CVD and COVID-19: Emerging Roles of Cardiac Fibroblasts and Myofibroblasts
Abstract
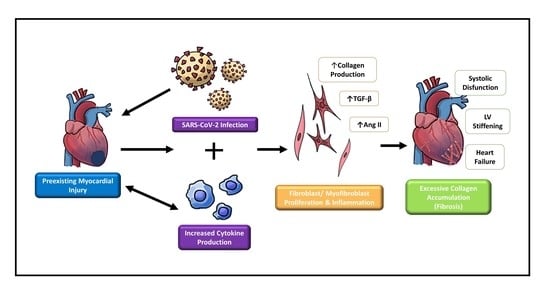
:1. Introduction
2. Cardiac Fibroblasts in Response to Injury
Cardiac Fibrosis and Adverse Remodeling
3. Emerging Cardiac Conditions Relating to COVID-19
3.1. COVID-19-Induced Cytokine Storm
3.2. ACE2’s Involvement in Viral Infection
3.3. MicroRNAs
3.4. TGF-β1’s Role in the Immune Systems of Severe COVID-19 Patients
3.5. Myocarditis Following mRNA COVID-19 Vaccination
4. Potential Treatments and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 16 December 2021).
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W. Heart Disease and Stroke Statistics—2021 Update. Circulation 2021, 143, 254–743. [Google Scholar] [CrossRef] [PubMed]
- Moody, W.E.; Liu, B.; Mahmoud-Elsayed, H.M.; Senior, J.; Lalla, S.S.; Khan-Kheil, A.M.; Brown, S.; Saif, A.; Moss, A.; Bradlow, W.M.; et al. Persisting Adverse Ventricular Remodeling in COVID-19 Survivors: A Longitudinal Echocardiographic Study. J. Am. Soc. Echocardiogr. 2021, 34, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Klingel, K.; Kindermann, I.; Brucato, A.; Rosa, F.G.D.; Adler, Y.; Ferrari, G.M.D. COVID-19 Pandemic and Troponin: Indirect Myocardial Injury, Myocardial Inflammation or Myocarditis? Heart 2020, 106, 1127–1131. [Google Scholar] [CrossRef]
- Chakinala, R.C.; Shah, C.D.; Rakholiya, J.H.; Martin, M.; Kaur, N.; Singh, H.; Okafor, T.L.; Nwodika, C.; Raval, P.; Yousuf, S.; et al. COVID-19 Outcomes Amongst Patients with Pre-Existing Cardiovascular Disease and Hypertension. Cureus 2021, 13, e13420. [Google Scholar] [CrossRef]
- Baudino, T.A.; Carver, W.; Giles, W.; Borg, T.K. Cardiac Fibroblasts: Friend or Foe? Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H1015–H1026. [Google Scholar] [CrossRef]
- Ivey, M.J.; Tallquist, M.D. Defining the Cardiac Fibroblast. Circ. J. 2016, 80, 2269–2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katwa, L.; Shashikant, C. Cardiac Remodeling and Fibrosis: Role of Myofibroblasts. In The Cardiac Fibroblast; Turner, N., Ed.; Research Signpost: Kerala, India, 2011; pp. 29–52. [Google Scholar]
- Travers, J.; Kamal, F.; Robbins, J.; Yutzey, K.; Blaxall, B. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [Green Version]
- Frangogiannis, N.G. Cardiac Fibrosis. Cardiovasc. Res. 2020, 117, 1450–1488. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The Pathogenesis of Cardiac Fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [Green Version]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxidative Med. Cell. Longev. 2017, 2017, e3920195. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, V.; Rai, R.; Place, A.T.; Murphy, S.B.; Verma, S.K.; Ghosh, A.K.; Vaughan, D.E. MiR-125b Is Critical for Fibroblast-to-Myofibroblast Transition and Cardiac Fibrosis. Circulation 2016, 133, 291–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feghali, C.A.; Wright, T.M. Cytokines in Acute and Chronic Inflammation. Front. Biosci. 1997, 2, d12–d26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in Fibrosis: Novel Roles and Mediators. Front. Pharm. 2014, 5, 123. [Google Scholar] [CrossRef] [Green Version]
- Kurose, H. Cardiac Fibrosis and Fibroblasts. Cells 2021, 10, 1716. [Google Scholar] [CrossRef]
- Ivey, M.J.; Kuwabara, J.T.; Pai, J.T.; Moore, R.E.; Sun, Z.; Tallquist, M.D. Resident Fibroblast Expansion during Cardiac Growth and Remodeling. J. Mol. Cell. Cardiol. 2018, 114, 161–174. [Google Scholar] [CrossRef]
- Hesse, M.; Welz, A.; Fleischmann, B.K. Heart Regeneration and the Cardiomyocyte Cell Cycle. Pflug. Arch.-Eur. J. Physiol. 2018, 470, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Tracy, E.; Rowe, G.; LeBlanc, A. Cardiac Tissue Remodeling in Healthy Aging: The Road to Pathology. Am. J. Physiol.-Cell Physiol. 2020, 319, C116–C182. [Google Scholar] [CrossRef]
