- 1The Department of Medical Imaging Guangdong Second Provincial General Hospital, Guangzhou, China
- 2The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
- 3The Department of Nuclear Medicine, Guangdong Second Provincial General Hospital, Guangzhou, China
Aim: Previously, neuroimaging studies on comorbid Posttraumatic-Major depression disorder (PTSD-MDD) comorbidity found abnormalities in multiple brain regions among patients. Recent neuroimaging studies have revealed dynamic nature on human brain activity during resting state, and entropy as an indicator of dynamic regularity may provide a new perspective for studying abnormalities of brain function among PTSD-MDD patients. During the COVID-19 pandemic, there has been a significant increase in the number of patients with PTSD-MDD. We have decided to conduct research on resting-state brain functional activity of patients who developed PTSD-MDD during this period using entropy.
Methods: Thirty three patients with PTSD-MDD and 36 matched TCs were recruited. PTSD and depression symptoms were assessed using multiple clinical scales. All subjects underwent functional magnetic resonance imaging (fMRI) scans. And the brain entropy (BEN) maps were calculated using the BEN mapping toolbox. A two-sample t-test was used to compare the differences in the brain entropy between the PTSD-MDD comorbidity group and TC group. Furthermore, correlation analysis was conducted between the BEN changes in patients with PTSD-MDD and clinical scales.
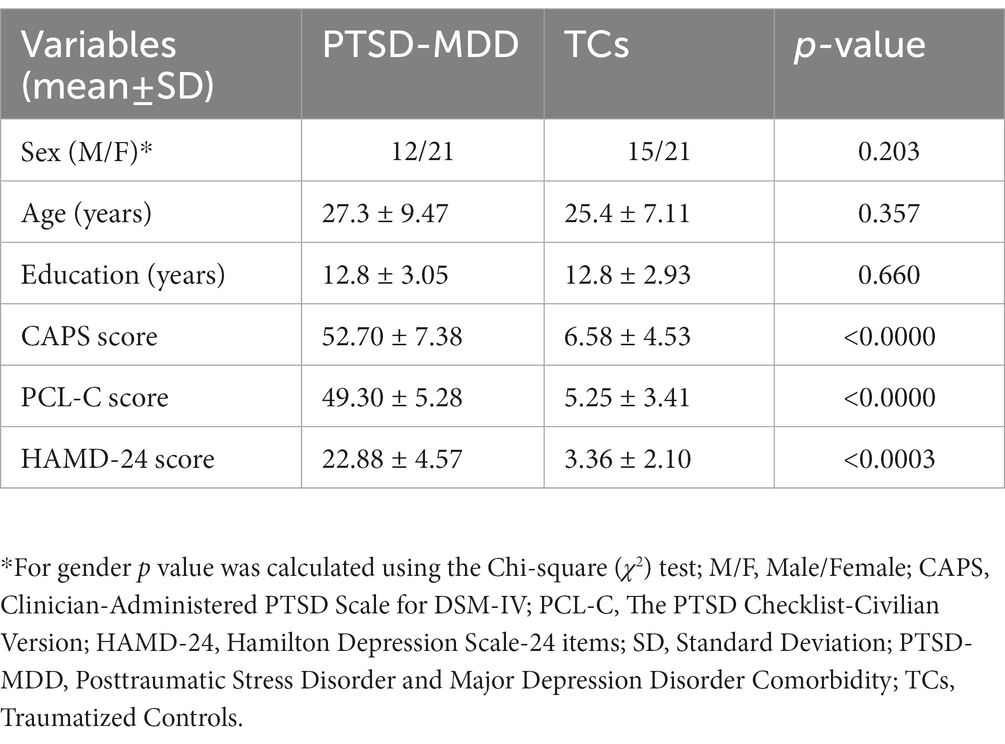
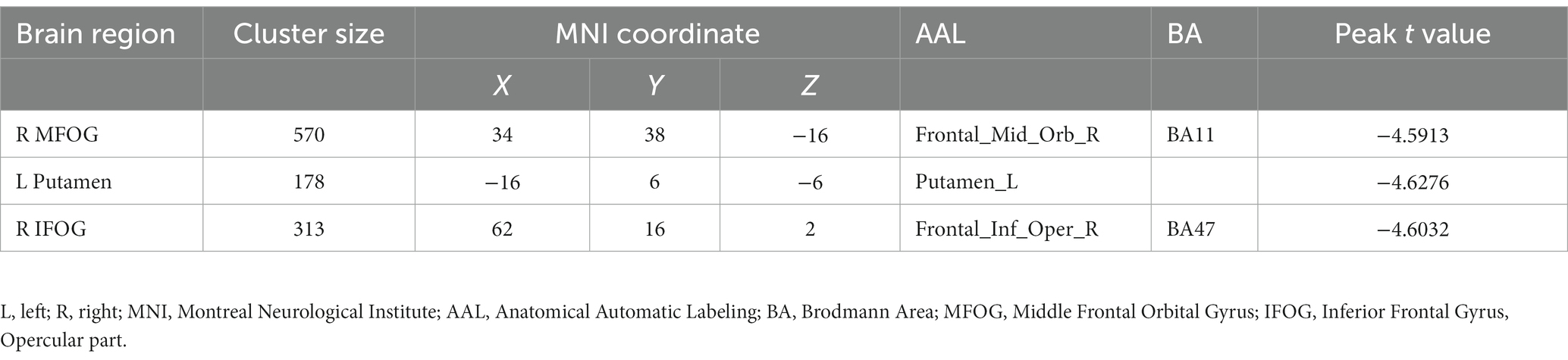
Results: Compared to the TCs, PTSD-MDD patients had a reduced BEN in the right middle frontal orbital gyrus (R_MFOG), left putamen, and right inferior frontal gyrus, opercular part (R_IFOG). Furthermore, a higher BEN in the R_MFOG was related to higher CAPS and HAMD-24 scores in the patients with PTSD-MDD.
Conclusion: The results showed that the R_MFOG is a potential marker for showing the symptom severity of PTSD-MDD comorbidity. Consequently, PTSD-MDD may have reduced BEN in frontal and basal ganglia regions which are related to emotional dysregulation and cognitive deficits.
