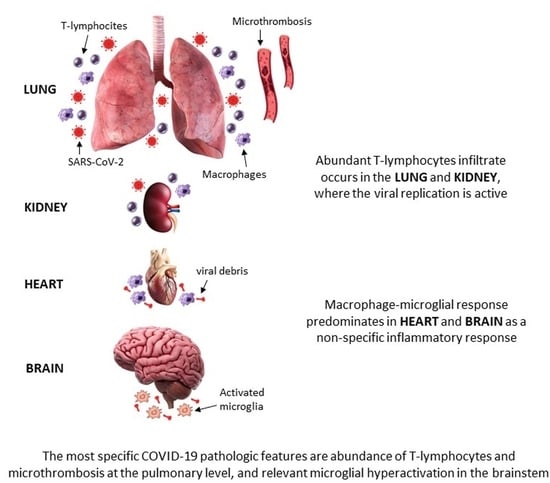
COVID-19 Pathology in the Lung, Kidney, Heart and Brain: The Different Roles of T-Cells, Macrophages, and Microthrombosis
Abstract
:1. Introduction
2. Materials & Methods
2.1. Study Design, Setting, Participants, and Clinical Data
2.2. Autopsies and Sampling of the Organs
2.3. Histology and Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. General and Clinical Characteristics
3.2. Pathological Findings in COVID-19 Cases
3.3. Pathological Findings in Control Non-COVID Cases (n = Five Lungs; n = Five Brains)
3.4. Comparison of Pathological Findings
4. Discussion
4.1. SARS-CoV-2 Antigens
4.2. Inflammatory Infiltrates
4.3. Comparison between COVID-19 and Other Types of Pneumonia
4.4. Comparison between COVID-19 and Control Brains
4.5. Microthrombosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, D.; Wu, T.; Liu, Q.; Yang, Z. The SARS-CoV-2 outbreak: What we know. Int. J. Infect. Dis. 2020, 94, 44–48. [Google Scholar] [CrossRef]
- Corman, V.M.; Muth, D.; Niemeyer, D.; Drosten, C. Hosts and Sources of Endemic Human Coronaviruses. Adv. Virus Res. 2018, 100, 163–188. [Google Scholar]
- Zhu, Z.; Lian, X.; Su, X.; Wu, W.; Marraro, G.A.; Zeng, Y. From SARS and MERS to COVID-19: A brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir. Res. 2020, 21, 224. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Deshmukh, V.; Motwani, R.; Kumar, A.; Kumari, C.; Raza, K. Histopathological observations in COVID-19: A systematic review. J. Clin. Pathol. 2021, 74, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Caramaschi, S.; Kapp, M.E.; Miller, S.E.; Eisenberg, R.; Johnson, J.; Epperly, G.; Maiorana, A.; Silvestri, G.; Giannico, G.A. Histopathological findings and clinicopathologic correlation in COVID-19: A systematic review. Mod. Pathol. 2021, 34, 1614–1633. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.L.; Klinkhammer, B.M.; Djudjaj, S.; Villwock, S.; Timm, M.C.; Buhl, E.M.; Wucherpfennig, S.; Cacchi, C.; Braunschweig, T.; Knüchel-Clarke, R.; et al. Multisystemic cellular tropism of SARS-CoV-2 in autopsies of COVID-19 patients. Cells 2021, 10, 1900. [Google Scholar] [CrossRef] [PubMed]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.; Shaik, R.A.; Ahmad, R.K.; Yusuf, M.; Khan, M.; Almutairi, A.B.; Alghuyaythat, W.K.Z.; Almutairi, S.B. “LONG COVID”: An insight. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5561–5577. [Google Scholar] [PubMed]
- Yende, S.; Parikh, C.R. Long COVID and kidney disease. Nat. Rev. Nephrol. 2021, 17, 792–793. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.R.; Barnby, J.M.; Hellyer, P.; et al. Cognitive deficits in people who have recovered from COVID-19. eClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef]
- Vasquez-Bonilla, W.O.; Orozco, R.; Argueta, V.; Sierra, M.; Zambrano, L.I.; Muñoz-Lara, F.; López-Molina, D.S.; Arteaga-Livias, K.; Grimes, Z.; Bryce, C.; et al. A review of the main histopathological findings in coronavirus disease 2019. Hum. Pathol. 2020, 105, 74–83. [Google Scholar] [CrossRef]
