- 1Center for Infectious Disease and Vaccine Research, La Jolla Institute for Immunology (LJI), La Jolla, CA, United States
- 2Translational Research Unit, National Institute for Infectious Diseases “Lazzaro Spallanzani”-IRCCS, Rome, Italy
- 3Ragon Institute of Massachusetts General Hospital (MGH), Massachusetts Institute of Technology (MIT), and Harvard, Cambridge, MA, United States
- 4Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS) MultiMedica, Milan, Italy
- 5Immunology and General Pathology Laboratory, Department of Biotechnology and Life Sciences, University of Insubria, Varese, Italy
- 6Department of Internal Medicine, Rush Medical College, Chicago, IL, United States
- 7IRCCS European Institute of Oncology IEO, Milan, Italy
Knowledge of aging biology needs to be expanded due to the continuously growing number of elderly people worldwide. Aging induces changes that affect all systems of the body. The risk of cardiovascular disease and cancer increases with age. In particular, the age-induced adaptation of the immune system causes a greater susceptibility to infections and contributes to the inability to control pathogen growth and immune-mediated tissue damage. Since the impact of aging on immune function, is still to be fully elucidated, this review addresses some of the recent understanding of age-related changes affecting key components of immunity. The emphasis is on immunosenescence and inflammaging that are impacted by common infectious diseases that are characterized by a high mortality, and includes COVID-19, HIV and tuberculosis.
1 Introduction
It has been estimated that in the next thirty years the number of individuals older than 60 years of age will double, increasing by over a billion individuals; and the number of individuals over age 80 may increase by as much as 300 million people (United Nations Secretariat, E.S.A., 2017). This will lead to a significant increase in age-related diseases. Aging has emerged as one of the greatest and most prevalent risk factors for the development of infectious diseases (1, 2). However, it has been observed that individuals have a different pace of aging which led to the concept of biologic aging. We refer to chronologic aging only with the passage of time, whereas we refer to the biologic aging in relation to functional decline. Biologic aging not only enhances the risk of disease, but further accelerates varied biological processes, which are defined aging hallmarks (3) or pillars (4, 5). Aging impacts nearly all aspects of cellular function, influencing the physiological performance of different organ systems, which induces a decline in immune resilience, with increased susceptibility to infections and mortality. Aging has effects on many cellular processes such as stem cell exhaustion, altered intercellular communication, genetic and epigenetic changes such as DNA methylation, histone modifications, nucleosome positioning, and telomere attrition, decreased capability of protein production and accumulation of misfolded proteins (i.e. loss of proteostasis), dysregulated nutrient sensing, mitochondrial dysfunction, and cellular senescence (5). Cardiovascular disease and cancer are strongly age-associated (3). As we age, the immune system undergoes immunosenescence, which is marked by a systemic process known as inflammaging along with a series of defects in immunological activity that results in poor responses to infectious agents, vaccination and cancer (6–8). Inflammaging is defined as a chronic, sterile, low-grade inflammation that associates with older age, and may contribute to the pathogenesis and clinical manifestations of other age-related diseases (6–8). Inflammaging is due to a loss of control over systemic inflammation leading to a chronic stimulation of innate immunity (7, 9–14). These changes in the immune system, which occur naturally in aging, are linked to an enhanced vulnerability of older adults to disease and death. In particular, aging reduces the ability of the anatomical sites to be barriers to infection, including thinning of the skin, reduced cough reflex, modifications in genitourinary anatomy and bacterial flora and impairment of the bladder capacity and emptying. Comorbidities such as diabetes mellitus and its complications may also weaken host immune defences, increasing risk of infections (i.e foot ulcers, skin and mucosa infections). Neurovascular alterations may enhance the risk of stroke resulting in swallowing dysfunction that may lead to aspiration pneumonia development. Parkinson, Alzheimer, and dementia are a burden of older age. Drug administration can also adversely affect host defence, as well implantable devices, and invasive procedures (15). Relatively healthy, older adults are mostly at risk of respiratory infections (bronchitis, pneumonia) mainly of bacterial origin, followed by urinary tract (in the non-catheterized person) and gastrointestinal infections. Older individuals with specific comorbidities have a higher risk of infection at that organ site (i.e endocarditis with a damaged heart valve, urosepsis with chronic catheter). The risk of infections in the elderly increases in hospitalized patients with aspiration pneumonia, urinary tract infections (for catheter-related issues), intravenous line infections, and infected pressure ulcers (bedridden, immobile). Being older than 85 years of age can be an independent risk factor for admission to the intensive care unit (ICU) and hospital mortality (16). COVID-19, tuberculosis (TB) and Human Immunodeficiency Virus (HIV) infection are the most important infectious disease worldwide accounting for the highest mortality among infectious diseases with more than 1.2 million cases in 2022 for COVID-19 (17–20), 1.6 million estimated deaths for TB in 2021 (21) and 650.000 estimated death for HIV infection in 2021 (22). There were approximately 38.4 million people living with HIV at the end of 2021 with 1.5 million people becoming newly infected with HIV in 2021 globally (22). These diseases have an important health and economic load globally with important consequences in the aging groups. In this review we will focus on immunosenescence during these most common infectious disease (Figure 1), highlighting the effect of aging on host innate and adaptive immunity in response to these infections and their contribution on the enhanced disease vulnerability seen in the fragile older adults.
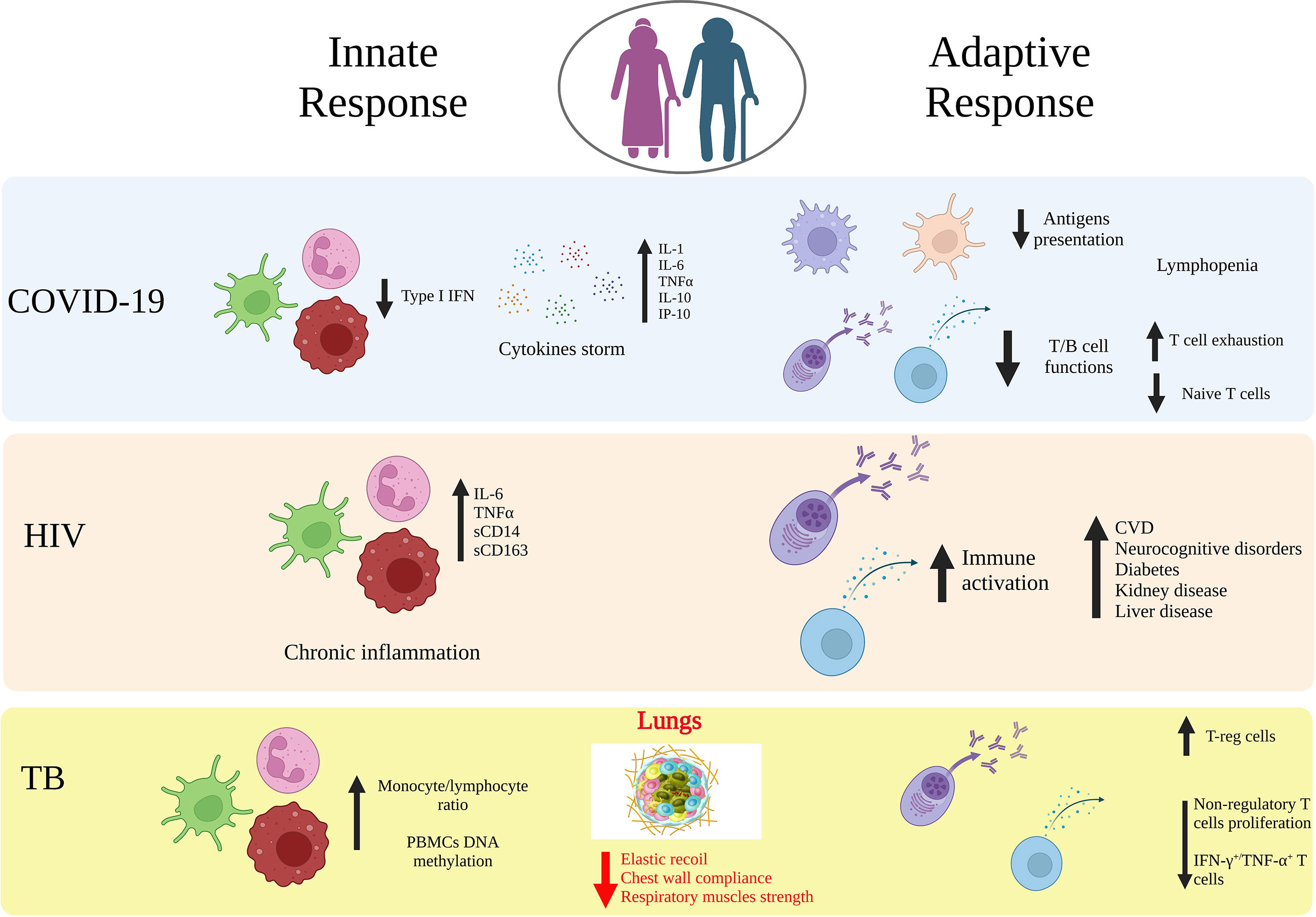
Figure 1 Age-related immune alterations in infectious diseases. Impact of age on the innate and adaptive immunity in COVID-19, HIV and tuberculosis (TB). Aging may lead to modifications of both innate and adaptive immunity arms that induce a dysfunctional immune response against viral or bacterial infections, which includes increased cytokines release, downregulation of APCs, T and B cell functions, upregulation of T-reg cells. Elderly people are an at-risk group for a more aggressive organ damage and the development of secondary diseases. Created with BioRender.com.
2 Aging immunity
2.1 Innate immunity in aging
The innate immune system acts as the first line of host defense against pathogens. Innate immune responses limit viral entry, replication, and assembly, identifying and removing infected cells, and coordinating the development of adaptive immunity. Innate immunity involves a broad range of cells, including those from the myeloid lineage: macrophages, monocytes, dendritic cells (DCs), neutrophils and granulocytes, myeloid derived suppressor cells, mast cells, and innate lymphoid cells (ILCs) such as natural killer (NK) cells. Innate immune cells can sense pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) by their pattern recognition receptors (PRRs) to induce inflammatory signaling pathways, followed by the production of inflammatory cytokines and the induction of cell death to clear infected cells (23, 24). Aging of the innate immune system in humans is notable for a paradoxical increase in levels of proinflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α (25). The innate immune system can be trained to respond more rapidly and effectively to infections and this phenomenon is called trained immunity or innate immune memory (26). Trained immunity is characterized by metabolic and epigenetic reprogramming in leukocytes in association with enhanced antimicrobial functions (27). Few studies are available on the effect of trained immunity in aging. Recently, it has been shown that innate immune training can be induced in aging healthy individuals as well as critically ill sepsis patients (28). It can be induced regardless of age and there are not significant differences in the immune trained phenotype as a function of age. Based on the few studies available, trained immunity seems preserved in aged people.