1. Introduction
Posttraumatic stress disorder (PTSD) and major depressive disorder (MDD) are common psychiatric mental disorders associated with the outbreak of the coronavirus disease 2019 (COVID-19); according to an epidemiological study, the prevalence of PTSD and depression was 21.94 and 15.97%, respectively (1). As a stressful event, the coronavirus disease 2019 (COVID-19) pandemic outbreak has had a critical impact on mental health and has been associated with both PTSD and depression (2–5). Therefore, unprecedented public health measures have been implemented to prevent the spread of the virus, as over half of the population was affected by this epidemic (6–8). Although home quarantine is an effective strategy to curtail virus transmission (9–11), it can have negative psychiatric effects simultaneously. Studies have reported the occurrence of negative psychological effects including PTSD, depression, anxiety, and insomnia during the home quarantine (12) and also showed that longer quarantine durations were associated with PTSD and depression (13–15).
PTSD is a highly comorbid disease, and epidemiological studies have reported that 52% of the patients with PTSD had comorbid depression (16–18). Recently, a genetic study indicated that PTSD is a subtype of MDD (19). In addition, studies have suggested that the co-occurrence of PTSD and MDD is linked with severe cognitive deficits, emotional symptoms, and a longer course than in patients with MDD or PTSD alone (20–22). For PTSD patients with comorbid depression, they require a larger dosage of psychotropic drugs compared to those with only PTSD (23). Although, a high incidence and severity of PTSD-MDD comorbidity have been established, the knowledge about its neurobiological mechanisms is limited.
The resting state functional magnetic resonance imaging (rs-fMRI) employs the blood oxygenation level-dependent (BOLD) signal to characterize the spontaneous activity of the brain (24). In addition, rs-fMRI is easy to implement and provides brain function time-resolved imaging at a relatively high spatial resolution compare to the EEG, making it a widely used tool for studying psychiatric diseases (25). Although rs-fMRI has been widely used in psychiatric disease, it is rarely applied in the study of PTSD-MDD comorbidity. A fMRI study suggesting that the subgenual anterior cingulate gyrus might be a potential neurobiological marker in distinguishing PTSD-MDD comorbidity from PTSD patients (26). Notably, current rs-fMRI studies have revealed the dynamic nature of the BOLD signals, which may reflect brain activity state or mental activity changes (27–30). Therefore, it may provide insight into the brain activity state in psychiatric disease by analyzing the dynamics regularity in fMRI time series. Wang et al. (31) developed a sample entropy (SampEn) toolbox to calculate the BEN maps based on the fMRI data. Although, the general algorithm for entropy needs a large dataset to precisely estimate the probability distribution function, whereas SampEn is an extension of Approximate Entropy and it showed preferable stability for different data lengths (32, 33). Therefore, SampEn is applicable for analyzing rs-fMRI data with an approximately small dataset.
Entropy indicates system irregularity. In the context of neural system time-based signals, it measures the irregularity of brain activity. Studies have suggested that the human brain should sustain entropy to maintain normal brain functioning (34, 35). Therefore, measuring the brain entropy (BEN) may be a physical means for characterizing brain activity state and its changes in psychological diseases. BEN has been characterized using electroencephalogram (EEG) data with relatively low spatial resolution (32, 36, 37). However, resting-state fMRI (rs-fMRI) provides an approximately high spatial resolution, that can be used for BEN mapping.
According to Wang et al. (31), over 1,000 healthy individuals’ SampEn maps showed significant lower entropy in the neocortex in compare with the rest of the brain. In addition, by comparing the changes in the BEN before and after caffeine intake in large samples of healthy subjects, Chang et al. (38) identified a significant caffeine-induced pattern of BEN increase in the whole brain. Furthermore, it has been shown in the study by Song et al. that BEN can provides features that cannot be fully described by other methods of resting-state brain activity, such as the cerebral blood flow (CBF) and the fractional amplitude of low-frequency fluctuation (ALFF) (39). Moreover, a current study also proposed the neurocognitive correlations of BEN in rs-fMRI (40). BEN alterations have been shown in various disorder conditions in clinical research, including schizophrenia (41, 42), MDD (43), attention deficit hyperactivity disorder (44), insomnia (45), and cocaine addiction (46). The BEN has been suggested as a potential biomarker for a number of psychiatric disorders. Studies on BEN represent a growing field on studying the dynamic nature of brain activities. we believe BEN can provide a novel insight into PTSD-MDD comorbidity.
The purpose of this study was to investigate the irregularity of the brain in patients with PTSD-MDD comorbidity by analyzing BEN in these patients and comparing them to traumatized controls. We observed that BEN had not been applied to the fMRI study PTSD or PTSD-MDD comorbidity; However, there are a few studies on MDD using the BEN. These studies propose that patients with MDD show BEN alterations in the brain regions associated with emotion regulation, such as the putamen and thalamus (47), and those vital for information processing, such as the medial orbital frontal cortex (43). Although these studies focused on MDD, previous studies have indicated that PTSD and MDD have quite a few overlapping symptoms, including anhedonia, sleep disturbance, and concentration difficulties (48). Therefore, we hypothesized that the BEN would differ between patients with comorbid PTSD-MDD and traumatized controls in the frontal regions and limbic systems, which are associated with information processing and emotion regulation.
2. Materials and methods
2.1. Participants
Participants include 33 drug-naive patients with PTSD-MDD comorbidity and 36 traumatized controls (TCs), who were enrolled at the Guangdong Second Provincial General Hospital in China and matched demographically. From December 2020 to October 2021, all the patients with PTSD-MDD were home-quarantined for 1 month or more during the COVID-19 epidemic, after which they received psychological consultation at the Guangdong Second Provincial General Hospital. The structured Mini-International Neuropsychiatric Interview for DSM-IV was administered by two experienced psychiatrists to assess PTSD-MDD comorbidity diagnosis. Subsequently, 33 patients were diagnosed with PTSD-MDD comorbidity. In addition, patients completed the PTSD Checklist Scale-Civilian (PCL-C) and the HAMD-24. TCs recruited from the local community were also quarantined for 1 month or more during the epidemic, and all of them showed no psychiatric symptoms after quarantine. The mental state of TCs subjects was also evaluated by two experienced psychiatrists. Lastly, each participant was matched according to sex, age, educational level, and hand dominance.
