. h Need for vasopressor support in MIS-C across the studies represented by Forest plot.
Results
Study characteristics
Our search strategy yielded a total of 862 articles out of which 665 abstracts were screened after the exclusion of duplicated articles. 618 articles were excluded based on exclusion criteria, yielding a total of 47 publications after add-ons from reference lists. Finally, 18 articles, comprising 8 case series,19,20,21,22,23,24,25,26 8 observational studies (prospective and retrospective)27,28,29,30,31,32,33,34, and 2 surveillance study35,36 with a total of 833 patient records were considered for quantitative analysis (refer to Fig. 3). Inter-reader reliability, assessed using Cohen’s κ, was found to be 0.772, which is equivalent to a moderate level of agreement.14 Dual reviewer quality assessment for assessment of the risk of bias on the NIH scale showed all the studies to be in the “fair” category (as depicted in Table 1). Heterogeneity assessment from Cochrane’s Q, I2 statistics, p-values for respective I2, and Forest plot is described in Table 2, Section A and Fig. 1. The degree of asymmetry of funnel plot was assessed using Egger’s and Begg’s test. Egger’s regression test for meta-bias analysis showed a bias coefficient of 4.40 with a 95% CI of 0.997–2.919, p = 0.0007 suggesting significant evidence for the presence of a small study effect. Kendall’s score obtained from Begg’s test was 44 with a standard error of 20.158, z of 2.13, and p of 0.0329, depicting a significant publication bias.
a Component bar diagram depicting clinical profile, complications, and management strategies of MIS-C in the included studies. b Box and Whisker plots depicting median with interquartile range of male vs female distribution. c Box and Whisker plots depicting median with interquartile range of racial distribution (Blacks- including Afro-Caribbean, Asians, Whites and Hispanics). d Box and Whisker plots depicting median with interquartile range of the rtPCR for SARS-CoV2 infection vs serological evidence.
Clinico-demographic analysis
Patient demographics
Analysis of 833 patient records showed a sex ratio of 1.31. The median age was 8.9 ± 1.9 years. Among the various racial groups, occurrence of MIS-C in black (including Afro-Caribbean), white, Hispanic and Asian ethnicity was 198/579 (34.2%) in 11 publications;19,20,21,22,23,24,25,26,27,28,30,31,33,35 114/530 (21.5%) in 8 articles;20,21,22,23,24,25,32,33,35 106/308 (34.8%) in 5 articles22,23,24,32,35 and 58/349 (16.6%) in 9 publications20,21,22,24,25,27,28,30,33, respectively. One-way ANOVA revealed a male predilection (p = 0.0085) for developing MIS-C in children with SARS-CoV-2 infection. The racial distribution pattern showed no definite predisposition. However, when compared between groups, children of the black population had a significantly greater risk when compared to Asians (p = 0.020). Co-morbidity profile projected overweight/obesity and chronic lung disease as the dominant pre-existing morbidity with a prevalence of 106/405 (28.6%) in 7 publications22,23,24,25,29,32,35 and 13/101(14.9%) in 4 studies,22,24,25,29 respectively. See Table S1 in Supplementary Appendix.