- Falasca, L.; Nardacci, R.; Colombo, D.; Lalle, E.; Di Caro, A.; Nicastri, E.; Antinori, A.; Petrosillo, N.; Marchioni, L.; Biava, G.; et al. Postmortem Findings in Italian Patients with COVID-19: A Descriptive Full Autopsy Study of Cases with and without Comorbidities. J. Infect. Dis. 2020, 222, 1807–1815. [Google Scholar] [CrossRef]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Quincy Brown, J.; Vander Heide, R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Grosse, C.; Grosse, A.; Salzer, H.J.F.; Dünser, M.W.; Motz, R.; Langer, R. Analysis of cardiopulmonary findings in COVID-19 fatalities: High incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc. Pathol. 2020, 49, 107263. [Google Scholar] [CrossRef]
- Edler, C.; Schröder, A.S.; Aepfelbacher, M.; Fitzek, A.; Heinemann, A.; Heinrich, F.; Klein, A.; Langenwalder, F.; Lütgehetmann, M.; Meißner, K.; et al. Dying with SARS-CoV-2 infection—An autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int. J. Leg. Med. 2020, 134, 1275–1284. [Google Scholar] [CrossRef]
- Borczuk, A.C.; Salvatore, S.P.; Seshan, S.V.; Patel, S.S.; Bussel, J.B.; Mostyka, M.; Elsoukkary, S.; He, B.; DEL Vecchio, C.; Fortarezza, F.; et al. COVID-19 pulmonary pathology: A multi-institutional autopsy cohort from Italy and New York City. Mod. Pathol. 2020, 33, 2156–2168. [Google Scholar] [CrossRef]
- Romanova, E.S.; Vasilyev, V.V.; Startseva, G.; Karev, V.; Rybakova, M.G.; Platonov, P.G. Cause of death based on systematic post-mortem studies in patients with positive SARS-CoV-2 tissue PCR during the COVID-19 pandemic. J. Intern. Med. 2021, 290, 655–665. [Google Scholar] [CrossRef]
- Schaller, T.; Hirschbühl, K.; Burkhardt, K.; Braun, G.; Trepel, M.; Märkl, B.; Claus, R. Postmortem Examination of Patients with COVID-19. JAMA 2020, 323, 2518–2520. [Google Scholar] [CrossRef]
- Bradley, B.T.; Maioli, H.; Johnston, R.; Chaudhry, I.; Fink, S.L.; Xu, H.; Najafian, B.; Deutsch, G.; Lacy, J.M.; Williams, T.; et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet 2020, 396, 320–332. [Google Scholar] [CrossRef]
- Duarte-Neto, A.N.; Monteiro, R.; Da Silva, L.F.F.; Malheiros, D.M.A.C.; De Oliveira, E.P.; Theodoro-Filho, J.; Pinho, J.R.R.; Gomes-Gouvêa, M.S.; Salles, A.P.M.; De Oliveira, I.R.S.; et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology 2020, 77, 186–197. [Google Scholar] [CrossRef]
- Elsoukkary, S.S.; Mostyka, M.; Dillard, A.; Berman, D.R.; Ma, L.X.; Chadburn, A.; Yantiss, R.K.; Jessurun, J.; Seshan, S.V.; Borczuk, A.C.; et al. Autopsy Findings in 32 Patients with COVID-19: A Single-Institution Experience. Pathobiology 2021, 88, 56–68. [Google Scholar] [CrossRef]
- Hariri, L.P.; North, C.M.; Shih, A.R.; Israel, R.A.; Maley, J.H.; Villalba, J.A.; Vinarsky, V.; Rubin, J.; Okin, D.A.; Sclafani, A.; et al. Lung Histopathology in Coronavirus Disease 2019 as Compared With Severe Acute Respiratory Sydrome and H1N1 Influenza: A Systematic Review. Chest 2021, 159, 73–84. [Google Scholar] [CrossRef]
- Santoriello, D.; Khairallah, P.; Bomback, A.S.; Xu, K.; Kudose, S.; Batal, I.; Barasch, J.; Radhakrishnan, J.; D’Agati, V.; Markowitz, G. Postmortem Kidney Pathology Findings in Patients with COVID-19. J. Am. Soc. Nephrol. 2020, 31, 2158–2167. [Google Scholar] [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.-P.; et al. Association of Cardiac Infection with SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef]