The inclusion criteria for patients with comorbid PTSD-MDD were as follows: (i) 1 month or more than 1 month of quarantine during the epidemic; (ii) met the criteria of DSM-IV (iii) PTSD Checklist-Civilian Version (PCL-C) score > 44 and HAMD score > 17; (iv) age > 18 years; and (iv) without a history of neurological disorders or psychiatric disorders. In contrast, the inclusion criteria for TCs were as follows: (i) age > 18 years; (ii) right-hand dominance; (iii) no psychiatric medications history; (iv) non-compliance with 3 Tesla fMRI safety standards. However, the groups did not differ in age or sex (Table 1). Furthermore, permission to conduct this study was granted by the ethics committee of Guangdong Second Provincial General Hospital. All the participants provided written informed consent in agreement with ethical approval from the Guangdong Second Provincial General Hospital committee.
2.2. Mental status assessment
The PTSD-MDD comorbidity diagnosis was determined according to the DSM-IV diagnostic criteria. Before undergoing the fMRI scanning, all the PTSD-MDD comorbidity patients were screened with the CAPS, PCL-C, and HAMD-24 to estimate the severity of the symptoms. Further structured clinical interview was conducted to assess other psychiatric comorbidities.
2.3. fMRI procedures
Scans were obtained in a single 3.0-T Philips MR scanner (Ingenia; Best, The Netherlands) equipped with a 32-channel head coil. The datasets include T2-FLAIR images, 3D T1-weighted images and gradient echo-planar images (EPI). The T2-FLAIR images were obtained to detect the participants with any brain lesions. The EPI data were acquired in an interleaved order to measure BOLD signal, the data were scanned approximately AP-PC line with the following parameters: repetition time/echo time (TR/TE) = 2,000/30 ms; matrix = 64 × 64, field of view (FOV) = 230 mm × 230 mm, flip angle (FA) = 90°, slice thickness = 3.6 mm slice, number of slices = 33, slice gap = 0.6 mm, 250 volumes were acquired within 500 s. 3D T1-weighted structural images were acquired for each participant with TR/TE = 25/4.1 ms; FA = 30°, matrix = 256 × 256, FOV = 230 mm × 230 mm, slice thickness = 1.0 mm; number of slice = 160, slice gap = 0. During the scan, the participants wore headphones to reduce noise, and placing soft pads on the sides of the head to minimize the head movement. All the participants were instructed to lie still, stay awake, and think of nothing in particular.
2.4. Data processing and BEN calculations
2.4.1. Preprocessing
MRI data were preprocessed using the Statistical Parametric Mapping version 12 (SPM12) software1. (1) Before preprocessing, the first 10 EPI volumes of each subject’s data were discarded to allow for image intensity to reach stable state. (2) The remaining volumes were performed with correction of intra-volume time delay using the middle slice as reference and inter-volume head motion using the first volume as the reference. (3) 3D T1-weighted images were registered into Montreal Neurological Institute (MNI) space, gray matter, white matter and cerebrospinal fluid maps were segmented and generated during this process. (4) The EPI images were spatially co-registered with the 3D T1-weighted structural images as mentioned above and resampled into 3 mm isotropic voxels. (5) We used the mean framewise displacement (FD) Jenkinson as the head motion reference standard. We eliminated the participants with motion (mean FD Jenkinson) > 2 × Standard Deviation (SD) above the group mean motion as we did in our previous study (49). (6) Temporal nuisance signals were regressed out including the head motion parameters (Friston 24 model), the cerebrospinal fluid signal, and white matter signal, global signal was not regressed out (50). Subsequently, linear detrend and bandpass filtering (0.01–0.08 Hz) were performed to minimize the low-frequency drift and high-frequency physiological noise. Finally, the data were spatially smoothed with a 10 mm full-width at half-maximum Gaussian kernel.
2.4.2. BEN calculation
A combination of home-designed Matrix Laboratory (MATLAB) code and the Brain Entropy Mapping toolbox (BENtbx) developed by Wang et al. (31) were used to calculate the sample entropy for each voxel after image preprocessing (33). SampEn is an extension of Approximate Entropy (ApEn), its determined from the temporal coherence of a time-series. SampEn is calculated the “logarithmic likelihood” that a small window (length “m”) of the data “matches” with other windows whether it will still “match” the other windows if the window length increases by 1 (length “m + 1”). The “match” depends on the tolerance threshold value <“r” times SD of the entire time series. Details of BEN calculation was described in the original BENtbx paper (31). Same as previous studies the “m” equal to 3 and the “r” equal to 0.6 and the threshold is r * SD (39).
2.4.3. Statistical analysis
A two-sample t-test was used to compare the differences in age and education level, While, a chi-square test was used to compare the gender composition between the two groups. A two-sample t-test was used to compare the differences in the brain entropy between the PTSD-MDD comorbidity group and TC group by using the DPABI toolbox,2 covariates included age, sex, and educational level. The false discovery rate (FDR) correction was used for the multiple comparison correction, and the significance level was set at p < 0.05. Therefore, to explore the relationship between the average BEN values of the ROIs and clinical indicators, we performed general linear models with the CAPS, PCL-C, HAMD-24, and BEN values from the clusters that showed significant group differences as independent variables, and age, gender, educational level, and head motion (mean FD Jenkinson) as covariates. Statistical analysis was performed using the Statistical Package Social Sciences (SPSS) software version 2.3, with a significance threshold set at p < 0.05.
3. Results
3.1. Demographic and clinical tests
There were no significant inter-group differences in terms of age, sex, or education level (all p > 0.05). However, in neuropsychology assessments, there were significant differences between patients with PTSD-MDD and the TCs in CAPS, PCL-C, and HAMD-24. Table 1 shows the demographic and neuropsychological assessment results.
3.2. Altered BEN values in patients with PTSD-MDD comorbidity
Figure 1 shows the results of the group-level analysis. The results showed that patients with PTSD-MDD had significantly decreased BEN values in the right middle frontal gyrus orbital part (R_MFOG), left putamen and right inferior frontal gyrus, opercular part (R_IFOG), after FDR correction compared to TCs (Table 2; Figure 1).