Although the increased inflammation with age is widely discussed, the key innate immune cells believed to facilitate the inflammaging phenomenon are monocytes and macrophages. Several reviews detail how the macrophage and monocyte phenotype and function changes with age (29), as well as neutrophils (30). Differently, how ILCs and NK cells are altered by aging has not been well described in the literature and will be the focus of our discussion below. ILCs represent a relatively recently identified heterogeneous family of mononuclear hematopoietic cells found mostly in solid tissues, which are defined by the expression of factors and cytokines previously associated with T cell lineages. Based on their morphology, gene expression and cytokine production ILCs are classified into three groups ILC1, ILC2, and ILC3. Different than T helper cells, ILCs are activated and regulated by accessory cytokines and germ-line-encoded receptors. Group 1 ILC populations include conventional NK cells and ILC1s and express the transcription factors T-bet or Hobit for their maintenance (31). ILC2s express mainly the transcription factor GATA-binding protein 3 (GATA3) and are associated with type 2 immunity, tissue fibrosis and epithelial repair, dependent on IL-33 (32–34). ILC3s are mainly divided into two subsets, NCR– and NCR+ ILC3 depending on the expression of natural cytotoxicity receptor (NCR) NKp46 or NKp44. ILC3s are grouped into more complex subsets on the basis of their expression of the transcription factors RORγt, RORα, c-Maf, and HIF1 (31). Frequencies and functional potentials of ILC subsets over the human life course have been studied (35). ILCs are more abundant in early life than in later years, but blood CD117+ ILC2 are preferentially maintained compared to their CD117- counterparts. ILC2 have been found decreased in aged mice (36), and ILC2-mediated functions repressed in response to aging and high-fat diet (34). ILC3 are important for the activation and activity of NK cells, including cell lysis and cytokine secretion. They produce anti-microbial peptides, IL-17 (important for neutrophil recruitment) and IL-22 (important for tissue repair). They play a role in viral and bacterial infections in the lung and gut (35, 37–39). They decrease with age (35, 38, 40) and this may likely contribute to susceptibility of infections of the elderly population. NK cells are ILCs involved in immunosurveillance by inducing cell lysis both directly (via perforin/granzyme system) or indirectly [via secretion of TNF-α and interferon (IFN)-γ]. One aspect of immunosenescence that has received less attention as compared to other innate immune cells is age-related NK cell dysfunction, characterized by reduced cytokine secretion and decreased target cell cytotoxicity, despite the increase with age (41). Two major human NK cell subsets are defined by the CD56dimCD16+ and CD56brightCD16-/+ phenotype. CD56brightCD16+ are lytic while CD56brightCD16- are cytokine producing (41, 42). Elderly individuals have NK cell subsets that are CD56lowCD16high and CD56-, and express check-point inhibitory receptors and activation marker phenotypes (43). Above 50 years of age increasing integrin α4β7 and decreasing C-C chemokine receptor type 7 (CCR7) has been observed (44). Elderly individuals >65 years accumulate the memory-like NK subset CD52+ NK2 (NK2.1) that exhibits a type I IFN response and displays proinflammatory characteristics (45). Beside the role that NK cells play against viral infections (33), they are important also in the immunosurveillance as they clear senescent cells, which is linked to age-associated diseases (37, 38). Indeed, senescent cells express NK activating ligands on their surface such as NKG2D ligands, MICA, MICB, CD112 or CD155 which bind to NKG2D receptors or DNAM-1 receptors present on NK cells, respectively, leading to the recognition and killing of senescent cells (46). Senescent cells can upregulate ligands that activate inhibitory signals such as HLA-E or PD-L2, which are ligands for NKG2A or PD-1 receptors expressed on NK cells, respectively (47, 48). NK cell dysfunction is linked to increased incidence of infections, cancer, inflammatory disorders and related to the accumulation of senescent cells with age (41).
In summary, age has an impact not only in the well-studied myeloid cells, but also in innate lymphoid cells and NK cells which are key players against infections. Understanding how NK cells clear senescent cells, as well as how age impacts NK cells is important for increasing our knowledge on immunopathogenesis of aging and for developing targeted therapy.
2.2 B cell immunity in aging
Antibodies represent the primary correlate of immunity against infection (49). However, aging is associated with a functional decline in the humoral immune response (50, 51), marked by poor antibody production and the accumulation of aberrant, age-associated B cells (ABCs) (52), compromised germinal center formation (53), and compromised T cell help (54). Specifically, aging is associated with altered responsiveness to vaccines, including reduced influenza vaccine-induced antibody neutralizing titers (51), reduced immunogenicity following COVID-19 vaccination, and reduced durability of vaccine-induced immune responses (55–57). However, in the setting of COVID-19 vaccination (58), the addition of a boost significantly augmented specific immunity in the elderly (59), suggesting that at least some immune defects may be rescued via the delivery of additional signals to potentiate the immune response. The induction of potent long-lived vaccine-induced humoral immune responses requires the delicate and coordinated interplay of vaccine antigen uptake by antigen-presenting cells (APCs) that must shuttle antigen to the lymph node and trigger helper-T cells and T-follicular helper cell (Thf) responses, while simultaneously driving antigen deposition on follicular dendritic cells (DCs) that must present antigen to circulating B cells (60). Aging has been associated with significant changes in all cell types involved in the vaccine-induced immune response (61) in terms of phagocytic activity, migratory capacity, inflammatory cytokine release resulting in less potent antigen presentation and priming activity. Interestingly, with aging, T-follicular regulatory cell (Tfr) frequencies decline in the blood and tissues, due to enhanced numbers of Tfr that attenuate Tfh and B cell activation (62). Moreover, as mentioned above, aging is marked by a dramatic shift in the phenotype and function of B cells (52). With aging B cell numbers do not change (63) whereas the bone marrow generates fewer B cells due to alterations in paired box protein (PAX)-5 expression, defects in pro-B cell response to IL-7 (64), and increased TNF-α secretion in the bone marrow, which attenuates the production of immature B cells (63). Instead, ABCs are characterized by distinct phenotypes (CD19+, CD21−, CD11c+, T-bet+), gene expression profiles, distinct survival requirements, different B cell repertoire and unique functions. ABCs are not derived from bone marrow B cells but emerge from follicular B cells after extensive affinity maturation, toll like receptor (TLR) stimulation, and proliferation. ABCs are particularly responsive to nucleic acid-sensitive TLRs, including TLR7 and TLR9, independent of B cell receptor stimulation (65). Importantly, these unique properties of ABCs accumulate in autoimmune conditions, and have been shown to contribute to autoantibody production (66). As mentioned above, despite the B cell compartment defects that accumulate with aging, additional boosts as well as novel immunization strategies, including the use of particular adjuvants, have the capacity to rescue these defects and drive robust immunity in the elderly (67). Precisely, against influenza virus, novel adjuvants including MF59 (68) and AS03 (69) have been shown to drive enhanced neutralizing antibody titers in aging populations. Additional boosts have equally been shown to normalize antibody levels to those observed in the general population (59). However, reduced durability of the antibody response remains a challenge, likely requiring additional strategies to promote enhanced T-help to drive longer-lived plasma cell formation.
In summary, antibodies are an important correlate of immunity against infections. Aging is associated with a numerical decline and impairment of humoral response that may limit the ability to contain pathogen growth and response to vaccines.
2.3 T cell immunity in aging
T cell responses are a key player of the adaptive immune response to infectious diseases. Adaptive immune responses in the course of a lifespan undergo numerous changes that lead to modifications in T cell subset composition, homeostasis and functionality (i.e. antigen processing and presentation, cell proliferation and activation and signalling pathways) (70). The changes observed in the T cell response can be summarized in the concepts of immunosenescence, inflammaging and enhanced immune suppression. Immunosenescence, defined as the impaired ability to mount immune responses against new infections, is characterized by a reduction of response, which is related to a decrease in the T Cell Receptor (TCR) repertoire diversity and consequent expansion of fewer memory T cell clones (71). As reported above, inflammaging describes the chronic inflammation observed in the elderly and is partially linked to self-reactive T cells inducing chronic tissue damage and low-grade inflammation. Finally, an enhanced immune suppression is observed and linked to an expansion and accumulation of T-reg and Th17 cells in peripheral lymphoid organs with a relative increase in the production of IL-17 and IL-22 cytokines, which are important regulators of proinflammatory cytokines such as IL-6 and TNF-α (72). All those changes in T cell responses are linked to thymic involution, primarily observable during the adolescence phase and characterized by a progressive decline with lymphoid organs being substituted by adipose tissue (8, 73, 74). A secondary atrophy of the thymus occurring around 40-50 years of age, is the one that ultimately reduces the production of naïve T cells (75), although the overall number does not show a comparable drastic decrease due to compensatory mechanisms such as the peripheral clonal expansions of CD4+ and CD8+ T cells also defined as homeostatic proliferation (76). An additional theory links T cell immunosenescence not only to the thymus but also to the presence of autoantibodies contributing to the deficiency of naïve T cells and increased T-cell activation (77). Therefore, regardless of the cause, with age we observe a progressive decrease of peripheral naïve T cells, in consort with an accumulation of memory T cells with an exhaustion phenotype, loss of the co-stimulatory molecules and acquisition of a differentiated phenotype (78, 79). The change in quality of the T cell responses as a function of age ultimately impairs the ability of T cells to mount a response against new antigens (80, 81). This is particularly evident in the context of CD8+T cells where the senescent immune system shows a specific reduction in effector capability, critical for the control of many viral infections (81).
In summary, T cell responses are important in protecting from disease severity. Aging is influencing the naïve T cell repertoire and capability to mount a rapid immune response against a novel viral threat. As we will describe below, this may lead to an increased risk of infectious disease severity as in COVID-19 or to the re-activation of latent infections as observed in the context of TB.
3 Specific infection diseases in elderly
3.1 COVID-19
Several studies suggest aging as a significant risk factor for severe COVID-19 and increased mortality rate and this is linked to age-related changes observed both in the innate and adaptive responses compartments (82–84) (Figure 1). In terms of innate immunity, the cytokines in COVID-19 exert both antiviral and inflammatory activities and promote disease-associated processes directly, such as epithelial cell death and immunothrombosis. Following SARS-CoV-2 exposure, the type I IFN production is increased (85–88). However, patients with severe COVID-19 have altered innate immune function, such as a reduced gene expression (i.e. IFN-β, IL4R; IL10RA, IFNAR1) and activity in response to type I IFN (i.e. TANK binding kinase 1 (TBK1), IFN-regulatory factor (IRF)3, IRF7, signal transducer and activator of transcription 1 (STAT1)/STAT2) (89, 90), thus strongly suggesting that the immunosenescence-associated high viral load could be due to a lower type I IFN response that limits the SARS-CoV-2 clearance. Angiotensin converting enzyme 2 (ACE2) expression levels, the receptor used by SARS-CoV-2 to infect host cells, has been also linked to COVID-19 severity and are affected by age. Counterintuitively, a decline in expression is associated both with age progression and severe disease. This is due to the fact that ACE2 enzyme exerts an innate anti-inflammatory function which is particularly important in the lungs in modulating an excessive inflammatory environment (84). Overall, the impairment of the innate immune response with a combined reduced type I IFN response, and ACE2 activity in the lungs, could contribute to the high levels of pro-inflammatory cytokines and chemokines released, promoting inflammation, vascular permeability, and poor outcomes in older COVID-19 patients (91). To expand more on the levels of pro-inflammatory cytokines, several studies have been shown that SARS-CoV-2 infection triggers in general an excessive induction of multiple cytokines beyond type I IFN, in the lungs and in the blood, which is associated with elevated levels of many pro-inflammatory cytokines, including IL-1, IL-2, IL-6, IL-10, IL-12, TNFα, CXCL10, CCL2, CCL3 (92–95). The levels of these cytokines are correlated with disease severity, and inversely correlated with survival rate. In the general population, inflammaging represents one common feature of biological aging with high circulating levels of pro-inflammatory molecules, such as TNF-α, IL-1, and IL-6 (9). Predisposition to severe symptoms and negative prognosis of COVID-19 has shown to be related with production of cytokines and pro-inflammatory molecules, dysregulation of mammalian target of rapamycin (mTOR), and alteration in the number of innate cells, their activation and polarization (96). Specifically, altered proinflammatory macrophages with consequent recruitment of granulocytes and monocytes in the lung tissue has been reported in severe cases (97, 98). Another hallmark characteristic of disease severity and connected with impaired innate immunity is the decrease of phagocytosis capabilities for neutrophils and macrophages, which is exacerbated by age. NK cell polarization is also affected in severe COVID-19 and age, particularly memory-like NK2.1 cells and related produced cytokines are associated with disease severity in COVID-19 (45). Specifically, IFN-γ due to an early excessive NK cell activation is involved in inflammatory response with a poor prognosis in elderly COVID-19 patients and TGF-β is associated with a shorter hospitalization time of adult patients suggesting a role for TGF-β in preventing an excessive NK cell activation and systemic inflammation (43). Overall, the changes to the innate immune system in older COVID-19 patients can contribute to a greater risk for cytokine storm, due to the proinflammatory environment and reduction in pathogens clearance making them more vulnerable to SARS-CoV-2, and eventually lead to severe cases, more complications, or deaths (10, 11, 99). Humoral responses and particularly neutralizing antibodies are a correlate of protection in COVID-19. The protective immune response against the viral spike protein that prevents SARS-CoV-2 infection has driven the development of vaccines. Four of the vaccines initially authorized for emergency use, Pfizer BNT162b2, Moderna mRNA‐1273, Janssen/J&J Ad26.COV2.S, and Novavax NVX‐CoV2373 COVID‐19 vaccines, were designed based on spike protein, and therefore, most of the knowledge has been obtained about immunity to this viral antigen and, particularly, to its receptor binding domain (RBD) in the context of vaccines. However, there