- Schurink, B.; Roos, E.; Radonic, T.; Barbe, E.; Bouman, C.S.C.; de Boer, H.H.; de Bree, G.J.; Bulle, E.B.; Aronica, E.M.; Florquin, S.; et al. Viral presence and immunopathology in patients with lethal COVID-19: A prospective autopsy cohort study. Lancet Microbe 2020, 1, e290–e299. [Google Scholar] [CrossRef]
- Hanley, B.; Naresh, K.N.; Roufosse, C.; Nicholson, A.G.; Weir, J.; Cooke, G.S.; Thursz, M.; Manousou, P.; Corbett, R.; Goldin, R.; et al. Articles Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 2020, 1, e245–e253. [Google Scholar] [CrossRef]
- Dal Ferro, M.; Bussani, R.; Paldino, A.; Nuzzi, V.; Collesi, C.; Zentilin, L.; Schneider, E.; Correa, R.; Silvestri, F.; Zacchigna, S.; et al. SARS-CoV-2, myocardial injury and inflammation: Insights from a large clinical and autopsy study. Clin. Res. Cardiol. 2021, 110, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Leone, O.; Rizzo, S.; De Gaspari, M.; Van Der Wal, A.C.; Aubry, M.-C.; Bois, M.C.; Lin, P.T.; Maleszewski, J.J.; Stone, J.R. Pathological features of COVID-19-associated myocardial injury: A multicentre cardiovascular pathology study. Eur. Heart J. 2020, 41, 3827–3835. [Google Scholar] [CrossRef] [PubMed]
- Sang, C.J.; Burkett, A.; Heindl, B.; Litovsky, S.H.; Prabhu, S.D.; Benson, P.V.; Rajapreyar, I. Cardiac pathology in COVID-19: A single center autopsy experience. Cardiovasc. Pathol. 2021, 54, 107370. [Google Scholar] [CrossRef] [PubMed]
- Poloni, T.E.; Medici, V.; Moretti, M.; Visonà, S.D.; Cirrincione, A.; Carlos, A.F.; Davin, A.; Gagliardi, S.; Pansarasa, O.; Cereda, C.; et al. COVID-19-related neuropathology and microglial activation in elderly with and without dementia. Brain Pathol. 2021, 31, 12997. [Google Scholar] [CrossRef]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef]
- Von Weyhern, C.H.; Kaufmann, I.; Neff, F.; Kremer, M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet 2020, 395, e109. [Google Scholar] [CrossRef]
- Al-Dalahmah, O.; Thakur, K.T.; Nordvig, A.S.; Prust, M.L.; Roth, W.; Lignelli, A.; Uhlemann, A.-C.; Miller, E.H.; Kunnath-Velayudhan, S.; del Portillo, A.; et al. Neuronophagia and microglial nodules in a SARS-CoV-2 patient with cerebellar hemorrhage. Acta Neuropathol. Commun. 2020, 8, 147. [Google Scholar] [CrossRef]
- Al-Sarraj, S.; Troakes, C.; Hanley, B.; Osborn, M.; Richardson, M.P.; Hotopf, M.; Bullmore, E.; Everall, I.P. Invited Review: The spectrum of neuropathology in COVID-19. Neuropathol. Appl. Neurobiol. 2021, 47, 3–16. [Google Scholar] [CrossRef]
- Deigendesch, N.; Sironi, L.; Kutza, M.; Wischnewski, S.; Fuchs, V.; Hench, J.; Frank, A.; Nienhold, R.; Mertz, K.D.; Cathomas, G.; et al. Correlates of critical illness-related encephalopathy predominate postmortem COVID-19 neuropathology. Acta Neuropathol. 2020, 140, 583–586. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Mahadeva, U.; Green, A.; Sekhawat, V.; Barrett, N.A.; Childs, L.; Shankar-Hari, M.; Thom, M.; Jäger, H.R.; Brandner, S. Microvascular injury and hypoxic damage: Emerging neuropathological signatures in COVID-19. Acta Neuropathol. 2020, 140, 397–400. [Google Scholar] [CrossRef]
- Kirschenbaum, D.; Imbach, L.L.; Rushing, E.J.; Frauenknecht, K.B.M.; Gascho, D.; Ineichen, B.V.; Keller, E.; Kohler, S.; Lichtblau, M.; Reimann, R.R.; et al. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol. Appl. Neurobiol. 2021, 47, 454–459. [Google Scholar] [CrossRef]