Clinical presentations and complications
In addition to the Kawasaki-like presentations, COVID-19 patients had gastrointestinal symptoms as the predominant feature that got reflected in all the included publications. Meta-analysis of 17 studies19,20,21,22,23,24,25,26,27,28,29,30,31,32,35 estimated an association of gastrointestinal symptoms with PMIS-TS in 603/715, (84.3%) of the subjects, with low evidence of heterogeneity (I2 = 24.36%, p = 0.43). This was followed by myocarditis, which was reported in 7 publications25,26,27,28,31,34,36 summing up to 191/309, (61.8%) of children, with a moderate degree of heterogeneity (I2 = 37.14, p = 0.12). There were 2 dedicated retrospective studies on acute myocarditis (n = 20) and acute heart failure (n = 35) by Grimaud et al.31 and Belhajder et al.29, respectively. Left ventricular dysfunction (ejection fraction < 50%) was reported in 190/422 (45.0%) of the subjects encompassing 8 publications19,20,22,24,25,29,32,35 with high heterogeneity (I2 = 81.14%, p < 0.01). Pericardial disease comprising echocardiographic pericardial effusion and pericarditis was reported in 135/436 (30.9% of patients across 10 studies),19,20,22,25,26,27,28,32,34,35 meta-analysis of which showed low heterogeneity (I2 = 49.23%, p = 0.04). Neurological symptoms which included meningeal signs, headache, confusion, irritability was reported in 138/602 (22.9%) of patients in 13 publications,19,20,21,22,23,24,25,26,27,28,29,32,33,34,35,36 with a high degree of heterogeneity (I2 = 75.50%, p < 0.01). Coronary artery abnormalities were detected in 117/681 (17.2%) of the study subjects in the 16 included articles,19,20,21,22,24,25,26,27,28,29,30,31,32,33,34,35 with moderate heterogeneity, (I2 = 66.26%, p < 0.01). Acute kidney injury was reported in 40/176 (22.7%) of children with MIS-C in 3 studies.23,24,25
SARS-CoV-2 diagnostics
Reverse transcriptase-polymerase chain reaction (rtPCR) from nasopharyngeal, tracheal, and feces sample specimen showed positivity in 291/797 (36.5%) patients across 18 publications compared to serology for SARS-CoV-2 antibodies (IgG, IgA, IgM) which had a much higher positivity rate of 495/749 (66.1%) of the tested patients (p < 0.001). COVID-19 was diagnosed based on the epidemiological context in the absence of evidence by rtPCR and serology for SARS-CoV-2 antibodies in 154/678 study subjects in 10 publications (22.7%).
Management strategies
Meta-analysis showed vasopressor support was needed by 458/783 (61.1%) children in the study population in 16 publications,19,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36 with evidence of high heterogeneity (I2 = 67.90%, p < 0.01). Invasive ventilation support was needed by 226/813 (27.8%) study subjects in 16 articles, with a high degree of heterogeneity (I2 = 88.31, p < 0.01). Intravenous immunoglobulin and aspirin were used as the first line of therapy in the majority of the patients in all the published literature. Corticosteroids were administered to 349/695 (50.2%) subjects.19,20,21,22,23,24,25,27,28,29,31,33,34,35,36 Retreatment with intravenous immunoglobulin was required in 66/266 subjects (24.8%).24,27,28,33,35 Second-line immunomodulators comprising anakinra, tocilizumab were used in 84/577 (14.6%) of the study subjects.19,25,26,27,30,32
Prognosis
A total of 13 fatalities were reported among 833 study subjects (1.6%) across 8 published literature.21,22,25,26,32,33,35,36 Sub-group analysis of all the deaths revealed massive ischemic infarctions of the brain with or without hemorrhage was the cause of death in 4/13 deaths in 4 of the articles. Out of the 4 deaths in the study by Feldstein et al., 2 were due to underlying clinical conditions (asthma and neurological co-morbidities) while one of the patients was negative with respect to both rtPCR and serology. Detailed mortality data was lacking in the rest of the studies. The study by Ramcharan et al.30 had follow-up data on 12 of the MIS-C patients, in which all the subjects had normal CT angiographic imaging.
Review of the studies excluded from meta-analysis
Data from the case reports, letters to the editors, correspondence articles, brief communication articles, and case series with less than 10 sample sizes, which were excluded from meta-analysis, are summarized in Table 2, Section B.