is still a lack of clear definition of a threshold of antibody response sufficient to provide protection against COVID-19 infection or development of severe disease when infection occurs. On the other hand, in several clinical studies of COVID-19 vaccine recipients, both neutralizing and binding antibodies were thought to be highly predictive of immune protection, although this relation is reduced to variants of SARS‐CoV‐2 (100–103). A single dose of mRNA-based vaccine (BNT162b2 or mRNA‐1273) induced IgG and neutralization antibodies, and subsequent doses induced a further increase in both uninfected healthy individuals and naturally infected convalescent individuals. At 6 months post‐vaccination, the antibody activities declined dramatically, and thus additional doses of vaccine are highly recommended to achieve maximum level of protection against COVID-19, especially against the current predominant variant Omicron BA.5 (104–106). Parallel studies have additionally shown that early kinetics of SARS-CoV-2-specific T cell responses and a coordinated adaptive immune response are associated with milder disease (107–110). Taken together, these findings suggest that T cells may act as second line of defense helping in reducing the viral spreading and potentially disease severity. Aging in COVID-19 affects multiple components of the immune system already challenged by the SARS-CoV-2 infection (82, 109, 111). Indeed, the delayed T cell kinetics are exacerbated by an impaired antigen presentation, frequently related to age, but also specifically induced by the SARS-CoV-2 infection (112) as well as a narrow antigen repertoire in contrast to what is observed in milder infection (110, 113). Another hallmark characteristic of the SARS-CoV-2 acute infection is the lymphopenia which has been shown to correlate with increased disease severity and poor outcome. In older patients the lymphopenia is more pronounced as compared to young severe cases, the lower T cell counts further reduce the pools of T cells responding to the infection and limiting its spreading (112). The number of naïve T cells in older adults is decreased, which reduces the development of a robust adaptive antiviral response, thus contributing to the generation of a severe form of COVID-19 (107, 114, 115). Quantity is not the only parameter to consider for T cells, similarly to the humoral response, the maturation status and functionality are also important. In terms of T cell maturation, previous studies have reported a negative correlation between disease severity and frequency of naïve T cells, which is even more pronounced and progressively decreased as a function of age (107, 116). In concomitance with a decline in the naïve repertoire, COVID-19 severe disease is also associated with more terminally differentiated CD8+ cytotoxic T cells with reduced functionality (116) and that is particularly common in patients over the age of 80 years (117). Another dysregulation of the T cell response that is common in aging and reported in COVID-19 patients is the T cell exhaustion, particularly for CD8+ T cells. However, it is worth noting that many of those conclusions are based on the expression of PD1 and TIM-3 markers (118), associated to T cell activation rather than functional exhaustion during acute viral infections (116, 119, 120). Overall, several reports conclude that a reduction in number and functionality of CD8+T cells contribute to severe disease. Conversely, contrasting findings emerge in terms of CD4+T cell effector functions where both a decrease in functionality, in terms of cytokine release, and no differences across disease severity have been independently reported (112, 121, 122). Most of the studies reviewed so far, focused on the role of T cell aging in contributing to COVID-19 severe disease. Since the introduction of COVID-19 vaccines, the field is now challenged in understanding how aging is affecting vaccine-induced T cell responses, particularly as age-dependent heterogeneity was observed in the context of the BNT162b2 vaccination (123). Limited studies have been performed so far on the effect of vaccination in elderly with particular emphasis on the T cell response. This remains an important gap of knowledge to address, given the importance of T cell responses in contributing to a protective SARS-CoV-2 specific response, and the resilience observed in cross-recognizing novel variants of concern following vaccination in immune competent (124–128) and immune deregulated individuals as those with multiple sclerosis (129, 130) or immune-mediated inflammatory diseases and/or Rheumatoid Arthritis (131–136). To evaluate potential therapeutic targets against SARS-CoV-2 infection factors influencing immunosenescence and inflammaging processes should be seriously considered. For example, more inflammation caused by innate cell activity has been reported in older males compared to age-matched females, while displaying less adaptive T- and B-cell activities, thus may explaining why older males more likely experienced a severe form of COVID-19 or even death (137, 138).
In summary, aging affects multiple components of the immune system. In the context of innate immunity, there is a direct effect on the viral infection (eg. expression levels of ACE2 that directly modulate infectivity) as well as an indirect effect due to altered functionality of other innate cells such as excessive production of pro-inflammatory cytokines and reduction of phagocytic capabilities directly linked to severe COVID-19. In parallel, adaptive immunity and mainly T cell responses are impacted, providing an additional explanation for the severe symptoms associated with age. Thus, severe COVID-19 is due to multifactorial immune components independently affected by age, highlighting geroscience’s importance in thoroughly dissecting all the pathogenetic mechanisms and providing better vaccine strategies and therapeutic approaches in the elderly population.
3.2 HIV
The field of HIV research has substantially advanced over the past forty years based on the introduction of highly active antiretroviral therapy. This has led to persons who are living with HIV (PLWH) to live to older ages even approaching normal life span (139). There exists however the potential that HIV infection may lead to accelerated or accentuated aging with the development of non-communicable diseases at an earlier age (140). This includes the development of cardiovascular, metabolic diabetes, neurocognitive, bone, liver and kidney disease (141–144). The underlying driver for developing these non-communicable diseases is a subject of active investigation. One of the major areas of study here is persistent inflammation in PLWH that has been recognized as a major contributor to these conditions (145). The main contributor to this inflammation appears to be the activated innate immune system (Figure 1). The markers of inflammation include finding persistent elevation of IL-6 and TNF-α as well as C reactive protein and D-dimer. The role of microbial translocation and the gut microbiome have received significant attention in contributing to the persistence of these inflammatory markers. The biomarkers of gut translocation include intestinal fatty acid binding protein, zonulin, lipopolysaccharide binding protein (LBP), and beta-D-Glucan. The bacterial translocation marker LBP (146) and the fungal biomarker beta-D-Glucan (147) have been shown to stimulate innate immune effector cells including monocyte/macrophages that leads to elevated levels of inflammatory mediators and drive the development of the non-communicable diseases in the aging HIV population (148). From the perspective of the microbiome changes in HIV-infected patients and aging we recognize the critical role of microbial metabolomic products. The most relevant of these are the short chain fatty acids which are reduced in PLWH and the loss of these metabolites has been shown to contribute to the persistent activation of innate immune effector cells and non-communicable diseases. We also recognize that other co-infections in PLWH may contribute to aging and these include Cytomegalovirus (CMV), Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) infections. The role of CMV in aging and on the immune system is especially critical as this virus can contribute significantly to T cell senescence (149). On a global basis other prominent infectious disease such as tuberculosis and arbovirus infections (i.e. dengue) found especially in resource limited settings, can be an important driver of inflammation and co-morbidities such as cardiovascular disease. Other critical contributors to inflammation include life style factors such as smoking, alcohol and diet. We recognize especially the critical interactions of alcohol with the immune system and the gastrointestinal tract that can contribute to microbial translocation and reductions in short chain fatty acid producing bacteria. The study of HIV disease has now become much more integrated into the aging biology field with the geroscience movement. The concepts of inflammaging and senescence-associated secretory phenotype (SASP) are being evaluated more and more in PLWH. We are working to move more concepts of gerontology into the HIV field including the evaluation of frailty as a critical way to focus on the functional assessment of the whole person (150). The features of both innate and adaptive immunity are now well integrated into our thinking of HIV pathogenesis and aging.
In summary, HIV can be considered a disease of accelerated and accentuated aging linked to multiple non-communicable disease outcomes. The cause of this aging phenotype in HIV is multifactorial and includes inflammaging, microbial translocation, coinfections, and life style factors. The study of HIV and aging now intersects much more with applications of geroscience providing novel insights into the pathogenesis of HIV.
3.3 Tuberculosis
It is known that aging is a major risk factor for developing TB disease (151). Although, TB notification rate decreased in the period 2016-2021, likely due to the COVID-19 pandemic (17–19), 10.6 million are the estimated new cases worldwide in 2021 and the elderly (>65 years) account for almost 1,400,000 cases (about 13% over total) (152). Elderly population is at higher risk for both the reactivation of TB infection and for new Mycobacterium tuberculosis (Mtb) infections, with higher mortality rates and adverse drug reactions (153–157). In addition to the presence of co-morbidities as diabetes (158) and concomitant drug use with decreased renal and hepatic drug clearance (159, 160), aging is associated with a progressive reduction in the respiratory system function. At an anatomical structural level, the lungs have a decreased elastic recoil, reduced chest wall compliance and show weakness of the respiratory muscles (161), thus compromising the efficacy of the airways secretion clearance. Although it is known that immune-senescence and inflammaging are natural processes that occur in both circulating cells and tissue immune cells, the influence of age on the interplay between these cells during Mtb infection is not understood and it is understudied. The host-Mtb interactions are complex and only partially explained. When Mtb invades a susceptible host, neutrophils, monocytes, DCs are infected in the lung and then an innate granuloma is formed. Then, T-cell specific response is generated in the draining lymph nodes and they reach the granuloma (162, 163). It has been demonstrated that the lungs of old mice have elevated levels of proinflammatory and Th1 cytokines (TNF-α, IFN-γ, IL-12) and the resident macrophages express elevated levels of IFN-γ-induced activation markers (IRGM-1, IRF-1, CIITA). Notably, those macrophages infected in vitro have increased Mtb uptake and phago-lysosomal (P-L) fusion but a defect in intracellular control of mycobacterial growth. These data indicate that age induces an inflammatory pulmonary environment which makes the resident macrophages more prone to be infected by Mtb, at least in mice (164). This is partially in agreement with the known altered monocyte proportion (increased monocyte/lymphocyte ratio) (165, 166) (Figure 1). T cells are crucial players in the host defence and in containing the spread of Mtb during acute infection and reactivation. T lymphocytes subsets are pivotal for the regulation of the immune response as demonstrated by the lack of Mtb containment during HIV-coinfection and increased HIV replication in coinfected patients (167–169) or by the impairment in coinfection TB-COVID-19 (170–173). It is reported that an imbalance between Th1- and Th2-like cells plays an important role in disease outcome in human TB, with a Th2 polarized response as the major contributing factor for immunosuppression and dissemination of the disease (169) whereas a Th1 polarized response is associated to cell–mediated host antimycobacterial defence (174). Conflicting results on the Mtb-T-cell-specific response of circulating cells in the elderly population are available. It has been reported that the IFN-γ production measured by IFN-γ release assays (IGRA) (175) is either negatively correlated with age (176) or not changed (166). It has been speculated that the PD-1/PD-L1 pathway impairs Th1 immune response in the late stage of infection (177). Interestingly, a trend for increased PD-1+ cells in both CD4+ and CD8+ lymphocytes in elderly patients with TB disease has been observed (166). However, the regulation of the Th1/Th2 balance is not sufficient to control Mtb replication (178). Other T cells such as T-reg cells are fundamental in the immune response against Mtb (179, 180). The frequency of circulating T-reg cells (CD4+ CD25+ FoxP3+) is higher in older TB patients as compared to younger counterparts (181). Interestingly, these cells have an increased proliferative rate in response to Mtb antigen stimulation in vitro, with the concomitant reduction of the proliferation of non-regulatory cells (CD4+ CD25− CD127+ FoxP3− Ki67+). Notably, the depletion of the T-reg population resulted in the rescue of effector T-cell functions as measured by the frequencies of IFN-γ+, TNF-α+ single, as well as polyfunctional (IFN-γ+ and TNF-α+) T cells (181). It has to be considered that differences in the memory immune cells repertoire may exist among the different anatomical sites, for instance peripheral blood vs tissue. Aged individuals with detectable circulating T cells responding to Mtb antigens were nonresponsive locally as evaluated by skin tests (182). Senescent or exhausted T cells display molecular signatures of aging, such as mitochondrial dysfunction and epigenetic remodelling (183). Interestingly, it has been reported that patients with TB have DNA hypermethylation of multiple genes of the IL-12/IFN-γ signalling pathway, associated with decreased immune responsiveness, IFN-γ–induced gene expression and decreased IL-12–inducible upregulation of IFN-γ (184). Moreover, it has been reported that peculiar DNA methylation signatures may influence individuals prone to be infected by Mtb (185). Overall, evidence strongly indicates that epigenetic modifications of immune cells of TB patients, in general and more specifically in the elderly, are critical for Mtb immune response. Human and non-human primates have unique Vγ2Vδ2 T cells, which are the sole γδ T-cell subpopulation capable of recognizing the microbial (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) produced by selected pathogens such as Mtb (186). Interestingly, it has been reported that aging induces a shift in the central and effector memory populations from Vγ9Vδ2 to Vγ2Vδ1, thus indicating that this T cell population may be involved in the higher susceptibility to TB of elderly people (187). Overall, the few data available on the aged TB patients show that aging is an important determinant for the quality and magnitude of systemic inflammatory and specific immune response to Mtb. Since it has been shown that in old people an elevated number of T-reg cells correlates with bacillary load and disease severity, it may be plausible that changes in the immune system related to senescence may contribute to the increased risk of either new infections or Mtb reactivation in the already infected people (181).