- Lee, M.-H.; Perl, D.P.; Nair, G.; Li, W.; Maric, D.; Murray, H.; Dodd, S.J.; Koretsky, A.P.; Watts, J.A.; Cheung, V.; et al. Microvascular Injury in the Brains of Patients with COVID-19. N. Engl. J. Med. 2021, 384, 481–483. [Google Scholar] [CrossRef]
- Reichard, R.R.; Kashani, K.B.; Boire, N.A.; Constantopoulos, E.; Guo, Y.; Lucchinetti, C.F. Neuropathology of COVID-19: A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020, 140, 1–6. [Google Scholar] [CrossRef]
- Solomon, I.H.; Normandin, E.; Bhattacharyya, S.; Mukerji, S.S.; Keller, K.; Ali, A.S.; Adams, G.; Hornick, J.L.; Padera, R.F., Jr.; Sabeti, P. Neuropathological Features of COVID-19. N. Engl. J. Med. 2020, 383, 989–992. [Google Scholar] [CrossRef]
- Bryce, C.; Grimes, Z.; Pujadas, E.; Ahuja, S.; Beth Beasley, M.; Albrecht, R.; Hernandez, T.; Stock, A.; Zhao, Z.; Al Rasheed, M.; et al. Pathophysiology of SARS-CoV-2: Targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. Mod. Pathol. 2021, 34, 1456–1467. [Google Scholar] [CrossRef]
- Kantonen, J.; Mahzabin, S.; Mäyränpää, M.I.; Tynninen, O.; Paetau, A.; Andersson, N.; Sajantila, A.; Vapalahti, O.; Carpén, O.; Kekäläinen, E.; et al. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020, 30, 1012–1016. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Liu, L.; Zhao, M.; Xiao, J.; Zhao, Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020, 40, 2095–2103. [Google Scholar] [CrossRef] [Green Version]
- Başkıran, A.; Akbulut, S.; Şahin, T.T.; Tunçer, A.; Kaplan, K.; Bayındır, Y.; Yılmaz, S. Coronavirus Precautions: Experience of High Volume Liver Transplant Institute. Turk. J. Gastroenterol. 2022, 33, 145–152. [Google Scholar] [CrossRef]
- Sahin, T.T.; Akbulut, S.; Yilmaz, S. COVID-19 pandemic: Its impact on liver disease and liver transplantation. World J. Gastroenterol. 2020, 26, 2987–2999. [Google Scholar] [CrossRef]
- Yachou, Y.; El Idrissi, A.; Belapasov, V.; Ait Benali, S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: Understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 2020, 41, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the Nervous System. Cell 2020, 183, 16–27.e1. [Google Scholar] [CrossRef]
- Moretti, M.; Malhotra, A.; Visonà, S.D.; Finley, S.J.; Osculati, A.M.M.; Javan, G.T. The roles of medical examiners in the COVID-19 era: A comparison between the United States and Italy. Forensic Sci. Med. Pathol. 2021, 17, 262–270. [Google Scholar] [CrossRef]
- Moretti, M.; Belli, G.; Morini, L.; Monti, M.C.; Osculati, A.M.M.; Visonà, S.D. Drug abuse-related neuroinflammation in human postmortem brains: An immunohistochemical approach. J. Neuropathol. Exp. Neurol. 2019, 78, 1059–1065. [Google Scholar] [CrossRef]
- Osborn, M.; Lucas, S.; Stewart, R.; Swift, B.; Youd, E. Briefing on COVID-19. Autopsy practice relating to possible cases of COVID-19 (2019-nCov, novel coronavirus from China 2019/2020). The Royal College of Pathologists. 2020. Available online: https://www.rcpath.org/uploads/assets/d5e28baf-5789-4b0f-acecfe370eee6223/fe8fa85a-f004-4a0c-81ee4b2b9cd12cbf/Briefing-on-COVID-19-autopsy-Feb-2020.pdf (accessed on 3 May 2020).