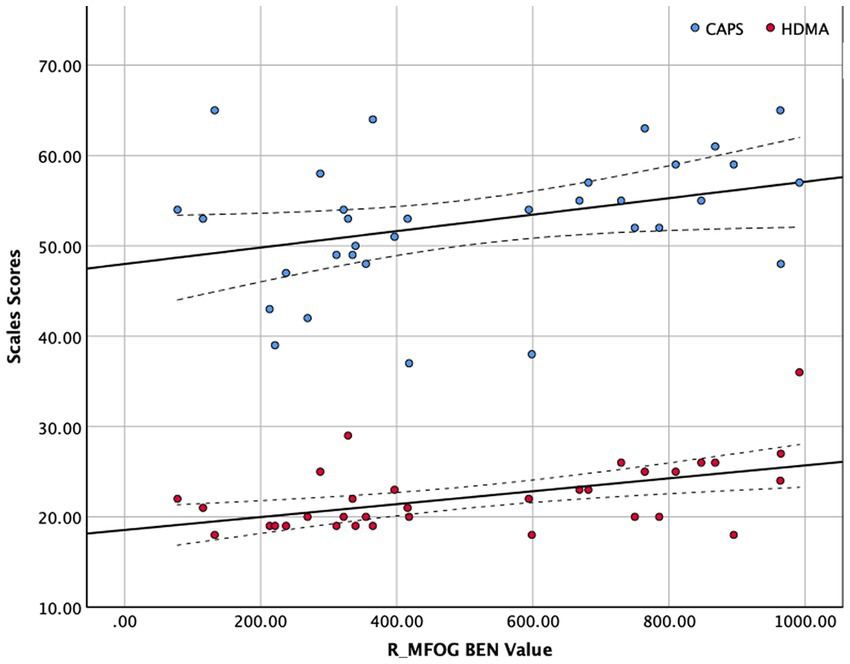
Figure 1. Two-sample t-test performed to test the differences in the brain entropy (BEN) maps between the posttraumatic stress disorder and major depressive disorder (PTSD-MDD) group and the traumatized controls (TCs) at each voxel. In addition, the false discovery rate (FDR) tests were performed for multiple comparison corrections (p < 0.05), PTSD-MDD group showed significantly reduced BEN value in the right middle frontal orbital gyrus (R_MFOG), left putamen, and the right inferior frontal gyrus, opercular part (R IFOG).
3.3. Correlations between BEN values of abnormal regions and CAPS score, HAMD score
The BEN values and clinical scales were correlated using Spearman’s correlation because of the limited sample size of this study (see in Figure 2). Figure 2 shows that R_MFOG BEN in was positively correlated with HAMD-24 (r = 0.479, p = 0.005) and CAPS (r = 0.366, p = 0.036).

Figure 2. Correlation between right middle frontal orbital gyrus (R_MFOG) brain entropy (BEN) values and Clinician-Administered PTSD Scale (CAPS) and Hamilton Depression Scale-24 items (HAMD-24) scores. The BEN values of the R_MFOG were positively correlated with CAPS scores and HAMD-24 scores (CAPS: r = 0.366, p = 0.036; HAMD-24: r = 0.479, p = 0.002).
4. Discussion
This study examined the BEN maps in PTSD-MDD by comparing the BEN maps in the PTSD-MDD and TCs. The following are two major findings derived from these results: (1) Patients with PTSD-MDD showed lower BEN in the R_MFOG, left putamen, and R_IFOG; and (2) BEN values in the R_MFOG was positively correlated with HAMD-24 and CAPS. These findings support our hypothesis that patients with PTSD-MDD show significant differences in the BEN in the frontal regions and limbic system.
The reduced BEN found in the R_MFOG during the resting state in patients with PTSD-MDD is partially consistent with those of previous studies (51–53). However, a resting-state perfusion study showed a reduced cerebral blood flow (CBF) in the R_MFOG, and the reduced CBF in the R_MFOG was negatively correlated with PTSD severity (51). Our study also showed reduced BEN in the R_MFOG, but the reduced BEN was positively correlated with PTSD severity and depression severity. A previous study showed BEN-CBF correlation especially in the MFOG and inferior temporal cortex (39), since the BOLD signal is mostly contributed by the CBF (54). These studies may explain the concordance between our results and the CBF results. Interestingly, a previous study indicated that BEN is mostly independent of CBF; they found that caffeine induced whole brain CBF decrease, but a large portion of BEN increased including the prefrontal cortex (38). These results suggest that caffeine induced CBF decrease, but caffeine induced BEN increase may suggest enhanced cognition in the subject. Electroencephalographic (EEG) studies have also indicated that BEN is associated with cognition and emotion (55, 56). Accordingly, we assumed that CBF reduction and BEN reduction in PTSD are independent, and that the correlation between PTSD severity and BEN may be related to emotional and cognitive symptoms.
Functional imaging studies of PTSD have reported hypoactivity in the ventromedial frontal cortex including in the R_MFOG (57, 58). Memory encoding and retrieval are affected by reduced activity of prefrontal cortex, resulting in difficulty in restructuring their traumatic memory in PTSD patients (59). The R_MFOG usually shows reduced connection in the EEG and fMRI studies, suggesting that the reduced connectivity in the R_MFOG might be related to dysregulation of the default mode network or self-referential processing process in patients with PTSD (60, 61). Furthermore, several neuroimaging findings propose that combined with the limbic system the orbital and medial frontal cortex is critical in the MDD aberrant network (62–64). Previous studies have suggested that the dysfunctional orbital frontal cortex is associated with suicidal symptoms, as it plays a key role in decision-making (65). On the other hand, the orbital frontal cortex is also involved in stressful events and has increased functional connectivity (FC) especially in self-referential processes, that can cause depression and frustration (66, 67). Accordingly, we believe the decreased BEN in the R_MFOG is associated with depression symptoms, and the BEN may reflect the severity of MDD.
Conversely, the reduced R_IFOG in this study are partly supported by a previous BEN study on MDD. A previous study found a decrease in the BEN value of the R_IFOG. However, they also observed a negative correlation between the HAMD-24 and the BEN value of the inferior frontal region (68). In addition, a previous task-based fMRI study reported increased FC in IFOG (69), proposing that the increased connectivity is associated with the fronto-temporo-parietal network, and the altered connectivity in this network may result from a compensatory mechanism, which is important for emotion regulation (70). There is evidence that IFOG is involved in cognition as well as emotional processing, including the association between MDD patients and the preference for processing negative information (71). A voxel-based morphometry study indicated that patients with MDD and patients with PTSD-MDD showed smaller volumes in the IFOG than in the healthy controls, and that the IFOG volume was normalized after medication and the depressive symptoms were also mitigated (72). Therefore, we assume that the reduced BEN in the R_IFOG might be associated with depression symptoms, and that abnormalities in the IFOG might indicate the depressed patients with anxiety comorbidities. Several structural studies have demonstrated that the reduced inferior frontal cortex in depressed patients is comorbid with anxiety symptoms (73, 74). In line with these studies, our findings provide evidence of the functional irregularity in the R_IFOG in patients with PTSD-MDD comorbidity.