In summary although almost 13% of TB patients are over the age of 65 (21) accounting for 1.4 million individuals, little research has been performed on this population. In the future for clinical and immunological research topics, it will be important to better understand in the elderly the immune mechanisms that may lead to TB progression versus Mtb containment. Since the average life span of the worlds’ population is constantly growing, progress towards the development of an effective vaccine and/or the development of adequate therapy for aged TB-infected people is needed. It is likely that a multidisciplinary approach involving infectious disease specialists and geriatricians will be required to improve clinical outcomes of Mtb infection in this vulnerable population. In fact, the Mtb-induced morbidity is not due exclusively to the pathogen loads but also to the capability of the host to resolve the inflammatory reaction.
4 Possibilities of strengthening immunity in old age
Aging is a very complex process which involves both biological features and environmental and psychological aspects. To improve immunity in the elderly it is, therefore, not sufficient to have treatments aimed at increasing the innate or specific immune response against microorganisms, it is also necessary to lower the inflammaging process. In other words, a holistic approach, which involves specific treatments, nutrition and life style is necessary to achieve this goal. Several approaches can be proposed to strengthen immunity against infections in old age, which includes vaccination, modulating inflammation and microbiome composition. Vaccinations are recommended in the elderly, with the purpose to generate specific immunity that reduces infections, disease severity and mortality. Recommendations are mainly for vaccinations against Influenza (188), SARS-CoV-2 (189), Pneumococcus (190) and Herpes Zoster (191). Vaccination against tuberculosis is not effective in adults or in the elderly to strengthen tuberculosis-specific immunity (192, 193). On the other hand, non-specific effects of vaccination have been reported (194) and may represent a way of overcoming immunosenescence in elderly people. Bacillus Calmette Guerin (BCG) vaccination is one of the most studied. It has been reported that BCG vaccination decreases the inflammatory state of elderly people, decreasing the plasma levels of proinflammatory cytokines (i.e. TNF-α, IL-6, and IL-1β), chemokines (i.e. CCL2 and CXCL10), matrix metalloproteinases, acute phase proteins (including C-Reactive Protein) one month after inoculation (195). Conflicting results have been reported on the role of BCG vaccination to protect elderly people from lower respiratory tract infections of viral origin including COVID-19 (196–198). It is however important to highlight that the BCG-vaccination improved the cytokine response and the production of antibody titers after COVID-19 infection (195, 197). In another trial, BCG vaccination decreased the risk of COVID-19 at six months post-vaccination while this approach failed at three months post-vaccination (175). Using a different vaccination strategy, live-attenuated Varicella Zoster (LAVZ) vaccine was shown to decrease the likelihood of being infected by SARS-CoV-2 in old people (199). The prophylactic function of BCG, LAVZ or other vaccinations has to be fully established; however, they may likely be used as a potential adjuvant to mitigate the effects of concurrent respiratory infections. Trained immunity has been hypothesised as a possible mechanism of action probably through the metabolic and epigenetic modifications induced by vaccination, as described for BCG (200). Since aging affects many intracellular processes, one possible strategy to improve immune cell fitness could be to provide elderly people with cellular supplement compounds such as antioxidants or nutrients. One example has been reported in HIV-infected individuals in which glutathione treatment for 13 weeks improved the levels of Th1-type cytokines circulating levels and in vitro reduced the intracellular survival of Mtb in infected PBMCs (201). Notably, supplementing aged people (61–80 years old) with a combination of the glutathione precursor amino acids glycine and cysteine (GlyNAC= glycine and N-acetylcysteine) for 16 weeks improved the oxidant damage, mitochondrial dysfunction, inflammation and endothelial dysfunction (202). A randomized case-controlled trial demonstrated that a supplement containing Sambucus nigra, zinc, tyndallized Lactobacillus acidophilus (HA122), vitamins (C, D, E) arabinogalactans, did improve both the inflammatory state and lymphocyte growth of people aged over 60 years (203). In line with these data are results of several clinical trials of elderly people receiving vitamin D supplementation that ameliorated the clinical score, the need for oxygen and intensive care support and survival rates (204). Along with these strategies, the microbiome, the community of microorganisms (i.e. fungi, bacteria, protozoa and viruses) present in the human body, can play a large part in inflammaging, especially when infectious diseases exert changes in the gut microbiome environment. The microbiome has a fundamental role in the development of the immune system throughout the lifespan of an individual. In advanced age, there are several aging-related changes that occur within the gut, including decreased epithelial cell proliferation, stem cell retention, and barrier function, as well as increased gut leakiness and proinflammatory dysbiosis (205). Aging-related changes to the microbiome can lead to changes in overall health of an individual, leading to further increases in inflammaging, and a proinflammatory state (206). There may be ways in which one could attempt to address or prevent aging-related deterioration of the microbiome. These approaches may include prebiotic supplementation which may be in conjunction with postbiotics to target an increase in beneficial microbial taxa and a reduction in taxa related to unhealthy aging (207, 208). Research is ongoing as to whether these types of interventions are beneficial or even functional in humans. Pilot studies of polyphenol-rich foods, prebiotics, and some probiotics have demonstrated some efficacy in older individuals with comorbidities (frailty, insulin resistance, obesity) (209). Interestingly, the use of probiotics as an adjuvant for influenza vaccination in a placebo-controlled, randomized, double-blind, clinical trial was found to be effective in reducing common infectious diseases symptoms and incidence (210). Other approaches to address inflammaging include the use of metformin or IL-6 inhibitors to reduce overall inflammation in the aging population. Metformin is widely used in the treatment of type 2 diabetes, and it may also promote healthy aging through a yet unknown mechanism, including potentially promoting a healthy microbiome in the gut (211). Efficacy of metformin may vary on an individual basis. As described above, the dysregulation of cytokines is implicated in inflammaging and in particular IL-6 is associated with increased age-related comorbidities, including frailty, risk for cardiovascular disease, diabetes, muscle loss, functional decline, and mortality (212–214). Therefore studies to evaluate the effect of IL-6 inhibitors in the aging populations may be beneficial to assess their role in preventing unhealthy aging. Further research is needed to conclusively determine interventions that will address aging-related changes to the microbiome that may lead to decreased risk of developing age-related systemic comorbidities.
In summary, there are a number of possible therapeutic areas including vaccination, inflammation modulators, vitamins, probiotics. Further studies are needed to identify those that may be pursued in ameliorating the impact of aging on infectious disease.
5 Conclusions
There is a strong link between the biological hallmarks of aging and the increased susceptibility to severe disease outcomes seen with biological aging. New research that may lead to the discovery of distinctive “biological hallmarks of aging” responsible for the immune deterioration and diminished resilience is often associated with aging (5). Novel therapeutic approaches need to be generated in order to improve infectious disease outcomes and enhance health in older adults.
Author contributions
DG, AG, AA, AL, GA: Conceptualization. AG, TA, DN, GA, AL searched literature. AG, TA, DG, AA, AL, GA wrote first draft. AG, AA, TA, GA, DN, DG wrote and edited overall. All authors contributed to the article and approved the submitted version.
Funding
DG and TA research is partially funded by the Italian Ministry of Health, Ricerca Corrente, Linea 4 and by TBVAC-HORIZON, funded by the European Union’s HORIZON program under Grant No. 101080309. This work was also supported by a grant from the Ministero della Salute COVID-2020-12371849 to DN and progetto RCR2021-23671212 on long COVID. Studies are partially funded by the Italian Ministry of Health Ricerca Corrente-IRCCS MultiMedica.