- Gagliardi, S.; Poloni, E.T.; Pandini, C.; Garofalo, M.; Dragoni, F.; Medici, V.; Davin, A.; Visonà, S.D.; Moretti, M.; Sproviero, D.; et al. Detection of SARS-CoV-2 genome and whole transcriptome sequencing in frontal cortex of COVID-19 patients. Brain Behav. Immun. 2021, 97, 13–21. [Google Scholar] [CrossRef]
- Poloni, T.E.; Medici, V.; Carlos, A.F.; Davin, A.; Ceretti, A.; Mangieri, M.; Cassini, P.; Vaccaro, R.; Zaccaria, D.; Abbondanza, S.; et al. Abbiategrasso Brain Bank Protocol for Collecting, Processing and Characterizing Aging Brains. J. Vis. Exp. 2020, 2020, e60296. [Google Scholar] [CrossRef]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L.V. Overview of Targets and Potential Drugs of SARS-CoV-2 According to the Viral Replication. J. Proteome Res. 2021, 20, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.-Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.-X.; Gong, L.; et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef]
- Farkash, E.A.; Wilson, A.M.; Jentzen, J.M. Ultrastructural Evidence for Direct Renal Infection with SARS-CoV-2. JASN 2020, 31, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.R.; Tang, J. COVID-19 and Kidney Injury. Rhode Isl. Med. J. 2020, 103, 24–28. [Google Scholar]
- Pan, X.W.; Xu, D.; Zhang, H.; Zhou, W.; Wang, L.H.; Cui, X.G. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis. Intensive Care Med. 2020, 46, 1114–1116. [Google Scholar] [CrossRef] [Green Version]
- Hassanein, M.; Radhakrishnan, Y.; Sedor, J.; Vachharajani, T.; Vachharajani, V.T.; Augustine, J.; Demirjian, S.; Thomas, G. COVID-19 and the kidney. Clevel. Clin. J. Med. 2020, 87, 619–631. [Google Scholar] [CrossRef]
- Mizuiri, S. ACE and ACE2 in kidney disease. World J. Nephrol. 2015, 4, 74–82. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Liu, Q.; Yao, Q.; Wang, X.; Zhang, H.; Chen, R.; Ren, L.; Min, J.; Deng, F.; et al. SARS-CoV-2 cell tropism and multiorgan infection. Cell Discov. 2021, 7, 17. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Barbosa Casaro, E.; Bevilaqua Rangel, É. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Boldrini, M.; Canoll, P.D.; Klein, R.S. How COVID-19 Affects the Brain. JAMA Psychiatry 2021, 78, 682–683. [Google Scholar] [CrossRef]
- Paul, J.F.; Charles, P.; Richaud, C.; Caussin, C.; Diakov, C. Myocarditis revealing COVID-19 infection in a young patient. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 776. [Google Scholar] [CrossRef]
- Mele, D.; Flamigni, F.; Rapezzi, C.; Ferrari, R. Myocarditis in COVID-19 patients: Current problems. Intern. Emerg. Med. 2021, 16, 1123–1129. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, J.; Daly, C.; Edroos, S.A. Acute pericarditis as a primary presentation of COVID-19. BMJ Case Rep. 2020, 13, e237617. [Google Scholar] [CrossRef]
- Lee, M.H.; Perl, D.P.; Steiner, J.; Pasternack, N.; Li, W.; Maric, D.; Safavi, F.; Horkayne-Szakaly, I.; Jones, R.; Stram, M.N.; et al. Neurovascular injury with complement activation and in fl ammation in COVID-19. Brain 2022, 145, 2555–2568. [Google Scholar] [CrossRef]