Discussion
Previous studies have shown a connection between Kawasaki disease and viral infections. Turnier et al. in a retrospective study showed that 192 out of 222 (86%) included patients had evidence of upper respiratory tract viral infection detected by PCR.37 The common virus detected was enterovirus/rhinovirus. However, there was no difference in the clinical profile in the groups without viral infection. Esper et al. in 2005, in his case–control study, showed an association between “New Heaven Coronavirus” (HCoV-NH) by rtPCR and Kawasaki disease in 8 out of 11 patients compared to controls (1 out of 22) rendering a p-value of 0.0015.38 Thus, perhaps the link between SARS-CoV-2 and MIS-C is not that surprising after all. Frequent clustering of simultaneous familial Kawasaki disease, as has been reported in several case reports and series, serves to reinforce this connection which we are witnessing now.39
Pathophysiology: the evidence so far
While the pathophysiology behind the late appearance of MIS-C associated with SARS-CoV-2 remains unproven, Rowley H has provided valuable insights, according to which, there may be the role of post-infectious IgG-mediated enhancement effect.40 This post-infectious immune dysregulation harbors at the core of pathomechanisms of SARS-CoV-2. Virus escapade from the futile efforts of phagocytes and its superantigenic character contributes to a hyperimmune response from the helper T cells and activation of macrophages, culminating in a potentially life-threatening cytokine storm.41 At the same time, humoral immune response, mediated by B1 or B2 cells result in the overproduction of IL-6, IL-1ß, IL-12, LAMP-1, IFNGR2, CD244, IgG, IgA, IgM, anti-La, anti aminoacyl t-RNA synthetase, and others.42,43 This paves the way for the formation and deposition of antigen-antibody complexes in the perivascular spaces which leads up to a massive increase in vascular permeability, activation of the complement system, chemotaxis of neutrophils, activation of CD16+ monocytes, decrease in NK cells, pCDc, mDC1, and non-classical monocytes. The resultant inflammatory milieu as a result of this type III hypersensitivity reaction draws forth secretion of CD54 (ICAM-1), CD64, and protease-mediated endothelial, mesothelial, and epithelial inflammation along with tissue damage that manifests as systemic symptoms.44 The fact that the majority of children with MIS-C were tested negative for SARS-CoV-2 rtPCR and instead were positive on serology serves as a crude marker of this hypothesis. However, no reported worsening of illness following convalescent plasma therapy so far somewhat contradicts this view on pathogenesis. High dose Immunoglobulin prior to serological testing could well be an important confounding factor that might have led to false-negative serological test among some of the patients.5 Computational models have also demonstrated the possibility of superantigenic property derived from the unique structural motif of SARS-CoV-2, which acts as a trigger for inflammatory cascade, climaxing to an event akin to Toxic Shock Syndrome.45 Cheng et al. have further demonstrated stronger interaction between human T cells and the virus with a rare mutation (D838Y/N/E) in the European strain of SARS-CoV-2. The higher prevalence of gastrointestinal symptoms as noted in our review can be accounted for by significant expression of Angiotensinogen converting enzyme receptor 2 (ACE-2) in the differentiated enterocytes. The study by Lamers et al. using human small intestinal organoids (hSIO) demonstrated a high affinity of SARS-CoV and SARS-CoV-2 virus to enterocytes.46 Gastrointestinal inflammation can be attributed to mucosal chemotaxis mediated by CCL20, CCL28, and is evident by elevation of the inflammatory markers in blood like MUC4, MUC15, and FOLH1. Similarly, myocarditis has been attributed to the affinity of SARS-CoV-2 to the ACE-2 receptors in the cardiomyocytes.47 Pericardial involvement has been linked with either direct invasion of the SARS-CoV2 or due to proinflammatory milieu induced by the virus.47,48 Inflammatory markers of endothelial dysfunction like P2RX4, ECE2, CLEC14A, VEGFA have been linked to cardiac involvement.42 Refer to Fig. 4 for further details.