Acknowledgments
We sincerely apologize to all those colleagues whose important work was not cited due to space constraints.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gonzalez-Gonzalez C, Palloni A, Wong R. Mortality and its association with chronic and infectious diseases in Mexico: a panel data analysis of the elderly. Salud publica Mexico (2015) 57 Suppl 1(0 1):S39–45. doi: 10.21149/spm.v57s1.7588
2. Torrance BL, Haynes L. Cellular senescence is a key mediator of lung aging and susceptibility to infection. Front Immunol (2022) 13:1006710. doi: 10.3389/fimmu.2022.1006710
3. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell (2013) 153(6):1194–217. doi: 10.1016/j.cell.2013.05.039
4. Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: linking aging to chronic disease. Cell (2014) 159(4):709–13. doi: 10.1016/j.cell.2014.10.039
5. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell (2023) 186(2):243–78. doi: 10.1016/j.cell.2022.11.001
6. Franceschi C, Valensin S, Bonafe M, Paolisso G, Yashin AI, Monti D, et al. The network and the remodeling theories of aging: historical background and new perspectives. Exp Gerontol (2000) 35(6-7):879–96. doi: 10.1016/s0531-5565(00)00172-8
7. Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev (2007) 128(1):92–105. doi: 10.1016/j.mad.2006.11.016
8. Fulop T, Larbi A. Biology of aging: paving the way for healthy aging. Exp Gerontol (2018) 107:1–3. doi: 10.1016/j.exger.2018.03.014
9. Franceschi C, Campisi J. Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci (2014) 69 Suppl 1:S4–9. doi: 10.1093/gerona/glu057
10. Bektas A, Schurman SH, Franceschi C, Ferrucci L. A public health perspective of aging: do hyper-inflammatory syndromes such as covid-19, sars, Ards, cytokine storm syndrome, and post-icu syndrome accelerate short- and long-term inflammaging? Immun Ageing (2020) 17:23. doi: 10.1186/s12979-020-00196-8
11. Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM, et al. Aging in covid-19: vulnerability, immunity and intervention. Ageing Res Rev (2021) 65:101205. doi: 10.1016/j.arr.2020.101205
12. Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, et al. Immunosenescence and its hallmarks: how to oppose aging strategically? a review of potential options for therapeutic intervention. Front Immunol (2019) 10:2247. doi: 10.3389/fimmu.2019.02247
13. Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev (2007) 128(1):83–91. doi: 10.1016/j.mad.2006.11.015
14. Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. The origins of age-related proinflammatory state. Blood (2005) 105(6):2294–9. doi: 10.1182/blood-2004-07-2599
15. Yoshikawa TT, Norman DC. Geriatric infectious diseases: current concepts on diagnosis and management. J Am Geriatr Soc (2017) 65(3):631–41. doi: 10.1111/jgs.14731
16. Dimopoulos G, Koulenti D, Blot S, Sakr Y, Anzueto A, Spies C, et al. Critically ill elderly adults with infection: analysis of the extended prevalence of infection in intensive care study. J Am Geriatr Soc (2013) 61(12):2065–71. doi: 10.1111/jgs.12544
17. Migliori GB, Thong PM, Akkerman O, Alffenaar JW, Alvarez-Navascues F, Assao-Neino MM, et al. Worldwide effects of coronavirus disease pandemic on tuberculosis services, January-April 2020. Emerg Infect Dis (2020) 26(11):2709–12. doi: 10.3201/eid2611.203163
18. Migliori GB, Thong PM, Alffenaar JW, Denholm J, Tadolini M, Alyaquobi F, et al. Gauging the impact of the covid-19 pandemic on tuberculosis services: a global study. Eur Respir J (2021) 58(5):2101786. doi: 10.1183/13993003.01786-2021
19. Migliori GB, Thong PM, Alffenaar JW, Denholm J, Tadolini M, Alyaquobi F, et al. Country-specific lockdown measures in response to the covid-19 pandemic and its impact on tuberculosis control: a global study. J Bras Pneumol (2022) 48(2):e20220087. doi: 10.36416/1806-3756/e20220087
20. Ong CWM, Migliori GB, Raviglione M, MacGregor-Skinner G, Sotgiu G, Alffenaar JW, et al. Epidemic and pandemic viral infections: impact on tuberculosis and the lung: a consensus by the world association for infectious diseases and immunological disorders (Waidid), global tuberculosis network (Gtn), and members of the European society of clinical microbiology and infectious diseases study group for mycobacterial infections (Esgmyc). Eur Respir J (2020) 56(4):2001727. doi: 10.1183/13993003.01727-2020
23. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol (2010) 11(5):373–84. doi: 10.1038/ni.1863
24. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev (2009) 22(2):240–73. doi: 10.1128/CMR.00046-08
25. Montgomery RR, Shaw AC. Paradoxical changes in innate immunity in aging: recent progress and new directions. J Leukoc Biol (2015) 98(6):937–43. doi: 10.1189/jlb.5MR0315-104R
26. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. Mtor- and hif-1alpha-Mediated aerobic glycolysis as metabolic basis for trained immunity. Science (2014) 345(6204):1250684. doi: 10.1126/science.1250684
27. Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, et al. Epigenetic programming of monocyte-to-Macrophage differentiation and trained innate immunity. Science (2014) 345(6204):1251086. doi: 10.1126/science.1251086
28. Gill PS, Ozment TR, Lewis NH, Sherwood ER, Williams DL. Trained immunity enhances human monocyte function in aging and sepsis. Front Immunol (2022) 13:872652. doi: 10.3389/fimmu.2022.872652
29. De Maeyer RPH, Chambers ES. The impact of ageing on monocytes and macrophages. Immunol Lett (2021) 230:1–10. doi: 10.1016/j.imlet.2020.12.003
30. Van Avondt K, Strecker JK, Tulotta C, Minnerup J, Schulz C, Soehnlein O. Neutrophils in aging and aging-related pathologies. Immunol Rev (2023) 314(1):357–75. doi: 10.1111/imr.13153
31. Schroeder JH, Howard JK, Lord GM. Transcription factor-driven regulation of Ilc1 and Ilc3. Trends Immunol (2022) 43(7):564–79. doi: 10.1016/j.it.2022.04.009
32. Peters AL, Luo Z, Li J, Mourya R, Wang Y, Dexheimer P, et al. Single cell rna sequencing reveals regional heterogeneity of hepatobiliary innate lymphoid cells in a tissue-enriched fashion. PloS One (2019) 14(4):e0215481. doi: 10.1371/journal.pone.0215481
33. Bernsmeier C, van der Merwe S, Perianin A. Innate immune cells in cirrhosis. J Hepatol (2020) 73(1):186–201. doi: 10.1016/j.jhep.2020.03.027
34. Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, et al. Interleukin-33 and interferon-gamma counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity (2015) 43(1):161–74. doi: 10.1016/j.immuni.2015.05.019
35. Darboe A, Nielsen CM, Wolf AS, Wildfire J, Danso E, Sonko B, et al. Age-related dynamics of circulating innate lymphoid cells in an African population. Front Immunol (2020) 11:594107. doi: 10.3389/fimmu.2020.594107
36. D'Souza SS, Shen X, Fung ITH, Ye L, Kuentzel M, Chittur SV, et al. Compartmentalized effects of aging on group 2 innate lymphoid cell development and function. Aging Cell (2019) 18(6):e13019. doi: 10.1111/acel.13019
37. Hoffmann JP, Kolls JK, McCombs JE. Regulation and function of Ilc3s in pulmonary infections. Front Immunol (2021) 12:672523. doi: 10.3389/fimmu.2021.672523
38. Vely F, Barlogis V, Vallentin B, Neven B, Piperoglou C, Ebbo M, et al. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol (2016) 17(11):1291–9. doi: 10.1038/ni.3553
39. Jarade A, Di Santo JP, Serafini N. Group 3 innate lymphoid cells mediate host defense against attaching and effacing pathogens. Curr Opin Microbiol (2021) 63:83–91. doi: 10.1016/j.mib.2021.06.005
40. Bennstein SB, Weinhold S, Manser AR, Scherenschlich N, Noll A, Raba K, et al. Umbilical cord blood-derived Ilc1-like cells constitute a novel precursor for mature Kir(+)Nkg2a(-) nk cells. Elife (2020) 9:e55232. doi: 10.7554/eLife.55232
41. Brauning A, Rae M, Zhu G, Fulton E, Admasu TD, Stolzing A, et al. Aging of the immune system: focus on natural killer cells phenotype and functions. Cells (2022) 11(6):1017. doi: 10.3390/cells11061017
42. Pinti M, Appay V, Campisi J, Frasca D, Fulop T, Sauce D, et al. Aging of the immune system: focus on inflammation and vaccination. Eur J Immunol (2016) 46(10):2286–301. doi: 10.1002/eji.201546178
43. Fionda C, Ruggeri S, Sciume G, Laffranchi M, Quinti I, Milito C, et al. Age-dependent nk cell dysfunctions in severe covid-19 patients. Front Immunol (2022) 13:1039120. doi: 10.3389/fimmu.2022.1039120
44. Kroll KW, Shah SV, Lucar OA, Premeaux TA, Shikuma CM, Corley MJ, et al. Mucosal-homing natural killer cells are associated with aging in persons living with hiv. Cell Rep Med (2022) 3(10):100773. doi: 10.1016/j.xcrm.2022.100773
45. Guo C, Wu M, Huang B, Zhao R, Jin L, Fu B, et al. Single-cell transcriptomics reveal a unique memory-like nk cell subset that accumulates with ageing and correlates with disease severity in covid-19. Genome Med (2022) 14(1):46. doi: 10.1186/s13073-022-01049-3
46. Kale A, Sharma A, Stolzing A, Desprez PY, Campisi J. Role of immune cells in the removal of deleterious senescent cells. Immun Ageing (2020) 17:16. doi: 10.1186/s12979-020-00187-9
47. Albini A, Indraccolo S, Noonan DM, Pfeffer U. Functional genomics of endothelial cells treated with anti-angiogenic or angiopreventive drugs. Clin Exp Metastasis (2010) 27(6):419–39. doi: 10.1007/s10585-010-9312-5
48. Pfeffer U, Ferrari N, Dell'eva R, Indraccolo S, Morini M, Noonan DM, et al. Molecular mechanisms of action of angiopreventive anti-oxidants on endothelial cells: microarray gene expression analyses. Mutat Res (2005) 591(1-2):198–211. doi: 10.1016/j.mrfmmm.2005.04.014
49. Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol (2010) 17(7):1055–65. doi: 10.1128/CVI.00131-10
50. Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing (2019) 16:25. doi: 10.1186/s12979-019-0164-9
51. Dugan HL, Henry C, Wilson PC. Aging and influenza vaccine-induced immunity. Cell Immunol (2020) 348:103998. doi: 10.1016/j.cellimm.2019.103998
52. Cancro MP. Age-associated b cells. Annu Rev Immunol (2020) 38:315–40. doi: 10.1146/annurev-immunol-092419-031130
53. Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev (1997) 160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x
54. Zhang W, Brahmakshatriya V, Swain SL. Cd4 T cell defects in the aged: causes, consequences and strategies to circumvent. Exp Gerontol (2014) 54:67–70. doi: 10.1016/j.exger.2014.01.002
55. Cunningham AL, McIntyre P, Subbarao K, Booy R, Levin MJ. Vaccines for older adults. BMJ (2021) 372:n188. doi: 10.1136/bmj.n188
56. Tortorella C, Aiello A, Gasperini C, Agrati C, Castilletti C, Ruggieri S, et al. Humoral- and T-Cell-Specific immune responses to sars-Cov-2 mrna vaccination in patients with Ms using different disease-modifying therapies. Neurology (2022) 98(5):e541–e54. doi: 10.1212/WNL.0000000000013108
57. D'Abramo A, Vita S, Maffongelli G, Mariano A, Agrati C, Castilletti C, et al. Prolonged and severe sars-Cov-2 infection in patients under b-Cell-Depleting drug successfully treated: a tailored approach. Int J Infect Dis (2021) 107:247–50. doi: 10.1016/j.ijid.2021.04.068
58. Somes MP, Turner RM, Dwyer LJ, Newall AT. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: a systematic review and meta-analysis. Vaccine (2018) 36(23):3199–207. doi: 10.1016/j.vaccine.2018.04.063
59. Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Amir O, Freedman L, et al. Protection by a fourth dose of Bnt162b2 against omicron in Israel. N Engl J Med (2022) 386(18):1712–20. doi: 10.1056/NEJMoa2201570
60. Langmann T, Rehli M. Immunobiology. editorial. Immunobiology (2010) 215(9-10):673. doi: 10.1016/j.imbio.2010.07.001
61. Agrawal A, Gupta S. Impact of aging on dendritic cell functions in humans. Ageing Res Rev (2011) 10(3):336–45. doi: 10.1016/j.arr.2010.06.004
62. Pallikkuth S, de Armas L, Rinaldi S, Pahwa S. T Follicular helper cells and b cell dysfunction in aging and hiv-1 infection. Front Immunol (2017) 8:1380. doi: 10.3389/fimmu.2017.01380
63. Ma S, Wang C, Mao X, Hao Y. B cell dysfunction associated with aging and autoimmune diseases. Front Immunol (2019) 10:318. doi: 10.3389/fimmu.2019.00318
64. Nguyen V, Mendelsohn A, Larrick JW. Interleukin-7 and immunosenescence. J Immunol Res (2017) 2017:4807853. doi: 10.1155/2017/4807853
65. Hao Y, O'Neill P, Naradikian MS, Scholz JL, Cancro MP. A b-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood (2011) 118(5):1294–304. doi: 10.1182/blood-2011-01-330530
66. Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. Tlr7 drives accumulation of abcs and autoantibody production in autoimmune-prone mice. Immunol Res (2013) 55(1-3):210–6. doi: 10.1007/s12026-012-8365-8
67. Nanishi E, Angelidou A, Rotman C, Dowling DJ, Levy O, Ozonoff A. Precision vaccine adjuvants for older adults: a scoping review. Clin Infect Dis (2022) 75(Suppl 1):S72–80. doi: 10.1093/cid/ciac302
68. Coleman BL, Sanderson R, Haag MDM, McGovern I. Effectiveness of the Mf59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir Viruses (2021) 15(6):813–23. doi: 10.1111/irv.12871
69. Ledgerwood JE. As03-adjuvanted influenza vaccine in elderly people. Lancet Infect Dis (2013) 13(6):466–7. doi: 10.1016/S1473-3099(13)70038-0
70. Nikolich-Zugich J, Rudd BD. Immune memory and aging: an infinite or finite resource? Curr Opin Immunol (2010) 22(4):535–40. doi: 10.1016/j.coi.2010.06.011
71. Stervbo U, Bozzetti C, Baron U, Jurchott K, Meier S, Malzer JN, et al. Effects of aging on human leukocytes (Part ii): immunophenotyping of adaptive immune b and T cell subsets. Age (Dordr) (2015) 37(5):93. doi: 10.1007/s11357-015-9829-2
72. Merino KM, Jazwinski SM, Rout N. Th17-type immunity and inflammation of aging. Aging (2021) 13(10):13378–9. doi: 10.18632/aging.203119
73. Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol (2009) 30(7):366–73. doi: 10.1016/j.it.2009.04.003
74. Nikolich-Zugich J, Bradshaw CM, Uhrlaub JL, Watanabe M. Immunity to acute virus infections with advanced age. Curr Opin Virol (2021) 46:45–58. doi: 10.1016/j.coviro.2020.09.007
75. Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. Sars-Cov-2 and covid-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience (2020) 42(2):505–14. doi: 10.1007/s11357-020-00186-0
76. Qi Q, Zhang DW, Weyand CM, Goronzy JJ. Mechanisms shaping the naive T cell repertoire in the elderly - thymic involution or peripheral homeostatic proliferation? Exp Gerontol (2014) 54:71–4. doi: 10.1016/j.exger.2014.01.005
77. Fuentes E, Fuentes M, Alarcon M, Palomo I. Immune system dysfunction in the elderly. Acad Bras Cienc (2017) 89(1):285–99. doi: 10.1590/0001-3765201720160487
78. Vicente R, Mausset-Bonnefont AL, Jorgensen C, Louis-Plence P, Brondello JM. Cellular senescence impact on immune cell fate and function. Aging Cell (2016) 15(3):400–6. doi: 10.1111/acel.12455
79. Elyahu Y, Hekselman I, Eizenberg-Magar I, Berner O, Strominger I, Schiller M, et al. Aging promotes reorganization of the Cd4 T cell landscape toward extreme regulatory and effector phenotypes. Sci Adv (2019) 5(8):eaaw8330. doi: 10.1126/sciadv.aaw8330
80. Weyand CM, Goronzy JJ. Aging of the immune system. mechanisms and therapeutic targets. Ann Am Thorac Soc (2016) 13 Suppl 5:S422–S8. doi: 10.1513/AnnalsATS.201602-095AW
81. Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int (2009) 22(11):1041–50. doi: 10.1111/j.1432-2277.2009.00927.x
82. Bajaj V, Gadi N, Spihlman AP, Wu SC, Choi CH, Moulton VR. Aging, immunity, and covid-19: how age influences the host immune response to coronavirus infections? Front Physiol (2020) 11:571416. doi: 10.3389/fphys.2020.571416
83. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to covid-19 in Italy. JAMA (2020) 323(18):1775–6. doi: 10.1001/jama.2020.4683
84. Albini A, Di Guardo G, Noonan DM, Lombardo M. The sars-Cov-2 receptor, ace-2, is expressed on many different cell types: implications for ace-inhibitor- and angiotensin ii receptor blocker-based cardiovascular therapies. Intern Emerg Med (2020) 15(5):759–66. doi: 10.1007/s11739-020-02364-6
85. Aiello A, Grossi A, Meschi S, Meledandri M, Vanini V, Petrone L, et al. Coordinated innate and T-cell immune responses in mild covid-19 patients from household contacts of covid-19 cases during the first pandemic wave. Front Immunol (2022) 13:920227. doi: 10.3389/fimmu.2022.920227
86. Chandran A, Rosenheim J, Nageswaran G, Swadling L, Pollara G, Gupta RK, et al. Rapid synchronous type 1 ifn and virus-specific T cell responses characterize first wave non-severe sars-Cov-2 infections. Cell Rep Med (2022) 3(3):100557. doi: 10.1016/j.xcrm.2022.100557
87. King C, Sprent J. Dual nature of type I interferons in sars-Cov-2-Induced inflammation. Trends Immunol (2021) 42(4):312–22. doi: 10.1016/j.it.2021.02.003
88. Sa Ribero M, Jouvenet N, Dreux M, Nisole S. Interplay between sars-Cov-2 and the type I interferon response. PloS Pathog (2020) 16(7):e1008737. doi: 10.1371/journal.ppat.1008737
89. Acharya D, Liu G, Gack MU. Dysregulation of type I interferon responses in covid-19. Nat Rev Immunol (2020) 20(7):397–8. doi: 10.1038/s41577-020-0346-x
90. Xia H, Cao Z, Xie X, Zhang X, Chen JY, Wang H, et al. Evasion of type I interferon by sars-Cov-2. Cell Rep (2020) 33(1):108234. doi: 10.1016/j.celrep.2020.108234
91. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med (2020) 383(23):2255–73. doi: 10.1056/NEJMra2026131
92. Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. Covid-19 cytokine storm: the anger of inflammation. Cytokine (2020) 133:155151. doi: 10.1016/j.cyto.2020.155151
93. Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe sars-Cov-2 infection: review of 3939 covid-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol (2020) 108(1):17–41. doi: 10.1002/JLB.3COVR0520-272R
94. Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, et al. Longitudinal analyses reveal immunological misfiring in severe covid-19. Nature (2020) 584(7821):463–9. doi: 10.1038/s41586-020-2588-y
95. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
96. Caci G, Albini A, Malerba M, Noonan DM, Pochetti P, Polosa R. Covid-19 and obesity: dangerous liaisons. J Clin Med (2020) 9(8):2511. doi: 10.3390/jcm9082511
97. Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, et al. Covid-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol (2020) 38(8):970–9. doi: 10.1038/s41587-020-0602-4
98. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with covid-19. Nat Med (2020) 26(6):842–4. doi: 10.1038/s41591-020-0901-9
99. Zazzara MB, Bellieni A, Calvani R, Coelho-Junior HJ, Picca A, Marzetti E. Inflammaging at the time of covid-19. Clin Geriatr Med (2022) 38(3):473–81. doi: 10.1016/j.cger.2022.03.003
100. Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mrna-1273 covid-19 vaccine efficacy clinical trial. Science (2022) 375(6576):43–50. doi: 10.1126/science.abm3425
101. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic sars-Cov-2 infection. Nat Med (2021) 27(7):1205–11. doi: 10.1038/s41591-021-01377-8
102. Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al. Correlates of protection against symptomatic and asymptomatic sars-Cov-2 infection. Nat Med (2021) 27(11):2032–40. doi: 10.1038/s41591-021-01540-1
103. Goldblatt D, Alter G, Crotty S, Plotkin SA. Correlates of protection against sars-Cov-2 infection and covid-19 disease. Immunol Rev (2022) 310(1):6–26. doi: 10.1111/imr.13091
104. Anderson M, Stec M, Rewane A, Landay A, Cloherty G, Moy J. Sars-Cov-2 antibody responses in infection-naive or previously infected individuals after 1 and 2 doses of the Bnt162b2 vaccine. JAMA network Open (2021) 4(8):e2119741. doi: 10.1001/jamanetworkopen.2021.19741
105. Moy JN, Anderson M, Shen X, Fu J, Stec M, Gosha A, et al. Neutralizing antibody activity to sars-Cov-2 delta (B.1.617.2) and omicron (B.1.1.529) after one and two doses of Bnt162b2 vaccine in infection-naive and previously-infected individuals. J Infect Dis (2022) 226(8):1407–11. doi: 10.1093/infdis/jiac261
106. Bordi L, Sberna G, Piscioneri CN, Cocchiara RA, Miani A, Grammatico P, et al. Longitudinal dynamics of sars-Cov-2 anti-receptor binding domain igg antibodies in a wide population of health care workers after Bnt162b2 vaccination. Int J Infect Dis (2022) 122:174–7. doi: 10.1016/j.ijid.2022.05.061
107. Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to sars-Cov-2 in acute covid-19 and associations with age and disease severity. Cell (2020) 183(4):996–1012 e19. doi: 10.1016/j.cell.2020.09.038
108. Tan AT, Linster M, Tan CW, Le Bert N, Chia WN, Kunasegaran K, et al. Early induction of functional sars-Cov-2-Specific T cells associates with rapid viral clearance and mild disease in covid-19 patients. Cell Rep (2021) 34(6):108728. doi: 10.1016/j.celrep.2021.108728
109. Sette A, Crotty S. Adaptive immunity to sars-Cov-2 and covid-19. Cell (2021) 184(4):861–80. doi: 10.1016/j.cell.2021.01.007
110. Tarke A, Potesta M, Varchetta S, Fenoglio D, Iannetta M, Sarmati L, et al. Early and polyantigenic Cd4 T cell responses correlate with mild disease in acute covid-19 donors. Int J Mol Sci (2022) 23(13):7155. doi: 10.3390/ijms23137155
111. Bertoletti A, Le Bert N, Qui M, Tan AT. Sars-Cov-2-Specific T cells in infection and vaccination. Cell Mol Immunol (2021) 18(10):2307–12. doi: 10.1038/s41423-021-00743-3
112. Bartleson JM, Radenkovic D, Covarrubias AJ, Furman D, Winer DA, Verdin E. Sars-Cov-2, covid-19 and the ageing immune system. Nat Aging (2021) 1(9):769–82. doi: 10.1038/s43587-021-00114-7
113. Tarke A, Sidney J, Kidd CK, Dan JM, Ramirez SI, Yu ED, et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of sars-Cov-2 epitopes in covid-19 cases. Cell Rep Med (2021) 2(2):100204. doi: 10.1016/j.xcrm.2021.100204
114. Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol (2018) 19(1):10–9. doi: 10.1038/s41590-017-0006-x
115. Nikolich-Zugich J. The aging immune system: challenges for the 21st century. Semin Immunol (2012) 24(5):301–2. doi: 10.1016/j.smim.2012.09.001
116. Rha MS, Shin EC. Activation or exhaustion of Cd8(+) T cells in patients with covid-19. Cell Mol Immunol (2021) 18(10):2325–33. doi: 10.1038/s41423-021-00750-4
117. Westmeier J, Paniskaki K, Karakose Z, Werner T, Sutter K, Dolff S, et al. Impaired cytotoxic Cd8(+) T cell response in elderly covid-19 patients. mBio (2020) 11(5):e02243-20. doi: 10.1128/mBio.02243-20
118. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (Covid-19). Front Immunol (2020) 11:827. doi: 10.3389/fimmu.2020.00827
119. de Alwis R, Bangs DJ, Angelo MA, Cerpas C, Fernando A, Sidney J, et al. Immunodominant dengue virus-specific Cd8+ T cell responses are associated with a memory pd-1+ phenotype. J Virol (2016) 90(9):4771–9. doi: 10.1128/JVI.02892-15
121. Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in covid-19 patients. Cell Mol Immunol (2020) 17(5):541–3. doi: 10.1038/s41423-020-0401-3
122. Mazzoni A, Maggi L, Capone M, Spinicci M, Salvati L, Colao MG, et al. Cell-mediated and humoral adaptive immune responses to sars-Cov-2 are lower in asymptomatic than symptomatic covid-19 patients. Eur J Immunol (2020) 50(12):2013–24. doi: 10.1002/eji.202048915
123. Collier DA, Ferreira I, Kotagiri P, Datir RP, Lim EY, Touizer E, et al. Age-related immune response heterogeneity to sars-Cov-2 vaccine Bnt162b2. Nature (2021) 596(7872):417–22. doi: 10.1038/s41586-021-03739-1
124. Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, et al. Sars-Cov-2 vaccination induces immunological T cell memory able to cross-recognize variants from alpha to omicron. Cell (2022) 185(5):847–59 e11. doi: 10.1016/j.cell.2022.01.015
125. Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, et al. T Cell responses to sars-Cov-2 spike cross-recognize omicron. Nature (2022) 603(7901):488–92. doi: 10.1038/s41586-022-04460-3
126. Gao Y, Cai C, Grifoni A, Muller TR, Niessl J, Olofsson A, et al. Ancestral sars-Cov-2-Specific T cells cross-recognize the omicron variant. Nat Med (2022) 28(3):472–6. doi: 10.1038/s41591-022-01700-x
127. GeurtsvanKessel CH, Geers D, Schmitz KS, Mykytyn AZ, Lamers MM, Bogers S, et al. Divergent sars-Cov-2 omicron-reactive T and b cell responses in covid-19 vaccine recipients. Sci Immunol (2022) 7(69):eabo2202. doi: 10.1126/sciimmunol.abo2202
128. Tarke A, Sidney J, Methot N, Yu ED, Zhang Y, Dan JM, et al. Impact of sars-Cov-2 variants on the total Cd4(+) and Cd8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep Med (2021) 2(7):100355. doi: 10.1016/j.xcrm.2021.100355
129. Petrone L, Tortorella C, Aiello A, Farroni C, Ruggieri S, Castilletti C, et al. Humoral and cellular response to spike of delta sars-Cov-2 variant in vaccinated patients with multiple sclerosis. Front Neurol (2022) 13:881988. doi: 10.3389/fneur.2022.881988
130. Madelon N, Heikkila N, Sabater Royo I, Fontannaz P, Breville G, Lauper K, et al. Omicron-specific cytotoxic T-cell responses after a third dose of mrna covid-19 vaccine among patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol (2022) 79(4):399–404. doi: 10.1001/jamaneurol.2022.0245
131. Petrone L, Picchianti-Diamanti A, Sebastiani GD, Aiello A, Lagana B, Cuzzi G, et al. Humoral and cellular responses to spike of delta sars-Cov-2 variant in vaccinated patients with immune-mediated inflammatory diseases. Int J Infect Dis (2022) 121:24–30. doi: 10.1016/j.ijid.2022.04.027
132. Picchianti-Diamanti A, Aiello A, Lagana B, Agrati C, Castilletti C, Meschi S, et al. Immunosuppressivetherapies differently modulate humoral- and T-Cell-Specific responses to covid-19 mrna vaccine in rheumatoid arthritis patients. Front Immunol (2021) 12:740249. doi: 10.3389/fimmu.2021.740249
133. Farroni C, Picchianti-Diamanti A, Aiello A, Nicastri E, Lagana B, Agrati C, et al. Kinetics of the b- and T-cell immune responses after 6 months from sars-Cov-2 mrna vaccination in patients with rheumatoid arthritis. Front Immunol (2022) 13:846753. doi: 10.3389/fimmu.2022.846753
134. Madelon N, Lauper K, Breville G, Sabater Royo I, Goldstein R, Andrey DO, et al. Robust T cell responses in anti-Cd20 treated patients following covid-19 vaccination: a prospective cohort study. Clin Infect Dis (2021) 75(1):e1037–e45. doi: 10.1093/cid/ciab954
135. Aiello A, Coppola A, Ruggieri S, Farroni C, Altera AMG, Salmi A, et al. Longitudinal characterisation of b and T-cell immune responses after the booster dose of covid-19 mrna-vaccine in people with multiple sclerosis using different disease-modifying therapies. J Neurol Neurosurg Psychiatry (2022) 94(4):290–9. doi: 10.1136/jnnp-2022-330175
136. Farroni C, Aiello A, Picchianti-Diamanti A, Laganà B, Petruccioli E, Agrati C, et al. Booster dose of sars-Cov-2 messenger rna vaccines strengthens the specific immune response of patients with rheumatoid arthritis: a prospective multicenter longitudinal study. Int J Infect Dis (2022) 125:195–208. doi: 10.1016/j.ijid.2022.10.035
137. Marquez EJ, Chung CH, Marches R, Rossi RJ, Nehar-Belaid D, Eroglu A, et al. Sexual-dimorphism in human immune system aging. Nat Commun (2020) 11(1):751. doi: 10.1038/s41467-020-14396-9
138. Marquez EJ, Trowbridge J, Kuchel GA, Banchereau J, Ucar D. The lethal sex gap: covid-19. Immun Ageing (2020) 17:13. doi: 10.1186/s12979-020-00183-z
139. Weinstein ER, Lee JS, Mendez NA, Harkness A, Safren SA, El-Sadr W. Hiv/Aids and aging: the new frontier for Hiv/Aids research and care. AIDS (2021) 35(12):2043–5. doi: 10.1097/QAD.0000000000003000
140. Pathai S, Bajillan H, Landay AL, High KP. Is hiv a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci (2014) 69(7):833–42. doi: 10.1093/gerona/glt168
141. Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in hiv-infected persons: the Swiss hiv cohort study. Clin Infect Dis (2011) 53(11):1130–9. doi: 10.1093/cid/cir626
142. Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among hiv-infected persons compared with the general population. Clin Infect Dis (2011) 53(11):1120–6. doi: 10.1093/cid/cir627
143. Greene M, Covinsky KE, Valcour V, Miao Y, Madamba J, Lampiris H, et al. Geriatric syndromes in older hiv-infected adults. J Acquir Immune Defic Syndr (2015) 69(2):161–7. doi: 10.1097/QAI.0000000000000556
144. Maciel RA, Kluck HM, Durand M, Sprinz E. Comorbidity is more common and occurs earlier in persons living with hiv than in hiv-uninfected matched controls, aged 50 years and older: a cross-sectional study. Int J Infect Dis (2018) 70:30–5. doi: 10.1016/j.ijid.2018.02.009
145. Deeks SG. Hiv infection, inflammation, immunosenescence, and aging. Annu Rev Med (2011) 62:141–55. doi: 10.1146/annurev-med-042909-093756
146. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic hiv infection. Nat Med (2006) 12(12):1365–71. doi: 10.1038/nm1511
147. Isnard S, Fombuena B, Sadouni M, Lin J, Richard C, Routy B, et al. Circulating beta-D-Glucan as a marker of subclinical coronary plaque in antiretroviral therapy-treated people with human immunodeficiency virus. Open Forum Infect Dis (2021) 8(6):ofab109. doi: 10.1093/ofid/ofab109
148. Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, et al. Hiv infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS (2012) 26(7):843–53. doi: 10.1097/QAD.0b013e328351f756
149. Gianella S, Moser C, Vitomirov A, McKhann A, Layman L, Scott B, et al. Presence of asymptomatic cytomegalovirus and Epstein–Barr virus DNA in blood of persons with hiv starting antiretroviral therapy is associated with non-aids clinical events. AIDS (2020) 34(6):849–57. doi: 10.1097/QAD.0000000000002484
150. Crane HM, Miller ME, Pierce J, Willig AL, Case ML, Wilkin AM, et al. Physical functioning among patients aging with human immunodeficiency virus (Hiv) versus hiv uninfected: feasibility of using the short physical performance battery in clinical care of people living with hiv aged 50 or older. Open Forum Infect Dis (2019) 6(3):ofz038. doi: 10.1093/ofid/ofz038
151. Caraux-Paz P, Diamantis S, de Wazieres B, Gallien S. Tuberculosis in the elderly. J Clin Med (2021) 10(24):5888. doi: 10.3390/jcm10245888
152. Jeremiah C, Petersen E, Nantanda R, Mungai BN, Migliori GB, Amanullah F, et al. The who global tuberculosis 2021 report - not so good news and turning the tide back to end Tb. Int J Infect Dis (2022) 124 (Suppl 1):S26–9. doi: 10.1016/j.ijid.2022.03.011
153. Di Gennaro F, Vittozzi P, Gualano G, Musso M, Mosti S, Mencarini P, et al. Active pulmonary tuberculosis in elderly patients: a 2016-2019 retrospective analysis from an Italian referral hospital. Antibiotics (Basel) (2020) 9(8):489. doi: 10.3390/antibiotics9080489
154. Oliveira-de-Souza D, Vinhaes CL, Arriaga MB, Kumar NP, Queiroz ATL, Fukutani KF, et al. Aging increases the systemic molecular degree of inflammatory perturbation in patients with tuberculosis. Sci Rep (2020) 10(1):11358. doi: 10.1038/s41598-020-68255-0
155. Bobak CA, Abhimanyu, Natarajan H, Gandhi T, Grimm SL, Nishiguchi T, et al. Increased DNA methylation, cellular senescence and premature epigenetic aging in Guinea pigs and humans with tuberculosis. Aging (2022) 14(5):2174–93. doi: 10.18632/aging.203936
156. Wu IL, Chitnis AS, Jaganath D. A narrative review of tuberculosis in the united states among persons aged 65 years and older. J Clin Tuberc Other Mycobact Dis (2022) 28:100321. doi: 10.1016/j.jctube.2022.100321
157. Vishnu Sharma M, Arora VK, Anupama N. Challenges in diagnosis and treatment of tuberculosis in elderly. Indian J Tuberc (2022) 69 Suppl 2:S205–S8. doi: 10.1016/j.ijtb.2022.10.001
158. Goletti D, Pisapia R, Fusco FM, Aiello A, Van Crevel R. Epidemiology, pathogenesis, clinical presentation and management of Tb in patients with hiv and diabetes. Int J Tuberc Lung Dis (2023) 27(4):284–90. doi: 10.5588/ijtld.22.0685
159. Duarte R, Lonnroth K, Carvalho C, Lima F, Carvalho ACC, Munoz-Torrico M, et al. Tuberculosis, social determinants and Co-morbidities (Including hiv). Pulmonology (2018) 24(2):115–9. doi: 10.1016/j.rppnen.2017.11.003
160. Kwon BS, Kim Y, Lee SH, Lim SY, Lee YJ, Park JS, et al. The high incidence of severe adverse events due to pyrazinamide in elderly patients with tuberculosis. PloS One (2020) 15(7):e0236109. doi: 10.1371/journal.pone.0236109
161. Ascher K, Elliot SJ, Rubio GA, Glassberg MK. Lung diseases of the elderly: cellular mechanisms. Clin Geriatr Med (2017) 33(4):473–90. doi: 10.1016/j.cger.2017.07.001
162. Cohen SB, Gern BH, Delahaye JL, Adams KN, Plumlee CR, Winkler JK, et al. Alveolar macrophages provide an early mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe (2018) 24(3):439–46 e4. doi: 10.1016/j.chom.2018.08.001
163. Cadena AM, Flynn JL, Fortune SM. The importance of first impressions: early events in mycobacterium tuberculosis infection influence outcome. mBio (2016) 7(2):e00342–16. doi: 10.1128/mBio.00342-16
164. Canan CH, Gokhale NS, Carruthers B, Lafuse WP, Schlesinger LS, Torrelles JB, et al. Characterization of lung inflammation and its impact on macrophage function in aging. J Leukoc Biol (2014) 96(3):473–80. doi: 10.1189/jlb.4A0214-093RR
165. La Manna MP, Orlando V, Dieli F, Di Carlo P, Cascio A, Cuzzi G, et al. Quantitative and qualitative profiles of circulating monocytes may help identifying tuberculosis infection and disease stages. PloS One (2017) 12(2):e0171358. doi: 10.1371/journal.pone.0171358
166. Ault R, Dwivedi V, Koivisto E, Nagy J, Miller K, Nagendran K, et al. Altered monocyte phenotypes but not impaired peripheral T cell immunity may explain susceptibility of the elderly to develop tuberculosis. Exp Gerontol (2018) 111:35–44. doi: 10.1016/j.exger.2018.06.029
167. Selwyn PA, Alcabes P, Hartel D, Buono D, Schoenbaum EE, Klein RS, et al. Clinical manifestations and predictors of disease progression in drug users with human immunodeficiency virus infection. N Engl J Med (1992) 327(24):1697–703. doi: 10.1056/NEJM199212103272401
168. Goletti D, Weissman D, Jackson RW, Graham NM, Vlahov D, Klein RS, et al. Effect of mycobacterium tuberculosis on hiv replication. Role Immune Activation. J Immunol (1996) 157(3):1271–8.