- Poloni, T.E.; Carlos, A.F.; Cairati, M.; Cutaia, C.; Medici, V.; Marelli, E.; Ferrari, D.; Galli, A.; Bognetti, P.; Davin, A.; et al. Prevalence and prognostic value of Delirium as the initial presentation of COVID-19 in the elderly with dementia: An Italian retrospective study. eClinicalMedicine 2020, 26, 100490. [Google Scholar] [CrossRef]
- Akbar, A.N.; Gilroy, D.W. Aging immunity may exacerbate COVID-19. Science. Am. Assoc. Adv. Sci. 2020, 369, 256–257. [Google Scholar]
- Mueller, A.L.; Mcnamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef] [PubMed]
- Salimi, S.; Hamlyn, J.M.; Le Couteur, D. COVID-19 and Crosstalk with the Hallmarks of Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, e34–e41. [Google Scholar] [CrossRef] [PubMed]
- Fullard, J.F.; Lee, H.-C.; Voloudakis, G.; Suo, S.; Javidfar, B.; Shao, Z.; Peter, C.; Zhang, W.; Jiang, S.; Corvelo, A.; et al. Single-nucleus transcriptome analysis of human brain immune response in patients with severe COVID-19. Genome Med. 2021, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, J.; Ma, X.; Tan, J.; Chen, L.; Liu, S.; Xin, Y.; Zhuang, L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed. Pharmacother. 2020, 131, 110678. [Google Scholar] [CrossRef]
- Ludlow, M.; Kortekaas, J.; Herden, C.; Hoffmann, B.; Tappe, D.; Trebst, C.; Griffin, D.E.; Brindle, H.; Solomon, T.; Brown, A.S.; et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 2016, 131, 159–184. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Pisano, T.J.; Hakkinen, I.; Rybinnik, I. Large Vessel Occlusion Secondary to COVID-19 Hypercoagulability in a Young Patient: A Case Report and Literature Review. J. Stroke Cerebrovasc. Dis. 2020, 29, 105307. [Google Scholar] [CrossRef]
- Baram, A.; Kakamad, F.H.; Abdullah, H.M.; Mohammed-Saeed, D.H.; Hussein, D.A.; Mohammed, S.H.; Abdulrahman, B.B.; Mirza, A.J.; Abdulla, B.A.; Rahim, H.M.; et al. Large vessel thrombosis in patient with COVID-19, a case series. Ann. Med. Surg. 2020, 60, 526–530. [Google Scholar] [CrossRef]
- Avila, J.; Long, B.; Holladay, D.; Gottlieb, M. Thrombotic complications of COVID-19. Am. J. Emerg. Med. 2021, 39, 213–218. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Thachil, J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 2103–2109. [Google Scholar] [CrossRef]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef]





| Code | PMD (Days/Hours) | General and Clinical Features | |||||
|---|---|---|---|---|---|---|---|
| Sex | Age (y/o) | Anamnesis; Cause of Death | NCD | DEL | SEP | ||
| COV2 | 7 d | M | 29 | NR; hemorrhagic shock | no | no | no |
| COV4 | 5 d | M | 67 | Obesity, HTN, and CVD; CIP and respiratory failure | no | no | yes |
| COV6 | 11 d | F | 90 | HTN and COPD; respiratory failure | no | no | no |
| COV3 | 7 d | M | 87 | T2D and CVD; respiratory failure | Mild (VCI) | Hyper/Hypo (early onset) | no |
| COV10 | 7 d | M | 81 | AF and paraparesis (previous GBS); respiratory failure | Mild (VCI) | Hypo (late onset) | yes |
