A case of Blaschkoid pityriasis rosea following COVID-19 vaccination: A rare exceptional occurrence
Aakanksha Arora, Alpana Mohta , Prasoon Soni
, Prasoon Soni
Department of Dermatology, Sardar Patel Medical College, Bikaner, Rajasthan, India
Corresponding author: Alpana Mohta
Submission: 16.01.2022; Acceptance: 19.05.2022
DOI: 10.7241/ourd.20223.10
Cite this article: Arora A, Mohta A, Soni P. A case of Blaschkoid pityriasis rosea following COVID-19 vaccination: A rare exceptional occurrence. Our Dermatol Online. 2022;13(3):286-288.
Citation tools:
Copyright information
© Our Dermatology Online 2022. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
The launch of COVID-19 vaccines in India has raised the expectations of the dreadful COVID-19 pandemic ending in the future. Various mild and benign cutaneous manifestations of the different forms of the COVID-19 vaccine have been documented. Herein, we are reporting a unique case of Blaschkoid pityriasis rosea (PR) developing after COVID-19 vaccination. A forty-two-year-old female presented with PR along a linear arbitrary zone on the back at the level of L1-L2 extending to involve the abdomen and an oblique zone on the thigh. She was vaccinated with the first dose of the COVISHIELD ChAdOx1/nCoV-19 (recombinant) coronavirus vaccine six days before the onset of the lesions. There are only several case reports of typical pityriasis rosea occurring after COVID-19 vaccination. Our unique case depicts the occurrence of atypical PR after COVID-19 vaccination.
Key words: Blaschkoid pityriasis rosea; COVID-19; COVISHIELD; lines of Blaschko; pityriasis rosea
INTRODUCTION
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection has taken a great toll on the world, causing over two million deaths and economical losses. The launch of COVID-19 vaccines in India has raised the expectations of this dreadful pandemic ending in the future. The Indian population has enthusiastically accepted the vaccine with millions of people being vaccinated. Various mild and benign cutaneous manifestations of different forms of the COVID-19 vaccine have been documented [1]. Herein, we are reporting a unique case of Blaschkoid pityriasis rosea (PR) developing after COVID-19 vaccination. Although PR is a common acute exanthematous disease, around 20% of patients with PR have atypical clinical presentations, posing a risk of misdiagnosis [2].
CASE REPORT
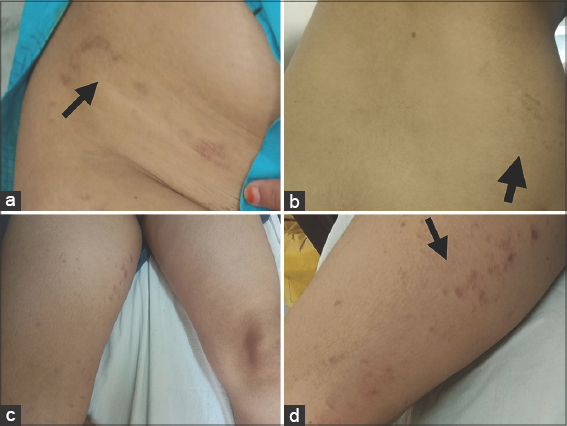
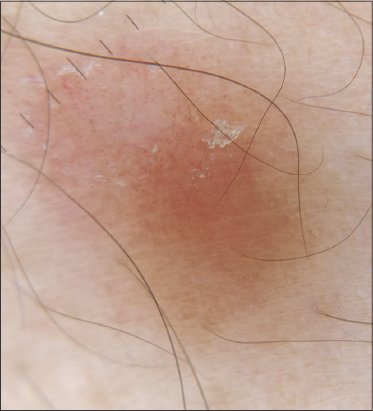
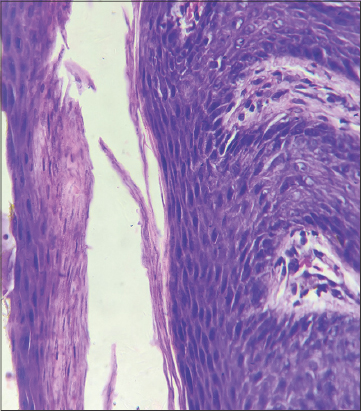
A forty-two-year-old female presented to our skin outpatient department with a well-defined, erythematous plaque, 2 × 3 cm in size, on the lower aspect of the right side of the abdomen. Two days later, secondary lesions developed around the primary herald patch progressing to involve the back, flank, and medial aspect of the thigh in a linear distribution. The distribution of the lesions lay along a linear arbitrary zone stretching from the midline of the back at the level of L1-L2 extending to involve the abdomen (Figs. 1a and 1b) and an oblique arbitrary zone on the thigh extending from the right anterior superior iliac spine to the medial aspect of the right thigh (Figs. 1c and 1d). All lesions had peripheral collarette scaling with a clear wrinkled center. The appearance of the lesions was not preceded by any prodrome. There was no significant history of allergic or irritant contact dermatitis, recent infections or drug intake, contact with a COVID-19-positive patient, a similar skin rash in the past, or a family history. However, the patient was vaccinated with the first dose of the COVISHIELD ChAdOx1/nCoV-19 (recombinant) coronavirus vaccine six days before the onset of the lesions. Routine laboratory investigations were normal. A SARS-CoV-2 PCR test performed from a nasopharyngeal swab sample was negative. Dermoscopy of the lesions revealed peripheral collarette scaling, blood spots, peripheral dotted vessels, and brown globules (Fig. 2). A punch biopsy taken from the lesions revealed psoriasiform hyperplasia, focal hyperkeratosis, slight parakeratosis, lymphocytic infiltrate around vessels, and red blood cell extravasation (Fig. 3). A possible differential diagnosis included various linear inflammatory skin disorders such as linear psoriasis, linear lichen planus, lichen striatus, and adult blaschkitis. Based on a detailed history and morphology of the lesions, we excluded the possibility of these disorders. The rash fulfilled the diagnostic criteria of pityriasis rosea [3]. Based on the strict unilateral distribution of the group of lesions along linear, oblique strips following the lines of Blaschko, a diagnosis of Blaschkoid PR was reached. The patient was prescribed oral antihistamines and topical betamethasone dipropionate lotion followed by complete recovery within fifteen days. However, the patient returned to the outpatient department two months later with similar lesions involving the same area following the second dose of the COVISHIELD vaccine with a latency period of four days. She was prescribed the same treatment and the lesions resolved in fifteen days.