169. Sharma SK, Mitra DK, Balamurugan A, Pandey RM, Mehra NK. Cytokine polarization in miliary and pleural tuberculosis. J Clin Immunol (2002) 22(6):345–52. doi: 10.1023/a:1020604331886
170. Petrone L, Petruccioli E, Vanini V, Cuzzi G, Gualano G, Vittozzi P, et al. Coinfection of tuberculosis and covid-19 limits the ability to in vitro respond to sars-Cov-2. Int J Infect Dis (2021) 113 Suppl 1:S82–S7. doi: 10.1016/j.ijid.2021.02.090
171. Najafi-Fard S, Petruccioli E, Farroni C, Petrone L, Vanini V, Cuzzi G, et al. Evaluation of the immunomodulatory effects of interleukin-10 on peripheral blood immune cells of covid-19 patients: implication for covid-19 therapy. Front Immunol (2022) 13:984098. doi: 10.3389/fimmu.2022.984098
172. du Bruyn E, Stek C, Daroowala R, Said-Hartley Q, Hsiao M, Schafer G, et al. Effects of tuberculosis and/or hiv-1 infection on covid-19 presentation and immune response in Africa. Nat Commun (2023) 14(1):188. doi: 10.1038/s41467-022-35689-1
173. Riou C, du Bruyn E, Stek C, Daroowala R, Goliath RT, Abrahams F, et al. Relationship of sars-Cov-2-Specific Cd4 response to covid-19 severity and impact of hiv-1 and tuberculosis coinfection. J Clin Invest (2021) 131(12):e149125. doi: 10.1172/JCI149125
174. Wu Y, Wu M, Ming S, Zhan X, Hu S, Li X, et al. Trem-2 promotes Th1 responses by interacting with the Cd3zeta-Zap70 complex following mycobacterium tuberculosis infection. J Clin Invest (2021) 131(17):e137407. doi: 10.1172/JCI137407
175. Goletti D, Delogu G, Matteelli A, Migliori GB. The role of igra in the diagnosis of tuberculosis infection, differentiating from active tuberculosis, and decision making for initiating treatment or preventive therapy of tuberculosis infection. Int J Infect Dis (2022) 124(Suppl 1):S12–9. doi: 10.1016/j.ijid.2022.02.047
176. Zhang BY, Yu ZM, Yang QL, Liu QQ, Chen HX, Wu J, et al. Serial anti-tuberculous immune responses during the follow-up of patients with tuberculous pleurisy. Medicine (2020) 99(2):e18367. doi: 10.1097/MD.0000000000018367
177. Sakai S, Kawamura I, Okazaki T, Tsuchiya K, Uchiyama R, Mitsuyama M. Pd-1-Pd-L1 pathway impairs T(H)1 immune response in the late stage of infection with mycobacterium bovis bacillus calmette-guerin. Int Immunol (2010) 22(12):915–25. doi: 10.1093/intimm/dxq446
178. Abebe F. Synergy between Th1 and Th2 responses during mycobacterium tuberculosis infection: a review of current understanding. Int Rev Immunol (2019) 38(4):172–9. doi: 10.1080/08830185.2019.1632842
179. Hougardy JM, Place S, Hildebrand M, Drowart A, Debrie AS, Locht C, et al. Regulatory T cells depress immune responses to protective antigens in active tuberculosis. Am J Respir Crit Care Med (2007) 176(4):409–16. doi: 10.1164/rccm.200701-084OC
180. Chiacchio T, Casetti R, Butera O, Vanini V, Carrara S, Girardi E, et al. Characterization of regulatory T cells identified as Cd4(+)Cd25(High)Cd39(+) in patients with active tuberculosis. Clin Exp Immunol (2009) 156(3):463–70. doi: 10.1111/j.1365-2249.2009.03908.x
181. Namdeo M, Kandel R, Thakur PK, Mohan A, Dey AB, Mitra DK. Old age-associated enrichment of peripheral T regulatory cells and altered redox status in pulmonary tuberculosis patients. Eur J Immunol (2020) 50(8):1195–208. doi: 10.1002/eji.201948261
182. Agius E, Lacy KE, Vukmanovic-Stejic M, Jagger AL, Papageorgiou AP, Hall S, et al. Decreased tnf-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory Cd4+ T cells during aging. J Exp Med (2009) 206(9):1929–40. doi: 10.1084/jem.20090896
183. Carrasco E, Gomez de Las Heras MM, Gabande-Rodriguez E, Desdin-Mico G, Aranda JF, Mittelbrunn M. The role of T cells in age-related diseases. Nat Rev Immunol (2022) 22(2):97–111. doi: 10.1038/s41577-021-00557-4
184. DiNardo AR, Rajapakshe K, Nishiguchi T, Grimm SL, Mtetwa G, Dlamini Q, et al. DNA Hypermethylation during tuberculosis dampens host immune responsiveness. J Clin Invest (2020) 130(6):3113–23. doi: 10.1172/JCI134622
185. Karlsson L, Das J, Nilsson M, Tyren A, Pehrson I, Idh N, et al. A differential DNA methylome signature of pulmonary immune cells from individuals converting to latent tuberculosis infection. Sci Rep (2021) 11(1):19418. doi: 10.1038/s41598-021-98542-3
186. Shen L, Huang D, Qaqish A, Frencher J, Yang R, Shen H, et al. Fast-acting gammadelta T-cell subpopulation and protective immunity against infections. Immunol Rev (2020) 298(1):254–63. doi: 10.1111/imr.12927
187. Kallemeijn MJ, Kavelaars FG, van der Klift MY, Wolvers-Tettero ILM, Valk PJM, van Dongen JJM, et al. Next-generation sequencing analysis of the human tcrgammadelta+ T-cell repertoire reveals shifts in vgamma- and vdelta-usage in memory populations upon aging. Front Immunol (2018) 9:448. doi: 10.3389/fimmu.2018.00448
189. Wong MK, Brooks DJ, Ikejezie J, Gacic-Dobo M, Dumolard L, Nedelec Y, et al. Covid-19 mortality and progress toward vaccinating older adults - world health organization, worldwide, 2020-2022. MMWR Morb Mortal Wkly Rep (2023) 72(5):113–8. doi: 10.15585/mmwr.mm7205a1
191. Mbinta JF, Nguyen BP, Awuni PMA, Paynter J, Simpson CR. Post-licensure zoster vaccine effectiveness against herpes zoster and postherpetic neuralgia in older adults: a systematic review and meta-analysis. Lancet Healthy Longev (2022) 3(4):e263–e75. doi: 10.1016/S2666-7568(22)00039-3
192. Goletti D, Al-Abri S, Migliori GB, Coler R, Ong CWM, Esposito SMR, et al. World tuberculosis day 2023 theme "Yes! we can end tb!". Int J Infect Dis (2023) S1201-9712(23)00137-6. doi: 10.1016/j.ijid.2023.04.006
193. Larsen SE, Baldwin SL, Coler RN. Tuberculosis vaccines update: is an rna-based vaccine feasible for tuberculosis? Int J Infect Dis (2023) S1201-9712(23)00114-5. doi: 10.1016/j.ijid.2023.03.035
194. de Bree LCJ, Koeken V, Joosten LAB, Aaby P, Benn CS, van Crevel R, et al. Non-specific effects of vaccines: current evidence and potential implications. Semin Immunol (2018) 39:35–43. doi: 10.1016/j.smim.2018.06.002
195. Pavan Kumar N, Padmapriyadarsini C, Rajamanickam A, Marinaik SB, Nancy A, Padmanaban S, et al. Effect of bcg vaccination on proinflammatory responses in elderly individuals. Sci Adv (2021) 7(32):eabg7181. doi: 10.1126/sciadv.abg7181
196. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in covid-19 patients with severe respiratory failure. Cell Host Microbe (2020) 27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009
197. Moorlag S, Taks E, Ten Doesschate T, van der Vaart TW, Janssen AB, Muller L, et al. Efficacy of bcg vaccination against respiratory tract infections in older adults during the coronavirus disease 2019 pandemic. Clin Infect Dis (2022) 75(1):e938–e46. doi: 10.1093/cid/ciac182
198. Tsilika M, Taks E, Dolianitis K, Kotsaki A, Leventogiannis K, Damoulari C, et al. Activate-2: a double-blind randomized trial of bcg vaccination against covid-19 in individuals at risk. Front Immunol (2022) 13:873067. doi: 10.3389/fimmu.2022.873067
199. Merzon E, Green I, Somekh E, Vinker S, Golan-Cohen A, Israel A, et al. The association of previous vaccination with live-attenuated varicella zoster vaccine and covid-19 positivity: an Israeli population-based study. Vaccines (Basel) (2022) 10(1):74. doi: 10.3390/vaccines10010074
200. Chen J, Gao L, Wu X, Fan Y, Liu M, Peng L, et al. Bcg-induced trained immunity: history, mechanisms and potential applications. J Transl Med (2023) 21(1):106. doi: 10.1186/s12967-023-03944-8
201. Ly J, Lagman M, Saing T, Singh MK, Tudela EV, Morris D, et al. Liposomal glutathione supplementation restores Th1 cytokine response to mycobacterium tuberculosis infection in hiv-infected individuals. J Interferon Cytokine Res (2015) 35(11):875–87. doi: 10.1089/jir.2014.0210
202. Kumar P, Liu C, Suliburk J, Hsu JW, Muthupillai R, Jahoor F, et al. Supplementing glycine and n-acetylcysteine (Glynac) in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, physical function, and aging hallmarks: a randomized clinical trial. J Gerontol A Biol Sci Med Sci (2023) 78(1):75–89. doi: 10.1093/gerona/glac135
203. Maselli Del Giudice A, La Mantia I, Barbara F, Ciccarone S, Ragno MS, de Robertis V, et al. Use of nutraceuticals in elderly to fight inflammation and immuno-senescence: a randomized case-control study. Nutrients (2022) 14(17):3476. doi: 10.3390/nu14173476
204. Annweiler G, Corvaisier M, Gautier J, Dubee V, Legrand E, Sacco G, et al. Vitamin d supplementation associated to better survival in hospitalized frail elderly covid-19 patients: the geria-covid quasi-experimental study. Nutrients (2020) 12(11):3377. doi: 10.3390/nu12113377
205. Walrath T, Dyamenahalli KU, Hulsebus HJ, McCullough RL, Idrovo JP, Boe DM, et al. Age-related changes in intestinal immunity and the microbiome. J Leukoc Biol (2021) 109(6):1045–61. doi: 10.1002/JLB.3RI0620-405RR
206. Bosco N, Noti M. The aging gut microbiome and its impact on host immunity. Genes Immun (2021) 22(5-6):289–303. doi: 10.1038/s41435-021-00126-8
207. Ghosh TS, Shanahan F, O'Toole PW. The gut microbiome as a modulator of healthy ageing. Nat Rev Gastroenterol Hepatol (2022) 19(9):565–84. doi: 10.1038/s41575-022-00605-x
208. Larson PJ, Zhou W, Santiago A, Driscoll S, Fleming E, Voigt AY, et al. Associations of the skin, oral and gut microbiome with aging, frailty and infection risk reservoirs in older adults. Nat Aging (2022) 2(10):941–55. doi: 10.1038/s43587-022-00287-9
209. Ntemiri A, Ghosh TS, Gheller ME, Tran TTT, Blum JE, Pellanda P, et al. Whole blueberry and isolated polyphenol-rich fractions modulate specific gut microbes in an in vitro colon model and in a pilot study in human consumers. Nutrients (2020) 12(9):2800. doi: 10.3390/nu12092800
210. Sandionigi A, De Giani A, Tursi F, Michelotti A, Cestone E, Giardina S, et al. Effectiveness of multistrain probiotic formulation on common infectious disease symptoms and gut microbiota modulation in flu-vaccinated healthy elderly subjects. BioMed Res Int (2022) 2022:3860896. doi: 10.1155/2022/3860896
211. Induri SNR, Kansara P, Thomas SC, Xu F, Saxena D, Li X. The gut microbiome, metformin, and aging. Annu Rev Pharmacol Toxicol (2022) 62:85–108. doi: 10.1146/annurev-pharmtox-051920-093829
212. Puzianowska-Kuznicka M, Owczarz M, Wieczorowska-Tobis K, Nadrowski P, Chudek J, Slusarczyk P, et al. Interleukin-6 and c-reactive protein, successful aging, and mortality: the polsenior study. Immun Ageing (2016) 13:21. doi: 10.1186/s12979-016-0076-x
213. Varadhan R, Yao W, Matteini A, Beamer BA, Xue QL, Yang H, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci (2014) 69(2):165–73. doi: 10.1093/gerona/glt023
Keywords: aging, innate cells, B cells, T cells, COVID-19, tuberculosis, HIV, immunosenescence
Citation: Grifoni A, Alonzi T, Alter G, Noonan DM, Landay AL, Albini A and Goletti D (2023) Impact of aging on immunity in the context of COVID-19, HIV, and tuberculosis. Front. Immunol. 14:1146704. doi: 10.3389/fimmu.2023.1146704
Received: 17 January 2023; Accepted: 11 May 2023;
Published: 24 May 2023.
Edited by:
Jonathan P. Butchar, The Ohio State University, United StatesReviewed by:
Petra Laznickova, International Clinical Research Center (FNUSA-ICRC), CzechiaMartin Helán, St. Anne’s University Hospital Brno, Czechia
Kamila Bendíčková, International Clinical Research Center (FNUSA-ICRC), Czechia
Copyright © 2023 Grifoni, Alonzi, Alter, Noonan, Landay, Albini and Goletti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delia Goletti, delia.goletti@inmi.it; Adriana Albini, albini.adriana@gmail.com
†These authors have contributed equally to this work
 Alba Grifoni
Alba Grifoni Tonino Alonzi2†
Tonino Alonzi2† Douglas McClain Noonan
Douglas McClain Noonan Alan L. Landay
Alan L. Landay Delia Goletti
Delia Goletti