a The early phase of SARS-CoV-2 infection is devoid of any immune response. As the disease progresses to the late phase, owing to its superantigenic property, the virus evades destruction by phagocytes. The mass proliferation of the virus subsequently triggers dysregulated activation of macrophages and initiates a hyperimmune response mediated by helper T cells. This subsequently leads to a tremendous efflux of IL-6, IL-1ß, IL-12, LAMP-1, IFNGR2, and CD244. The humoral response, mediated by the proliferation of B1 or B2 cells leads to the overproduction of antibodies (IgG, IgA, Anti-La, aminoacyl t-RNA synthetase). The antigen-antibody complexes, thus formed mediates a Type III hypersensitivity reaction resulting in further activation of mast cells, neutrophils, macrophages, secretion of CD54 (ICAM-1), CD64 (FcγR1), CD16+ monocytes, and activation of complement pathways. At the same time, there is a decrease in levels of CD56 bright and CD56 dim NK cells, pCDc, mDC1 and non-classical monocytes. The resultant inflammatory milieu causes protease-mediated endothelial, mesothelial, and epithelial inflammation with subsequent tissue damage leading to systemic symptoms. b Depicting the pathogenesis of the most commonly encountered organ systems involved in MIS-C. Gastrointestinal symptoms, attributed mostly to mucosal chemotaxis, are mediated by CCL20 and CCL28. This leads to elevation of the several inflammatory markers in blood like MUC4, MUC15, and FOLH1. Cardiac symptoms are attributed to inflammatory mediators of endothelial dysfunction such as P2RX4, ECE2, CLEC14A, and VEGFA. c Delineates the role of STING (stimulator of interferon gene) pathway. Transmembrane protein 173 (TMEM173), located in chromosome 5q31.2, a ~7-kb-long gene encodes the STING protein. STING can be activated by cytosolic DNA (damaged self-DNA) and RNA viruses leading to activation of NFκß, IRF-3, and subsequently overproduction of interferon ß and cytokines. This results in STING-associated vasculopathy with onset in infancy (SAVI) like manifestations characterized by fever, rash, pulmonitis, myositis, lymphopenia, inflammatory vasculopathy, and rarely acral necrosis. d Role of STING pathway in SARS-CoV pathogenesis in the early phase “During its early phase of SARS-CoV was shown to cause downregulation of the STING pathway through nsp3, nsp 16”. e While the role of the STING pathway in the early phase of SARS-CoV-2 infections remains unknown, COVID-19, in its late phase, leads to excessive upregulation of STING protein which results in massive stimulation of NF-κß and IRF-3, thereby causing a huge surge in the levels of interferon ß and cytokines. f The human tissues expressing STING protein include alveoli of the lung, endothelial cells, and spleen. g Depicts the various polymorphisms of TMEM173 gene.
Role of the STING pathway
While the early phase in the pathogenesis of SARS-CoV2-mediated MIS-C largely remains unexplored, extrapolation of knowledge from SARS-CoV pathomechanisms hints at a possible role of the STING pathway (stimulator of interferon-gamma). STING protein, encoded by TMEM173 (transmembrane protein) and expressed in alveoli, endothelial cells, and spleen can be activated by DNA debris of host cells, degraded mitochondrial DNA as well as RNA viruses. ACE-2 activation mediated by SARS-CoV-2 has also been found to upregulate this pathway. Subsequently, there is a massive release of interferon ß and cytokines resulting from activation of NF-κß and IRF-3. This leads to STING-associated vasculopathy with onset in infancy (SAVI) like manifestations49,50 typified by fever, lung injury, vascular inflammation, myositis, dermatologic manifestations, acral necrosis, and arterial aneurysms. Polymorphisms of TMEM173 among various racial subgroups perhaps account for the variations in clinical presentation and severity of the disease manifestations.
Kawasaki-MIS-C: the mismatch
While the connection between the two disease entities is still hypothetical, the differences albeit, are glaring. The median age of children in this review, 8.9 ± 1.9 years, is quite different from the typical age group in which Kawasaki disease usually manifests. The 5-year National surveillance study of the Netherlands by Take et al. has shown median age of 2.4 years.9 The data from all over the globe have suggested an overwhelming predominance of the disease among the under-fives.51,52,53,54,55 In contrary to the widespread prevalence of Kawasaki disease in the Asian continent, the prevalence of MIS-C has not been that striking even though the number of cases of COVID-19 has also been quite significant in this part of the globe. In fact, in this review, the blacks had a significantly greater incidence compared to the Asians. A majority of the reported cases meeting the criteria of MIS-C didn’t meet the American Heart Association (AHA) criteria for complete Kawasaki disease, another pointer of mismatch between these two clinical conditions. The frequent occurrences of gastrointestinal symptoms, neurological features, myocarditis, left ventricular dysfunction with a relative sparing effect on renal functions as evidenced from this review throw striking daylight on the genuine differences in clinical presentations of these two clinical entities.