- Ono, K.; Ohtomo, T.; Ninomiya-Tsuji, J.; Tsuchiya, M. A Dominant Negative TAK1 Inhibits Cellular Fibrotic Responses Induced by TGF-Beta. Biochem. Biophys. Res. Commun. 2003, 307, 332–337. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Zhang, C.; Li, P.; Wu, Y.; Wang, C.; Bond Lau, W.; Ma, X.; Du, J. Cardiac Fibroblast-Specific Activating Transcription Factor 3 Protects Against Heart Failure by Suppressing MAP2K3-P38 Signaling. Circulation 2017, 135, 2041–2057. [Google Scholar] [CrossRef]
- Wang, X.-M.; Liu, X.-M.; Chen, Z.-Y. Activating Transcription Factor 3 (ATF3) Regulates Cell Growth, Apoptosis, Invasion and Collagen Synthesis in Keloid Fibroblast through Transforming Growth Factor Beta (TGF-Beta)/SMAD Signaling Pathway. Bioengineered 2021, 12, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Babapoor-Farrokhran, S.; Gill, D.; Walker, J.; Rasekhi, R.T.; Bozorgnia, B.; Amanullah, A. Myocardial Injury and COVID-19: Possible Mechanisms. Life Sci. 2020, 253, 117723. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and Impact of Cardiovascular Metabolic Diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The Cytokine Storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The Cytokine Storm in COVID-19: An Overview of the Involvement of the Chemokine/Chemokine-Receptor System. Cytokine Growth Factor Rev. 2020, 53, 25. [Google Scholar] [CrossRef]
- Morgan, P.; Arnold, S.J.; Hsiao, N.-W.; Shu, C.-W. A Closer Look at Dexamethasone and the SARS-CoV-2-Induced Cytokine Storm: In Silico Insights of the First Life-Saving COVID-19 Drug. Antibiotics 2021, 10, 1507. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in COVID-19: A Systematic Review and Meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Gomolak, J.R.; Didion, S.P. Angiotensin II-Induced Endothelial Dysfunction Is Temporally Linked with Increases in Interleukin-6 and Vascular Macrophage Accumulation. Front. Physiol. 2014, 5, 396. [Google Scholar] [CrossRef]
- Didion, S.P. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-Cell RNA-Seq Data Analysis on the Receptor ACE2 Expression Reveals the Potential Risk of Different Human Organs Vulnerable to 2019-NCoV Infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-Converting Enzyme 2 (ACE2) Expression and Tissue Susceptibility to SARS-CoV-2 Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Marques, O.; Halpert, G.; Schimke, L.F.; Ostrinski, Y.; Vojdani, A.; Baiocchi, G.C.; Freire, P.P.; Filgueiras, I.S.; Zyskind, I.; Lattin, M.T.; et al. Autoantibodies Targeting GPCRs and RAS-Related Molecules Associate with COVID-19 Severity. Nat. Commun. 2022, 13, 1220. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Duffy, F.; Hadlock, J.; Raappana, A.; Styrchak, S.; Beck, I.; Mast, F.D.; Miller, L.R.; Chour, W.; Houck, J.; et al. Angiotensin II Receptor I Auto-Antibodies Following SARS-CoV-2 Infection. PLoS ONE 2021, 16, e0259902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, M.; Zhang, X.; Liu, T.; Libby, P.; Shi, G.-P. COVID-19, the Pandemic of the Century and Its Impact on Cardiovascular Diseases. Cardiol. Discov. 2021, 1, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G. Sex-Biased Mechanisms of Cardiovascular Complications in COVID-19. Physiol. Rev. 2022, 102, 333–337. [Google Scholar] [CrossRef]
- Califf, R.M. Avoiding the Coming Tsunami of Common, Chronic Disease. Circulation 2021, 143, 1831–1834. [Google Scholar] [CrossRef]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular Complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef]
- Unudurthi, S.D.; Luthra, P.; Bose, R.J.C.; McCarthy, J.R.; Kontaridis, M.I. Cardiac Inflammation in COVID-19: Lessons from Heart Failure. Life Sci. 2020, 260, 118482. [Google Scholar] [CrossRef]
- Lu, D.; Chatterjee, S.; Xiao, K.; Riedel, I.; Wang, Y.; Foo, R.; Bär, C.; Thum, T. MicroRNAs Targeting the SARS-CoV-2 Entry Receptor ACE2 in Cardiomyocytes. J. Mol. Cell. Cardiol. 2020, 148, 46–49. [Google Scholar] [CrossRef]