The finding of reduced BEN in putamen is partly in agreement with a previous study of BEN in patients with MDD (68). Not only that, but a previous fMRI study showed a decreased FC between the basolateral amygdala and putamen in PTSD-MDD comorbidity versus PTSD alone. Furthermore, they found a negative correlation between the basolateral amygdala-putamen FC and the HAMD-24 scores (75). These results indicate that the FC difference between the PTSD-MDD and PTSD groups may be more closely associated with MDD symptoms. Although we could not find correlations between the HAMD-24 scores and the BEN value in the putamen, several studies have suggested that putamen is associated with depressive symptoms (76, 77). In addition, a previous MDD study also showed weaker FC between the amygdala and the cortico-striatal-pallidal-thalamic circuit, which maintains information in working memory (78). There is a correlation between working memory, as a main cognitive deficit, and PTSD (79, 80). Accordingly, we believe that the reduced BEN in the putamen may meditate an impaired working memory in patients with comorbid PTSD-MDD.
This study had some limitations. First, this study lacked a PTSD-only group or MDD-only group, making it impossible to evaluate whether patients with PTSD-only patients or MDD-only patients had different BEN than those with PTSD-MDD. Second, as all our patients were exposed to trauma, most people in the urban areas had home-quarantine experience, and the social panic following the outbreak is an important traumatic factor. Third, the small sample size may have resulted in insufficient statistical power. Fourth, although previous studies have indicated the BEN reflects the human brain complexity of human brain, the exact neurophysiological basis remains unclear, limiting the interpretation of our findings.
In conclusion, this study revealed a decreased BEN in patients with PTSD-MDD who were home-quarantined for 1 month or more during the COVID-19 epidemic. Therefore, these results not only provide evidence for the BEN research on PTSD-MDD comorbidity but also provide evidence that pandemic-related psychiatric disease affects the brain irregularities in brain activity. Furthermore, the brain regions that show significant BEN alterations agree with previous fMRI studies, and correlations were established between the BEN in the right MFOG and the symptom severity (CAPS, HAMD-24), suggesting that MFOG is a potential marker for revealing the mechanism of PTSD and MDD and may inform future clinical interventions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Guangdong Second Provincial General Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was funded by grants from the National Natural Science Foundation of China (Grant Numbers: U1903120, 81771807, 81901729, and 82001792) and the Science and Technology Planning Project of Guangzhou (Grant Number: 202002030234) and the Science Foundation of Guangdong Second Provincial General Hospital (No. 3D-A2021009).
Acknowledgments
The authors would like to acknowledge the support of Ze Wang and Donghui Song.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Cénat, JM, Blais-Rochette, C, Kokou-Kpolou, CK, Noorishad, P-G, Mukunzi, JN, McIntee, S-E, et al. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Res. (2021) 295:113599–17. doi: 10.1016/j.psychres.2020.113599
2. Schwartz, RM, Rasul, R, Gargano, LM, Lieberman-Cribbin, W, Brackbill, RM, and Taioli, E. Examining associations between hurricane Sandy exposure and posttraumatic stress disorder by Community of Residence. J Trauma Stress. (2019) 32:677–87. doi: 10.1002/jts.22445
3. Cénat, JM, Felix, N, Blais-Rochette, C, Rousseau, C, Bukaka, J, Derivois, D, et al. Prevalence of mental health problems in populations affected by Ebola virus disease: A systematic review and meta-analysis. Psychiatry Res. (2020) 289:113033. doi: 10.1016/j.psychres.2020.113033
4. Cénat, JM, McIntee, S-E, and Blais-Rochette, C. Symptoms of posttraumatic stress disorder, depression, anxiety and other mental health problems following the 2010 earthquake in Haiti: A systematic review and meta-analysis. J Affect Disord. (2020) 273:55–85. doi: 10.1016/j.jad.2020.04.046
5. Zhang, Z, Ran, MS, Li, YH, Ou, GJ, Gong, RR, Li, RH, et al. Prevalence of post-traumatic stress disorder among adolescents after the Wenchuan earthquake in China. Psychol Med. (2011) 42:1687–93. doi: 10.1017/s0033291711002844
6. Qiu, J, Shen, B, Zhao, M, Wang, Z, Xie, B, and Xu, Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: implications and policy recommendations. Gen Psychiatry. (2020) 33:e100213. doi: 10.1136/gpsych-2020-100213
7. Jernigan, DB. Team CC-19 R. update: public health response to the coronavirus disease 2019 outbreak — United States, February 24, 2020. Morbidity Mortal Wkly Rep. (2020) 8:216–9. doi: 10.15585/mmwr.mm6908e1
8. Prem, K, Liu, Y, Russell, TW, Kucharski, AJ, Eggo, RM, and Davies, N. Group C for the MM of IDC-19 W, Flasche S, Clifford S, Pearson CAB, et al. the effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Heal. (2020) 5:e261–70. doi: 10.1016/s2468-2667(20)30073-6
9. Wang, Y. Analyzing Wuhan’s strategies for controlling Covid-19 epidemic. J Phys Conf Ser. (2021) 1993:012033. doi: 10.1088/1742-6596/1993/1/012033
10. Xu, Z-Q, Wang, J-Z, Wang, H-R, He, J-F, Wang, B, Yang, Y-C, et al. Effects of home quarantine for COVID-19 community control in Shenzhen, China JAMA Netw Open. (2020) 3:e2012934. doi: 10.21203/rs.3.rs-34537/v1,
11. Zhu, P, and Tan, X. Is compulsory home quarantine less effective than centralized quarantine in controlling the COVID-19 outbreak? Evidence from Hong Kong. Sustain Cities Soc. (2021) 74:103222–2. doi: 10.1016/j.scs.2021.103222
12. Brooks, SK, Webster, RK, Smith, LE, Woodland, L, Wessely, S, Greenberg, N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. (2020) 395:912–20. doi: 10.1016/s0140-6736(20)30460-8
13. Hawryluck, L, Gold, WL, Robinson, S, Pogorski, S, Galea, S, and Styra, R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. (2004) 10:1206–12. doi: 10.3201/eid1007.030703
14. REYNOLDS, DL, GARAY, JR, DEAMOND, SL, MORAN, MK, GOLD, W, and STYRA, R. Understanding, compliance and psychological impact of the SARS quarantine experience. Epidemiol Infect. (2007) 136:997–1007. doi: 10.1017/s0950268807009156
15. Hamaideh, SH, Al-Modallal, H, Tanash, M, and Hamdan-Mansour, A. Depression, anxiety and stress among undergraduate students during COVID-19 outbreak and “home-quarantine.”. Nurs Open. (2022) 9:1423–31. doi: 10.1002/nop2.918
16. Rytwinski, NK, Scur, MD, Feeny, NC, and Youngstrom, EA. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: A Meta-analysis. J Trauma Stress. (2013) 26:299–309. doi: 10.1002/jts.21814
17. Brady, KT, Killeen, TK, Brewerton, T, and Lucerini, S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry. (2000) 61:22–32.