| COV1 | 7 d | F | 74 | NR; respiratory failure | Major (AD) | Hyper (early onset) | no |
| COV5 | 3 d | F | 94 | T2D, HTN, CVD, and AF; multiorgan failure | Major (AD + VaD) | Hypo (early onset) | yes |
| COV8 | 13 d | F | 83 | HTN; respiratory failure | Major (AD) | no | no |
| COV9 | 6 d | M | 92 | HTN and cerebrovascular disease; respiratory failure | Major (AD + VaD) | Hyper/Hypo (late onset) | no |
| L1 | 4 d | F | 76 | AF; multiorgan failure 7 days after head trauma | no | no | no |
| L2 | 5 d | M | 92 | HTN CVD, and cerebrovascular disease; respiratory failure | Major (VaD) | Hypo (late onset) | no |
| L3 | 6 d | F | 60 | CVD; multiorgan failure 3 days after head trauma | no | no | no |
| L4 | 4 d | M | 62 | HTN; respiratory failure | no | no | yes |
| L5 | 8 d | M | 74 | HTN and COPD; multiorgan failure 10 days after intestinal perforation | Major (AD + VaD) | Hypo (late onset) | yes |
| B1 | 3 h | M | 79 | T2D; liver cancer | no | no | no |
| B2 | 8 h | M | 79 | HTN, CVD, and cerebrovascular disease; cachexia | Mild (VCI) and hemiparesis | no | no |
| B3 | 16 h | F | 83 | HTN, CVD, and cerebrovascular disease; CHF | Major (AD + VaD) | no | no |
| B4 | 15 h | F | 85 | CVD and cerebrovascular disease; CHF | Major (AD + VaD) | Hyper (prev. ep) | no |
| B5 | 15 h | F | 89 | HTN, COPD, and cerebrovascular disease; cachexia | Major (AD) | Hyper (prev. ep) | no |
| General Pathological Features | Specific Pathological Features | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung | Kidney | Heart | Brain | Lung | Kidney | Heart | Brain-Frontal Lobe | Brain-Pons | |||||||||||||||||||||||
| CASE | (Acute; chronic findings) | (Acute; chronic findings) | (Acute; chronic findings) | (Acute; chronic findings) | M | TL | BL | AP | VA | M | TL | BL | AP | VA | M | TL | BL | AP | VA | M | TL | BL | AP | VA | Vr | Vd | M | TL | BL | AP | VA |
| COV2 | Mild C, AE, interstitial- subpleural IF; F, CE, A | Severe C | MA | HA; no AD, no SVD, mild gliosis-RA | + | - | - | - | n/a | n/a | - | ||||||||||||||||||||
| COV4 | Severe C, interstitial-subpleural AH, AE, E, DAD, BS, IF; CE, A | Severe C, cortical-medullary IF; GS | MV, BS, MA, Subepicardial IF; F, CH | HA; no AD, SVD, mild gliosis | - | - | - | - | - | + | - | ||||||||||||||||||||
| COV6 | AE, E, interstitial IF; F, A | Cortical IF; F | Mild C | HA; low AD, mild gliosis | - | - | - | - | - | + | - | ||||||||||||||||||||
| COV3 | Severe C, AE, DAD, interstitial IF; AS, CE, A | Severe C, AGA, cortical-medullary IF; F, AS, GS | MV, MA; F, AS | HA; low AD, SVD, mild gliosis | + | - | - | - | - | + | + | ||||||||||||||||||||
| COV10 | Severe C, AE, BS, interstitial IF; AS | Severe C, AGA; F | MA, interstitial-subepicardial IF; F | HA; no AD, SVD, perivascular gliosis | + | + | + | - | - | + | - | ||||||||||||||||||||
| COV1 | C, AE, AH, E, DAD, BS, interstitial IF; F, CE | Cortical IF; F, GS | MA, BS; F | HA; high AD, perivascular gliosis | + | - | - | - | - | - | - | ||||||||||||||||||||