Key aspects of the review
From the evidence gathered from this systematic review and meta-analysis, we suggest this unique combination of Kawasaki-like illness with a definite consortium with gastrointestinal symptoms, neurologic manifestations including meningeal signs, myocarditis with or without left ventricular dysfunction, and symptomatic or echocardiographic pericardial involvement, be assigned a different nosology called Systemic inflammatory syndrome in COVID-19 or SISCoV. This will enable faster characterization of this clinical entity, the inclusion of relevant investigations, and appropriate evidence-based management thereby preventing fatalities.
Research gaps
The wide geographic distribution of the studies, observational design, and lack of follow-up are some of the key factors reducing the robustness of this review. As the objectives differed among various studies, the data representation was less adequate in some of the variables. A dearth of detailed patient-wise distribution in many of the published articles including their Supplementary Appendices was a significant hindrance to the use of advanced statistical analysis. The natural course of the coronary artery abnormalities remains an unexplored frontier which will only be revealed in due course of time through follow-up studies.
Conclusion
With the emergence and subsequent uprise of MIS-C, there needs to be strict vigilance among children presenting to the emergency department or Pediatric Intensive Care Unit. A high index of suspicion should be maintained in patients reporting gastrointestinal symptoms, evidence of circulatory failure, central nervous system symptoms, left ventricular dysfunction, and symptomatic or echocardiographic pericardial effusion even if rtPCR for SARS-CoV-2 is negative. It is important to abide by the guidelines for diagnosis and management laid by WHO, UKRCPCH, and CDC. Early management with intravenous immunoglobulins and aspirin has been found to have a favorable outcome. Certain groups of patients require retreatment with IVIg, while others require initiation of second-line immunomodulation. A baseline evaluation for coronary artery abnormalities forms an essential component for future surveillance. Fatalities due to MIS-C are usually rare especially when suspected early, diagnosed promptly, investigated rationally, and treated methodically.
References
Choi, S. H., Kim, H. W., Kang, J. M., Kim, D. H. & Cho, E. Y. Epidemiology and clinical features of coronavirus disease 2019 in children. Pediatr. Infect. Vaccin. 27, 11–23 (2020).
Bialek, S. et al. Coronavirus disease 2019 in children — United States, February 12–April 2, 2020. Morb. Mortal. Wkly. Rep. 69, 422–426 (2020).
Docherty, A. B. et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. Preprint at https://www.medrxiv.org/content/10.1101/2020.04.23.20076042v1 (2020).
Jiang, L. et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 20, e276–88 (2020).
Verdoni, L. et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 6736, 1–8 (2020).
Viner, R. M. & Whittaker, E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet 6736, 19–20 (2020).
Kawasaki, T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 16, 178–222 (1967).
Komatsu, H. & Fujisawa, T. Kawasaki disease and infection. Nihon Rinsho 66, 278–282 (2008).
Tacke, C. E. et al. Five years of Kawasaki disease in the Netherlands a national surveillance study. Pediatr. Infect. Dis. J. 33, 793–797 (2014).
Chang, L. Y. et al. Viral infections associated with Kawasaki disease. J. Formos. Med. Assoc. 113, 148–154 (2014).
Heald, R. C. Guidance paediatric multisystem inflammatory syndrome temporallly associated with Cov-19. R. Coll. Paediatr. Child Heal. 1–6 (2020).
Kest, H., Kaushik, A., DeBruin, W., Colletti, M. & Goldberg, D. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with 2019 Novel Coronavirus (SARS-CoV-2) Infection. Case Rep. Pediatr. 2020, 4 (2020).
WHO. Multisystem Inflammatory Syndrome in Children and Adolescents with COVID-19 1–3 (2020).
McHugh, M. L. Interrater reliability: the kappa statistic. Biochem. Med. 22, 276–282 (2012).
Grant, J. & Hunter, A. Measuring inconsistency in knowledgebases. J. Intell. Inf. Syst. 27, 159–184 (2006).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test measures of funnel plot asymmetry. BMJ 315, 629–634 (1997).