18. Pietrzak, RH, Goldstein, RB, Southwick, SM, and Grant, BF. Medical comorbidity of full and partial posttraumatic stress disorder in US adults: results from wave 2 of the National Epidemiologic Survey on alcohol and related conditions. Psychosom Med. (2011) 73:697–707. doi: 10.1097/psy.0b013e3182303775
19. Zhang, F, Rao, S, Cao, H, Zhang, X, Wang, Q, Xu, Y, et al. Baranova a genetic evidence suggests posttraumatic stress disorder as a subtype of major depressive disorder. J Clin Investigat. (2022) 132:e145942. doi: 10.1172/jci145942
20. Nijdam, MJ, Gersons, BPR, and Olff, M. The role of major depression in neurocognitive functioning in patients with posttraumatic stress disorder. Eur J Psychotraumatol. (2013) 4:19979. doi: 10.3402/ejpt.v4i0.19979
21. Radell, ML, Hamza, EA, and Moustafa, AA. Depression in post-traumatic stress disorder. Rev Neurosci. (2020) 31:703–22. doi: 10.1515/revneuro-2020-0006
22. Angelakis, S, and Nixon, RDV. The comorbidity of PTSD and MDD: implications for clinical practice and future research. Behav Change. (2015) 32:1–25. doi: 10.1017/bec.2014.26
23. CHIBA, H, Oe, M, and Uchimura, N. Patients with posttraumatic stress disorder with comorbid major depressive disorder require a higher dose of psychotropic drugs. Kurume Med J. (2015) 62:23–8. doi: 10.2739/kurumemedj.ms65010
24. Biswal, BB, Mennes, M, Zuo, X-N, Gohel, S, Kelly, C, Smith, SM, et al. Toward discovery science of human brain function. Proc National Acad Sci USA. (2010) 107:4734–9. doi: 10.1073/pnas.0911855107
25. Barkhof, F, Haller, S, and Rombouts, SARB. Resting-state functional MR imaging: a new window to the brain. Radiology. (2014) 272:29–49. doi: 10.1148/radiol.14132388
26. Kennis, M, Rademaker, AR, Van, RSJH, Kahn, RS, and Geuze, E. Altered functional connectivity in posttraumatic stress disorder with versus without comorbid major depressive disorder: a resting state fMRI study. F1000research. (2014) 2:289. doi: 10.12688/f1000research.2-289.v2
27. Chang, C, and Glover, GH. Time–frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage. (2010) 50:81–98. doi: 10.1016/j.neuroimage.2009.12.011
28. Hutchison, RM, Womelsdorf, T, Gati, JS, Everling, S, and Menon, RS. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp. (2012) 34:2154–77. doi: 10.1002/hbm.22058
29. Keilholz, SD, Magnuson, ME, Pan, W-J, Willis, M, and Thompson, GJ. Dynamic properties of functional connectivity in the rodent. Brain Connect. (2013) 3:31–40. doi: 10.1089/brain.2012.0115
30. Keilholz, SD. The neural basis of time-varying resting-state functional connectivity. Brain Connect. (2014) 4:769–79. doi: 10.1089/brain.2014.0250
31. Wang, Z, Li, Y, Childress, AR, and Detre, JA. Brain Entropy Mapping Using fMRI. PLoS One. (2014) 9:e89948. doi: 10.1371/journal.pone.0089948
32. Richman, JS, and Moorman, JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. (2000) 278:H2039–49. doi: 10.1152/ajpheart.2000.278.6.h2039
33. Lake, DE, Richman, JS, Griffin, MP, and Moorman, JR. Sample entropy analysis of neonatal heart rate variability. Am J Physiol Regul Integr Comp Physiol. (2002) 283:R789–97. doi: 10.1152/ajpregu.00069.2002
34. Bergström, RM. An entropy model of the developing brain. Dev Psychobiol. (1969) 2:139–52. doi: 10.1002/dev.420020304
35. Singer, W. The brain, a complex self-organizing system. Eur Rev. (2009) 17:321–9. doi: 10.1017/s1062798709000751
36. Rezek, IA, and Roberts, SJ. Stochastic complexity measures for physiological signal analysis. IEEE Trans Bio Med Eng. (1998) 45:1186–91. doi: 10.1109/10.709563
37. Fernández, A, Hornero, R, Gómez, C, Turrero, A, Gil-Gregorio, P, Matías-Santos, J, et al. Complexity analysis of spontaneous brain activity in Alzheimer disease and mild cognitive impairment: an MEG study. Alz Dis Assoc Dis. (2010) 24:182–9. doi: 10.1097/wad.0b013e3181c727f7
38. Chang, D, Song, D, Zhang, J, Shang, Y, Ge, Q, and Wang, Z. Caffeine caused a widespread increase of resting brain entropy. Sci Rep. (2018) 8:2700. doi: 10.1038/s41598-018-21008-6
39. Song, D, Chang, D, Zhang, J, Ge, Q, Zang, Y-F, and Wang, Z. Associations of brain entropy (BEN) to cerebral blood flow and fractional amplitude of low-frequency fluctuations in the resting brain. Brain Imaging Behav. (2019) 13:1486–95. doi: 10.1007/s11682-018-9963-4
40. Wang, Z. The neurocognitive correlates of brain entropy estimated by resting state fMRI. NeuroImage. (2021) 232:117893. doi: 10.1016/j.neuroimage.2021.117893
41. Xue, S-W, Yu, Q, Guo, Y, Song, D, and Wang, Z. Resting-state brain entropy in schizophrenia. Compr Psychiatry. (2019) 89:16–21. doi: 10.1016/j.comppsych.2018.11.015
42. Sokunbi, MO, Gradin, VB, Waiter, GD, Cameron, GG, Ahearn, TS, Murray, AD, et al. Nonlinear complexity analysis of brain fMRI signals in schizophrenia. PLoS One. (2014) 9:e95146. doi: 10.1371/journal.pone.0095146