DISCUSSION
PR is a common, acute, self-limiting exanthema, yet its exact cause remains unknown. Classical PR is characterized by the appearance of a herald patch followed by discrete lesions, with a symmetrical distribution involving the trunk and proximal parts of the limbs. However, atypical and rare variants of pityriasis rosea have been reported in the literature. Based on the atypical sites and distributions, the lesions may be described as inverse, acral, Blaschkoid, unilateral, limb girdle, mucosal, localized, etc. [4]. Blaschkoid PR is a highly rare variant of PR [5]. The term suggests that the individual lesions coalesce into a group, with the entire group following and extending along Blaschko’s lines [6]. The orientation of the individual lesions is not included in this term. The distribution of the lesions is strictly unilateral in Blaschkoid PR. Blaschko’s lines are representative of the route of migration of embryonic ectodermal cells. Grosshans hypothesized that the unmasking of tolerance or susceptibility of abnormal keratinocyte clones by an underlying disease leads to the Blaschko linear distribution of lesions in various acquired dermatoses [7]. Genetic mosaicism to the offending infectious agent trigger an abnormal response of the susceptible clone of a keratinocyte [6,7]. Blaschkoid PR is an extremely rare variant of PR. As of today, we were able to find only two case reports on adults by the Medline search queries “pityriasis rosea” and “lines of Blaschko” (Table 1). Our case was unique in that this rare form of PR was preceded by the administration of the COVISHIELD ChAdOx1/nCoV-19 (recombinant) coronavirus vaccine. There are only several case reports of pityriasis rosea occurring after COVID-19 vaccination [8,9]. The exact pathogenesis behind PR after vaccination is unknown. We hypothesized that the immune response triggered by the vaccine elevated the plasma cytokine levels and distracted the T-cell-mediated control on the latent infections, unmasking the tolerance of abnormal keratinocytes along the particular Blaschkoid’s lines [10]. Case reports of typical PR following COVID-19 vaccination already exist in the literature. However, the triggering of atypical forms such as Blaschkoid PR following a COVID-19 vaccine is a highly rare occurrence.
 |
Table 1: Cases of Blaschkoid pityriasis rosea in adults identified by PubMed compared with the case reported herein. |
CONCLUSION
Several case reports of typical PR following COVID-19 vaccination already exist in the literature. However, the triggering of atypical forms such as Blaschkoid PR following a COVID-19 vaccine is a highly rare occurrence. A dermatologist must always be on the lookout for these rare cutaneous adverse effects of COVID-19 vaccines. Therefore, we claim that COVID-19 vaccination may be a potential trigger of PR.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination:A registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
2. Chuh A, Zawar V, Lee A. Atypical presentations of pityriasis rosea:Case presentations. JEur Acad Dermatol Venereol. 2005;19:120-6.
3. Zawar V, Chuh A. Applicability of proposed diagnostic criteria of pityriasis rosea:Results of a prospective case-control study in India. Indian J Dermatol. 2013;58:439-42.
4. Mahajan K, Relhan V, Relhan AK, Garg VK. Pityriasis rosea:An update on etiopathogenesis and management of difficult aspects. Indian J Dermatol. 2016;61:375-84.
5. Zawar V, Chuh A. Pityriasis rosea along Blaschko’s lines:A rare variant. Indian J Dermatol Venereol Leprol. 2017;83:516.
6. Ang CC, Tay YK. Blaschkoid pityriasis rosea. J Am Acad Dermatol. 2009;61:906-8.
7. Grosshans EM. Acquired Blaschko linear dermatoses. Am J Med Genet. 1999;85:334-7.
8. Marcantonio-SantaCruz OY, Vidal-Navarro A, PesquéD, Giménez-Arnau AM, Pujol RM, Martin-Ezquerra G. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:721-22.
9. Abdullah L, Hasbani D, Kurban M, Abbas O. Pityriasis rosea after mRNA COVID-19 vaccination. Int J Dermatol. 2021;60:1150-51.
10. Drago F, Ciccarese G, Javor S, Parodi A. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions:A review of the literature. J Eur Acad Dermatol Venereol. 2016;30:54.
Notes
Source of Support: Nil,
Conflict of Interest: None declared.
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
|



Comments are closed.