Begg, C. B. & Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 50, 1088 (1994).
Armstrong, R. A. When to use the Bonferroni correction. Ophthal. Physiol. Opt. 34, 502–508 (2014).
Verdoni, L. et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395, 1771–1778 (2020).
Cheung, E. W. et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA https://doi.org/10.1001/jama.2020.10374 (2020).
Whittaker, E. et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 324, 259–269 (2020).
Kaushik, S. et al. Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection: a multi-institutional study from New York City. J. Pediatr. 224, 24–29 (2020).
Miller, J. et al. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology 159, 1571–1574 (2020).
Rasmussen, S. A., Smulian, J. C., Lednicky, J. A., Wen, T. S. & Jamieson, D. J. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am. J. Obstet. Gynecol. 222, 415–426 (2020). (2020).
Dufort, E. M. et al. Multisystem inflammatory syndrome in children in New York State. N. Engl. J. Med. 383, 347–358 (2020).
Shema, H. et al. Spectrum of imaging findings on chest radiographs, US, CT, and MRI images in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Radiology 298, E1–10 (2021).
Toubiana, J. et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 369, m2094 (2020).
Pouletty, M. et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann. Rheum. Dis. 79, 999–1006 (2020).
Belhadjer, Z. et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic Running title: Belhadjer et al.; Pediatric acute heart failure and SARS-CoV-2 infection Covid-19. Circulation 142, 429–436 (2020).
Ramcharan, T. et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK Tertiary Paediatric Hospital. Pediatr. Cardiol. 41, 1391–1401 (2020).
Grimaud, M. et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann. Intens. Care. 10, 69 (2020).
Riollano-Cruz, M. et al. Multisystem inflammatory syndrome in children (MIS-C) related to COVID-19: a New York City experience. J. Med Virol. 93, 424–433 (2020).
Davies, P. et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc. Heal 4, 669–677 (2020).
Ouldali, N. et al. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc. Heal. 4, 662–668 (2020).
Feldstein, L. R. et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 383, 334–346 (2020).
Belot, A. et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France 1 March to 17 May 2020. Eurosurveillance 25 (2020).
Turnier, J. L. et al. Concurrent respiratory viruses and Kawasaki disease. Pediatrics 136, e609–14 (2015).
Esper, F. et al. Association between a novel human coronavirus and kawasaki disease. J. Infect. Dis. 191, 499–502 (2005).
Ebina-Shibuya, R., Namkoong, H., Shibuya, Y. & Horita, N. Multisystem inflammatory syndrome in children (MIS-C) with COVID-19: Insights from simultaneous familial Kawasaki Disease cases. Int. J. Infect. Dis. 97, 371–373 (2020).
Rowley, A. H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat. Rev. Immunol. 20, 453–454 (2020).
Gruber, C. N. et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell 183, 982–95.e14 (2020).
Giamarellos-Bourboulis, E. J. et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27, 992–1000.e3 (2020).
Rawat, M., Chandrasekharan, P., Hicar, M. D. & Lakshminrusimha, S. COVID-19 in newborns and infants-low risk of severe disease: silver lining or dark cloud? Am. J. Perinatol. 37, 845–849 (2020).
Roe, K. A viral infection explanation for Kawasaki disease in general and for COVID-19 virus-related Kawasaki disease symptoms. Inflammopharmacology 28, 1219–1222 (2020).
Cheng, M. H. et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc. Natl. Acad. Sci. U. S. A. 117, 25254–25262 (2020).
Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54 (2020)
Siripanthong, B. et al. Recognizing COVID-19–related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Hear. Rhythm. 17, 1463–1471 (2020).
Farina, A., Uccello, G., Spreafico, M., Bassanelli, G. & Savonitto, S. SARS-CoV-2 detection in the pericardial fluid of a patient with cardiac tamponade. Eur. J. Intern. Med. 76, 100–101 (2020).
Berthelot, J. M. & Lioté, F. COVID-19 as a STING disorder with delayed over-secretion of interferon-beta. EBioMedicine 56, 6–8 (2020).