43. Liu, X, Song, D, Yin, Y, Xie, C, Zhang, H, Zhang, H, et al. Altered brain entropy as a predictor of antidepressant response in major depressive disorder. J Affect Disord. (2020) 260:716–21. doi: 10.1016/j.jad.2019.09.067
44. Sokunbi, MO, Fung, W, Sawlani, V, Choppin, S, Linden, DEJ, and Thome, J. Resting state fMRI entropy probes complexity of brain activity in adults with ADHD. Psychiatry Res Neuroimaging. (2013) 214:341–8. doi: 10.1016/j.pscychresns.2013.10.001
45. Zhou, F, Huang, S, Gao, L, Zhuang, Y, Ding, S, and Gong, H. Temporal regularity of intrinsic cerebral activity in patients with chronic primary insomnia: a brain entropy study using resting-state fMRI. Brain Behav. (2016) 6:e00529. doi: 10.1002/brb3.529
46. Wang, Z, Suh, J, Duan, D, Darnley, S, Jing, Y, Zhang, J, et al. A hypo-status in drug-dependent brain revealed by multi-modal MRI. Addict Biol. (2017) 22:1622–31. doi: 10.1111/adb.12459
47. Lin, C, Lee, S-H, Huang, C-M, Chen, G-Y, Ho, P-S, Liu, H-L, et al. Increased brain entropy of resting-state fMRI mediates the relationship between depression severity and mental health-related quality of life in late-life depressed elderly. J Affect Disord. (2019) 250:270–7. doi: 10.1016/j.jad.2019.03.012
48. Flory, JD, and Yehuda, R. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues Clin Neurosci. (2015) 17:141–50. doi: 10.31887/DCNS.2015.17.2/jflory
49. Fu, S, Ma, X, Wu, Y, Bai, Z, Yi, Y, Liu, M, et al. Jiang G altered local and large-scale dynamic functional connectivity variability in posttraumatic stress disorder: A resting-state fMRI study. Front Psych. (2019) 10:234. doi: 10.3389/fpsyt.2019.00234
50. Saad, ZS, Gotts, SJ, Murphy, K, Chen, G, Jo, HJ, Martin, A, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. (2012) 2:25–32. doi: 10.1089/brain.2012.0080
51. Zhe, X, Liu, K, Mu, Y-F, Qi, S, Xi, Y-B, Du, P, et al. Decreased regional cerebral perfusion at resting state in acute posttraumatic stress disorder resulting from a single, prolonged stress event. Acad Radiol. (2016) 23:1083–90. doi: 10.1016/j.acra.2016.05.002
52. Detre, JA, Wang, J, Wang, Z, and Rao, H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol. (2009) 22:348–55. doi: 10.1097/wco.0b013e32832d9505
53. Molina, ME, Isoardi, R, Prado, MN, and Bentolila, S. Basal cerebral glucose distribution in long-term post-traumatic stress disorder. World J Biol Psychiatry. (2010) 11:493–501. doi: 10.3109/15622970701472094
54. Mulderink, TA, Gitelman, DR, Mesulam, M-M, and Parrish, TB. On the use of caffeine as a contrast booster for BOLD fMRI studies. NeuroImage. (2002) 15:37–44. doi: 10.1006/nimg.2001.0973
55. Rutkowski, TM, Abe, MS, Komendziński, T, and Otake-Matsuura, M. Older adult mild cognitive impairment prediction from multiscale entropy EEG patterns in reminiscent interior image working memory paradigm. Annu Int Conf IEEE Eng Med Biol Soc. (2021) 2021:6345–8. doi: 10.1109/embc46164.2021.9629480
56. Wei, L, Li, Y, Ye, J, Yang, X, and Wang, J. Emotion-induced higher wavelet entropy in the EEG with depression during a cognitive task. Annu Int Conf IEEE Eng Med Biol Soc. (2009) 2009:5018–21. doi: 10.1109/iembs.2009.5334603
57. Francati, V, Vermetten, E, and Bremner, JD. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress Anxiety. (2007) 24:202–18. doi: 10.1002/da.20208
58. Jackowski, AP, De A, FGM, De, AAG, De, ACM, Reis, M, Nery, F, et al. The involvement of the orbitofrontal cortex in psychiatric disorders: an update of neuroimaging findings. Rev Bras Psiquiatr. (2012) 34:207–12. doi: 10.1590/s1516-44462012000200014
59. Hull, AM. Neuroimaging findings in post-traumatic stress disorder. Brit J Psychiatry. (2002) 181:102–10. doi: 10.1192/bjp.181.2.102
60. Toll, RT, Wu, W, Naparstek, S, Zhang, Y, Narayan, M, Patenaude, B, et al. An electroencephalography Connectomic profile of posttraumatic stress disorder. Am J Psychiatry. (2020) 177:233–43. doi: 10.1176/appi.ajp.2019.18080911
61. Clausen, AN, Francisco, AJ, Thelen, J, Bruce, J, Martin, LE, McDowd, J, et al. PTSD and cognitive symptoms relate to inhibition-related prefrontal activation and functional connectivity. Depress Anxiety. (2017) 34:427–36. doi: 10.1002/da.22613
62. Price, JL, and Drevets, WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. (2010) 35:192–216. doi: 10.1038/npp.2009.104
63. Hamani, C, Mayberg, H, Stone, S, Laxton, A, Haber, S, and Lozano, AM. The Subcallosal cingulate Gyrus in the context of major depression. Biol Psychiatry. (2011) 69:301–8. doi: 10.1016/j.biopsych.2010.09.034
64. Samara, Z, Evers, EAT, Peeters, F, Uylings, HBM, Rajkowska, G, Ramaekers, JG, et al. Orbital and medial prefrontal cortex functional connectivity of major depression vulnerability and disease. Biol Psychiatry Cogn Neurosci Neuroimag. (2018) 3:348–57. doi: 10.1016/j.bpsc.2018.01.004