- Wang, H.; Cai, J. The Role of MicroRNAs in Heart Failure. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ning, Q.; Wang, J. Angiotensin II Induced Differentially Expressed MicroRNAs in Adult Rat Cardiac Fibroblasts. J. Physiol. Sci. 2013, 63, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Sun, X.; Shan, H.; Wang, N.; Wang, J.; Ren, J.; Feng, S.; Xie, L.; Lu, C.; Yuan, Y.; et al. MicroRNA-101 Inhibited Postinfarct Cardiac Fibrosis and Improved Left Ventricular Compliance via the FBJ Osteosarcoma Oncogene/Transforming Growth Factor-B1 Pathway. Circulation 2012, 126, 840–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghazavi, A.; Ganji, A.; Keshavarzian, N.; Rabiemajd, S.; Mosayebi, G. Cytokine Profile and Disease Severity in Patients with COVID-19. Cytokine 2021, 137, 155323. [Google Scholar] [CrossRef]
- Witkowski, M.; Tizian, C.; Ferreira-Gomes, M.; Niemeyer, D.; Jones, T.C.; Heinrich, F. Untimely TGFβ Responses in COVID-19 Limit Antiviral Functions of NK Cells. Nature 2021, 600, 295–301. [Google Scholar] [CrossRef]
- Cooper, L.T. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef] [Green Version]
- Gargano, J.W. Use of MRNA COVID-19 Vaccine after Reports of Myocarditis among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 MRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Nguyen, M.; Martin, D.; DeStefano, F. Safety Monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015, 33, 4398–4405. [Google Scholar] [CrossRef] [Green Version]
- Marshall, M.; Ferguson, I.D.; Lewis, P.; Jaggi, P.; Gagliardo, C.; Collins, J.S.; Shaughnessy, R.; Caron, R.; Fuss, C.; Corbin, K.J.E.; et al. Symptomatic Acute Myocarditis in 7 Adolescents after Pfizer-BioNTech COVID-19 Vaccination. Pediatrics 2021, 148, e2021052478. [Google Scholar] [CrossRef]
- Dionne, A.; Sperotto, F.; Chamberlain, S.; Baker, A.L.; Powell, A.J.; Prakash, A.; Castellanos, D.A.; Saleeb, S.F.; de Ferranti, S.D.; Newburger, J.W.; et al. Association of Myocarditis with BNT162b2 Messenger RNA COVID-19 Vaccine in a Case Series of Children. JAMA Cardiol. 2021, 6, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Farinholt, T.; Doddapaneni, H.; Qin, X.; Menon, V.; Meng, Q.; Metcalf, G.; Chao, H.; Gingras, M.-C.; Avadhanula, V.; Farinholt, P.; et al. Transmission Event of SARS-CoV-2 Delta Variant Reveals Multiple Vaccine Breakthrough Infections. BMC Med. 2021, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 14 December 2021).
- Zhang, L.; Li, Q.; Liang, Z.; Li, T.; Liu, S.; Cui, Q.; Nie, J.; Wu, Q.; Qu, X.; Huang, W.; et al. The Significant Immune Escape of Pseudotyped SARS-CoV-2 Variant Omicron. Emerg. Microbes Infect. 2022, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic Approaches for Cardiac Regeneration and Repair. Nat. Rev. Cardiol. 2018, 15, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, J.; Robuffo, I.; Spalletta, S.; Giambuzzi, G.; De Iuliis, V.; Toniato, E.; Martinotti, S.; Conti, P.; Flati, V. The Epithelial-to-Mesenchymal Transition as a Possible Therapeutic Target in Fibrotic Disorders. Front. Cell Dev. Biol. 2020, 8, 1591. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Quaggin, S.E.; Vaughan, D.E. Molecular Basis of Organ Fibrosis: Potential Therapeutic Approaches. Exp. Biol. Med. 2013, 238, 461–481. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katwa, L.C.; Mendoza, C.; Clements, M. CVD and COVID-19: Emerging Roles of Cardiac Fibroblasts and Myofibroblasts. Cells 2022, 11, 1316. https://doi.org/10.3390/cells11081316
Katwa LC, Mendoza C, Clements M. CVD and COVID-19: Emerging Roles of Cardiac Fibroblasts and Myofibroblasts. Cells. 2022; 11(8):1316. https://doi.org/10.3390/cells11081316
Chicago/Turabian StyleKatwa, Laxmansa C., Chelsea Mendoza, and Madison Clements. 2022. "CVD and COVID-19: Emerging Roles of Cardiac Fibroblasts and Myofibroblasts" Cells 11, no. 8: 1316. https://doi.org/10.3390/cells11081316