Berthelot, J. M., Drouet, L. & Lioté, F. Kawasaki-like diseases and thrombotic coagulopathy in COVID-19: delayed over-activation of the STING pathway? Emerg. Microbes Infect. 9, 1514–1522 (2020).
Nakamura, Y. et al. Epidemiologic features of Kawasaki disease in Japan: Results of the 2009-2010 nationwide survey. J. Epidemiol. 22, 216–221 (2012).
Lue, H. C. et al. Epidemiological features of Kawasaki disease in Taiwan, 1976-2007: results of five nationwide questionnaire hospital surveys. Pediatr. Neonatol. 55, 92–96 (2014).
Kim, G. B. et al. Epidemiologic features of Kawasaki disease in South Korea: data from nationwide survey, 2009-2011. Pediatr. Infect. Dis. J. 33, 24–27 (2014).
Harnden, A. et al. Kawasaki disease in England: ethnicity, deprivation, and respiratory pathogens. Pediatr. Infect. Dis. J. 28, 21–24 (2009).
Beom, K. G. Reality of kawasaki disease epidemiology. Korean J. Pediatr. 62, 292–296 (2019).
Jones, V. G. et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp. Pediatr. 10, 537–540 (2020).
Rauf, A., Vijayan, A., John, S. T., Krishnan, R. & Latheef, A. Multisystem inflammatory syndrome with features of atypical Kawasaki disease during COVID-19 pandemic. Indian J. Pediatr. 87, 745–747 (2020).
Labé, P. et al. Erythema multiforme and Kawasaki disease associated with COVID‐19 infection in children. J. Eur. Acad. Dermatol. Venereol. 34, e539–41 (2020).
Dollinger, M. T. et al. Pediatric Crohn’s disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J. Chem. Inf. Model. 53, 287 (2008).
Greene, A. G., Saleh, M., Roseman, E. & Sinert, R. Toxic shock-like syndrome and COVID-19: A case report of multisystem inflammatory syndrome in children (MIS-C). Am. J. Emerg. Med. 38, 2492.e5–2 (2020).
Chiu, J. S., Lahoud-Rahme, M., Schaffer, D., Cohen, A. & Samuels-Kalow, M. Kawasaki disease features and myocarditis in a patient with COVID-19. Pediatr. Cardiol. 41, 1526–1528 (2020).
Yozgat, C. Y. et al. Dermatological manifestation of pediatrics multisystem inflammatory syndrome associated with COVID ‐19 in a 3‐year‐old girl. Dermatol. Ther. 33, e13770 (2020).
Leon, M. P. D. et al. COVID-19 associated pediatric multi-system inflammatory syndrome. J. Pediatr. Infect. Dis. Soc. 9, 407–408 (2020).
Rodriguez-gonzalez, M. et al. New onset severe right ventricular failure associated with COVID-19 in a young infant without previous heart disease. Cardiol. Young-. 30, 1346–1359 (2020).
Alizargar, J. The novel coronavirus (COVID-19) and the risk of Kawasaki disease in children. J. Formos. Med. Assoc. 119, 1713–1714 (2020).
Ng, K. F. et al. COVID-19 multisystem inflammatory syndrome in three teenagers with confirmed SARS-CoV-2 infection. J. Med. Virol. 92, 2880–2886 (2020).
Regev, T. et al. Pediatric inflammatory multisystem syndrome with central nervous system involvement and hypocomplementemia following Sars-Cov-2 infection. Pediatr. Infect. Dis. J. 39, e206–e207 (2020).
Hutchison, L., Plichta, A. M., Lerea, Y., Madora, M. & Ushay, H. M. Neuropsychiatric symptoms in an adolescent boy with multisystem inflammatory syndrome in children (MIS-C). Psychosomatics 61, 739–744 (2020).
Wolfe, D. M., Nassar, G. N., Divya, K., Krilov, L. R. & Noor, A. Young children presenting with fever and rash in the midst of SARS-CoV-2 outbreak in New York. Clin. Pediatr. 59, 1112–1118 (2020).
Wacker, J. et al. Coronary artery dilatation in a child with hyperinflammatory syndrome with SARS-CoV-2-positive serology. Eur. Heart J. 41, 3103 (2020).