65. Monkul, ES, Hatch, JP, Nicoletti, MA, Spence, S, Brambilla, P, Lacerda, ALT, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry. (2007) 12:360–6. doi: 10.1038/sj.mp.4001919
66. Kühn, S, and Gallinat, J. Resting-state brain activity in schizophrenia and major depression: A quantitative Meta-analysis. Schizophrenia Bull. (2013) 39:358–65. doi: 10.1093/schbul/sbr151
67. Sheline, YI, Price, JL, Yan, Z, and Mintun, MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc National Acad Sci USA. (2010) 107:11020–5. doi: 10.1073/pnas.1000446107
68. Xue, S-W, Wang, D, Tan, Z, Wang, Y, Lian, Z, Sun, Y, et al. Disrupted brain entropy and functional connectivity patterns of thalamic subregions in major depressive disorder. Neuropsych Dis Treat. (2019) 15:2629–38. doi: 10.2147/ndt.s220743
69. Keller, M, Mendoza-Quiñones, R, Muñoz, AC, Iglesias-Fuster, J, Virués, AV, Zvyagintsev, M, et al. Transdiagnostic alterations in neural emotion regulation circuits – neural substrates of cognitive reappraisal in patients with depression and post-traumatic stress disorder. BMC Psychiatry. (2022) 22:173. doi: 10.1186/s12888-022-03780-y
70. Mathiak, K, and Weber, R. Toward brain correlates of natural behavior: fMRI during violent video games. Hum Brain Mapp. (2006) 27:948–56. doi: 10.1002/hbm.20234
71. Gollan, JK, Connolly, M, Buchanan, A, Hoxha, D, Rosebrock, L, Cacioppo, J, et al. Neural substrates of negativity bias in women with and without major depression. Biol Psychol. (2015) 109:184–91. doi: 10.1016/j.biopsycho.2015.06.003
72. Dai, D, Lacadie, CM, Holmes, SE, Cool, R, Anticevic, A, Averill, C, et al. Ketamine normalizes the structural alterations of inferior frontal Gyrus in depression. Chronic Stress. (2020) 4:2470547020980681. doi: 10.1177/2470547020980681
73. Peng, W, Jia, Z, Huang, X, Lui, S, Kuang, W, Sweeney, JA, et al. Brain structural abnormalities in emotional regulation and sensory processing regions associated with anxious depression. Prog Neuro-psychopharmacol Biol Psychiatry. (2019) 94:109676. doi: 10.1016/j.pnpbp.2019.109676
74. Canu, E, Kostić, M, Agosta, F, Munjiza, A, Ferraro, PM, Pesic, D, et al. Brain structural abnormalities in patients with major depression with or without generalized anxiety disorder comorbidity. J Neurol. (2015) 262:1255–65. doi: 10.1007/s00415-015-7701-z
75. Yuan, M, Pantazatos, SP, Zhu, H, Li, Y, Miller, JM, Rubin-Falcone, H, et al. Altered amygdala subregion-related circuits in treatment-naïve post-traumatic stress disorder comorbid with major depressive disorder. Eur Neuropsychopharmacol. (2019) 29:1092–101. doi: 10.1016/j.euroneuro.2019.07.238
76. Lawrence, NS, Williams, AM, Surguladze, S, Giampietro, V, Brammer, MJ, Andrew, C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. (2004) 55:578–87. doi: 10.1016/j.biopsych.2003.11.017
77. Phan, KL, Wager, T, Taylor, SF, and Liberzon, I. Functional Neuroanatomy of emotion: A Meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. (2002) 16:331–48. doi: 10.1006/nimg.2002.1087
78. Yang, J, Yin, Y, Svob, C, Long, J, He, X, Zhang, Y, et al. Yuan Y amygdala atrophy and its functional disconnection with the Cortico-striatal-Pallidal-thalamic circuit in major depressive disorder in females. PLoS One. (2017) 12:e0168239. doi: 10.1371/journal.pone.0168239
79. Wisdom, NM, Pastorek, NJ, Miller, BI, Booth, JE, Romesser, JM, Linck, JF, et al. PTSD and cognitive functioning: importance of including performance validity testing. Clin Neuropsychol. (2013) 28:128–45. doi: 10.1080/13854046.2013.863977
Keywords: posttraumatic stress disorder, major depressive disorder, brain entropy, fMRI, resting-state
Citation: Fu S, Liang S, Lin C, Wu Y, Xie S, Li M, Lei Q, Li J, Yu K, Yin Y, Hua K, Li W, Wu C, Ma X and Jiang G (2023) Aberrant brain entropy in posttraumatic stress disorder comorbid with major depressive disorder during the coronavirus disease 2019 pandemic. Front. Psychiatry. 14:1143780. doi: 10.3389/fpsyt.2023.1143780
Edited by:
Huanzhong Liu, Chaohu Hospital of Anhui Medical University, ChinaReviewed by:
Donghui Song, Beijing Normal University, ChinaDa Chang, Beijing Normal University, China
Copyright © 2023 Fu, Liang, Lin, Wu, Xie, Li, Lei, Li, Yu, Yin, Hua, Li, Wu, Ma and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofen Ma, xiaofenma12@163.com; Guihua Jiang, jianggh@gd2h.org.cn
†These authors have contributed equally to this work
 Shishun Fu
Shishun Fu Sipei Liang2†
Sipei Liang2† Yunfan Wu
Yunfan Wu Meng Li
Meng Li Qiang Lei
Qiang Lei Jianneng Li
Jianneng Li Yi Yin
Yi Yin Kelei Hua
Kelei Hua Xiaofen Ma
Xiaofen Ma Guihua Jiang
Guihua Jiang