| COV5 | Severe C, AH, AE, E, DAD, BS, interstitial IF; A, CE | Severe C, cortical-IF, AGA; GS | MV, MA, subepicardial IF; F, CH | HA; intermediate AD, SVD, mild gliosis-RA | + | + | - | - | - | + | - | ||||||||||||||||||||
| COV8 | Severe C, interstitial IF; CE, A | Cortical-medullary IF; AS | F | HA; intermediate AD, perivascular gliosis-RA | - | - | - | - | - | + | - | ||||||||||||||||||||
| COV9 | Severe C, AH, interstitial IF; CE, A | AS | MA, subepicardial IF; F, AS | HA; intermediate AD, SVD, perivascular gliosis-RA | - | + | - | - | - | + | - | ||||||||||||||||||||
| C:L1 / B1 | Mild C, E, DAD, interstitial IF; F, A | n/a | n/a | No AD, no SVD | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | - | - | - | n/a | ||||||||||||
| C:L2 / B2 | AE, interstitial-subpleural IF; F, CE, A | n/a | n/a | No AD, stroke and SVD | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | ||||||||||||
| C:L3 / B3 | Mild C, interstitial IF; E, DAD, BS | n/a | n/a | Intermediate AD, stroke, severe gliosis | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | ||||||||||||
| C:L4 / B4 | Severe C, AE, BS, interstitial-subpleural IF; A | n/a | n/a | High AD, SVD, severe gliosis | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | - | - | n/a | ||||||||||||
| C:L5 / B5 | Mild C, E, BS, interstitial IF; F, CE | n/a | n/a | High AD, severe gliosis | - | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | ||||||||||||
 .
.| χ2 | p-Value | |
|---|---|---|
| Friedman rank sum test | 31.8 | <0.001 |
| Pairwise comparisons (Durbin–Conover) | t | p-value |
| Sum of T-B Lymphocytes Lungs vs. Kidneys | 0.438 | 0.665 |
| Sum of T-B Lymphocytes Lungs vs. Heart | 10.506 | <0.001 |
| Sum of T-B Lymphocytes Lungs vs. Brain frontal lobe | 8.536 | <0.001 |
| Sum of T-B Lymphocytes Lungs vs. Brain pons | 11.162 | <0.001 |
| Sum of T-B Lymphocytes Kidneys vs. Heart | 10.068 | <0.001 |
| Sum of T-B Lymphocytes Kidneys vs. Brain frontal lobe | 8.098 | <0.001 |
| Sum of T-B Lymphocytes Kidneys vs. Brain frontal pons | 10.725 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poloni, T.E.; Moretti, M.; Medici, V.; Turturici, E.; Belli, G.; Cavriani, E.; Visonà, S.D.; Rossi, M.; Fantini, V.; Ferrari, R.R.; et al. COVID-19 Pathology in the Lung, Kidney, Heart and Brain: The Different Roles of T-Cells, Macrophages, and Microthrombosis. Cells 2022, 11, 3124. https://doi.org/10.3390/cells11193124
Poloni TE, Moretti M, Medici V, Turturici E, Belli G, Cavriani E, Visonà SD, Rossi M, Fantini V, Ferrari RR, et al. COVID-19 Pathology in the Lung, Kidney, Heart and Brain: The Different Roles of T-Cells, Macrophages, and Microthrombosis. Cells. 2022; 11(19):3124. https://doi.org/10.3390/cells11193124
Chicago/Turabian StylePoloni, Tino Emanuele, Matteo Moretti, Valentina Medici, Elvira Turturici, Giacomo Belli, Elena Cavriani, Silvia Damiana Visonà, Michele Rossi, Valentina Fantini, Riccardo Rocco Ferrari, and et al. 2022. "COVID-19 Pathology in the Lung, Kidney, Heart and Brain: The Different Roles of T-Cells, Macrophages, and Microthrombosis" Cells 11, no. 19: 3124. https://doi.org/10.3390/cells11193124