Nguyen, D. C., Haydar, H., Pace, E. R., Zhang, X. S. & Dobbs, R. K. Pediatric case of severe COVID-19 with shock and multisystem inflammation. Cureus 12, e8915 (2020).
Vari, D. et al. Cardiac dysfunction in a patient with multisystem inflammatory syndrome in children associated with COVID-19: retrospective diagnosis of a puzzling presentation. A case report. Prog. Pediatr. Cardiol. 58, 101270 (2020).
Bapst, T., Romano, F., Müller, M. & Rohr, M. Special dermatological presentation of paediatric multisystem inflammatory syndrome related to COVID-19: erythema multiforme. BMJ Case Rep. 13, e236986 (2020).
Giannattasio, A. et al. A child with a severe multisystem inflammatory syndrome following an asymptomatic COVID‐19 infection: a novel management for a new disease? J. Med. Virol. 93, 112–114 (2020).
Klocperk, A. et al. Case report: systemic inflammatory response and fast recovery in a case report: systemic inflammatory response and fast recovery in a pediatric patient With COVID-19. Front. Immunol. 11, 1665 (2020).
Saeed, A., Sanaei, A., Ghotababadi, S. H. & Shorafa, E. Case report: Pediatric patients with COVID-19 presented as multi-system inflammatory syndrome. Res. Square 1–11 (2020).
Licciardi, F. et al. SARS-CoV-2-induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics 146, e20201711 (2020).
Chiotos, K. et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J. Pediatr. Infect. Dis. Soc. 9, 393–398 (2020).
Waltuch, T. et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am. J. Emerg. Med. 38, 2246.e3–2246.e6 (2020).
Riphagen, S., Gomez, X., Gonzalez-Martinez, C., Wilkinson, N. & Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395, 1607–1608 (2020).
Blondiaux, E. et al. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology 297, E283–E288 (2020).
Dallan, C. et al. Septic shock presentation in adolescents with COVID-19. Lancet Child Adolesc. Heal 4, e21–23 (2020).
Acknowledgements
Our heartfelt acknowledgment to late Dr. Tomisaku Kawasaki, who departed from us on June 5, 2020, leaving behind a legacy that will remain unmatched in years to come. We sincerely acknowledge the efforts of all the clinicians and healthcare workers doing their utmost in these challenging times.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the formulation of this study. Authors D.D., T.D., M.M.S, H.P., A.C., N.P., S.M.S, P.K., A.P., and A.G. were involved in conceptualization. Review of literature review, data extraction, and data synthesis were done by D.D., M.M.S., N.P., S.R., A.G., and A.P. Quality assessment of the studies were done by D.D., M.M.S, H.P, N.P., C.S.R, and M.K. Statistical analyses were carried out by T.D. and A.C. Drafting, and framing of images was done by D.D. and M.M.S. Analysis and interpretation of results were carried out by D.D., M.M.S., T.D., H.P, C.S.R., M.K., and S.R. All authors played an active role in the revision of the manuscript. Final approval was obtained from all authors prior to submission of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Consent from the patients was not required in the conduct of this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dhar, D., Dey, T., Samim, M.M. et al. Systemic inflammatory syndrome in COVID-19–SISCoV study: systematic review and meta-analysis. Pediatr Res 91, 1334–1349 (2022). https://doi.org/10.1038/s41390-021-01545-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01545-z
This article is cited by
-
Nasopharyngeal angiotensin converting enzyme 2 (ACE2) expression as a risk-factor for SARS-CoV-2 transmission in concurrent hospital associated outbreaks
BMC Infectious Diseases (2024)
-
Multisystem inflammatory syndrome in children: an Umbrella review
Journal of Anesthesia (2024)
-
Myocarditis mortality with and without COVID-19: insights from a national registry
Clinical Research in Cardiology (2024)
-
Epidemiology, Clinical Features, and Outcomes of Multisystem Inflammatory Syndrome in Children (MIS-C) and Adolescents—a Live Systematic Review and Meta-analysis
Current Pediatrics Reports (2022)