Abstract
Introduction
COVID-19 long-haulers, also decribed as having “long-COVID” or post-acute COVID-19 syndrome, represent 10% of COVID-19 patients and remain understudied.
Methods
In this prospective study, we recruited 30 consecutive patients seeking medical help for persistent symptoms (> 30 days) attributed to COVID-19. All reported a viral illness compatible with COVID-19. The patients underwent a multi-modal evaluation, including clinical, psychologic, virologic and specific immunologic assays and were followed longitudinally. A group of 17 convalescent COVID-19 individuals without persistent symptoms were included as a comparison group.
Results
The median age was 40 [interquartile range: 35–54] years and 18 (60%) were female. At a median time of 152 [102–164] days after symptom onset, fever, cough and dyspnea were less frequently reported compared with the initial presentation, but paresthesia and burning pain emerged in 18 (60%) and 13 (43%) patients, respectively. The clinical examination was unremarkable in all patients, although the median fatigue and pain visual analog scales were 7 [5–8] and 5 [2–6], respectively. Extensive biologic studies were unremarkable, and multiplex cytokines and ultra-sensitive interferon-α2 measurements were similar between long-haulers and convalescent COVID-19 individuals without persistent symptoms. Using SARS-CoV-2 serology and IFN-γ ELISPOT, we found evidence of a previous SARS-CoV-2 infection in 50% (15/30) of patients, with evidence of a lack of immune response, or a waning immune response, in two patients. Finally, psychiatric evaluation showed that 11 (36.7%), 13 (43.3%) and 9 (30%) patients had a positive screening for anxiety, depression and post-traumatic stress disorder, respectively.
Conclusions
Half of patients seeking medical help for post-acute COVID-19 syndrome lack SARS-CoV-2 immunity. The presence of SARS-CoV-2 immunity, or not, had no consequence on the clinical or biologic characteristics of post-acute COVID-19 syndrome patients, all of whom reported severe fatigue, altered quality of life and psychologic distress.
Similar content being viewed by others
Why carry out this study? |
Long-COVID, or post-acute COVID-19 syndrome, has been reported to occur in up to 10% of all COVID-19 patients. |
Little information is available on the clinical characteristics and pathophysiology of this syndrome. In the present study, we aim to describe these characteristics and to investigate potential underlying mechanisms. |
What was learned from this study |
Among 30 consecutive patients reporting persistent symptoms (median 6 months) self-attributed to COVID-19, pain, fatigue and disability were reported in virtually all patients. |
More than one third of patients suffer from psychologic disorders such as anxiety, depression and/or post-traumatic stress disorder, regardless of SARS-CoV-2 immunity. |
At the time of evaluation, only 50% of patients had cellular and/or humoral signs of a past SARS-CoV-2, and serology positivity varied depending on the kit used. |
Exhaustive clinical, biologica and immunologic evaluations failed to find an alternative diagnosis or to identify a specific cytokine signature including type I interferon. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14786100.
Introduction
After SARS-CoV-2 infection, some patients may continue to experience symptoms for several months [1], a condition which has been termed “long-COVID,” “COVID-19 long-haulers,” or post-acute COVID-19 syndrome [2]. These long-term symptoms contribute to the worldwide COVID-19 burden and have been extensively communicated by patients and physicians [3, 4]. Manifestations of post-acute COVID-19 syndrome are clinically diverse, and multiple underlying mechanisms are likely to be implicated. Patients who underwent extensive hospitalization due to severe disease may exhibit chronic lung or heart injury due to the heightened immune response [5, 6], micro- or macrovascular thrombotic neurologic complications [7] and/or physical deconditioning [8]. Indeed, a recent cohort study found that among 1733 hospitalized COVID-19 patients, 76% reported persistence of at least one symptom after 6 months, and objective pulmonary abnormalities were found in > 20% [9]. However, these pathologic mechanisms are less likely to explain the manifestations in patients with initially non-severe COVID-19 who go on to experience long-lasting symptoms. Among those plausible mechanisms, viral-induced autoimmunity should be investigated in light of the accumulating data reporting this phenomenon during or following acute COVID-19 [10]. Although 10% of COVID-19 patients may show chronic symptoms (> 12 weeks) [11], data on the clinical presentation, biologic characteristics and overall prognosis of such patients are scarce [9, 12]. Moreover, the virologic definition of post-acute COVID-19 syndrome in terms of serology and other specific immunologic assays remains to be precisely established [12, 13].
The aim of our study was to describe the clinical and biologic characteristics of post-acute COVID-19 syndrome by conducting a multimodal evaluation of consecutive patients seeking medical help for persistent symptoms attributed to COVID-19 and to compare their characteristics to convalescent COVID-19 individuals without persistent symptoms. This evaluation also aimed to investigate the potential underlying mechanisms, including autoimmunity and psychologic distress.
Methods
Patients and Ethical Considerations
We screened 34 consecutive patients seeking medical help for persistent symptoms attributed to COVID-19 for participation in this systematic, prospective study. Patients were recruited in the “Grand-Est” region of France, the area with the highest incidence of SARS-CoV-2 infection during the first epidemic wave in France (February to April 2020) [14]. Information about a post-acute COVID-19 syndrome consultation in our tertiary center was advertised through local media and social networks, and interested patients were then asked to participate in the study. All patients provided written informed consent. The data from 17 age-matched patients with a history of non-severe COVID-19 (confirmed with RT-PCR or serology) at least 3 months before immuno-virologic assays, and without persistent symptoms, were included as controls (these patients were initially recruited as part of the Study “SeroCOVHUS,” ClinicalTrials.gov Identifier: NCT04441684, Ethical Committee authorization from SUD MEDITERRANEE III, No. 2020.04.15 bis_ 20.04.10.66856). The sera from 5 patients with acute severe COVID-19 (hospitalized in the intensive care unit) and from 18 patients with active systemic lupus erythematosus were used as a positive control for multiplex cytokine and interferon-α2 measurements, respectively. The present study is part of the “COVID-HUS” study, which was approved by the Ethics Committee of the University Hospital of Strasbourg (NCE–2020–51).
Medical Evaluation
All patients were evaluated at our reference center for rare systemic and autoimmune diseases. Five physicians (MS, RF, EC, LP and JS) conducted the clinical evaluation with a physical examination and a standardized questionnaire on medical and SARS-CoV-2–related infection history. Dedicated questionnaires were used to evaluate subjective symptoms: DN4 for neuropathic pain [15], Fibromyalgia Rapid Screening Tool (FiRST [16]) and visual analog scales for fatigue/pain/dryness. Additionally, patients underwent systematic blood testing to rule out alternative diagnoses (full list in Supplementary File 1).
Virologic and Immunologic Evaluation
As a second step to the clinical evaluation, immuno-virologic assays were conducted. Testing included an extensive serology evaluation using four commercial assays: a lateral flow assay testing for the receptor-binding domain (RBD) of the spike protein as an antigenic source (Biosynex BSS IgM/IgG assay); two enzyme-linked immunosorbent assays (ELISA) targeting the RBD (Wantai total antibody assay) and the S1 domain (Euroimmun IgG assay); a third ELISA testing for anti-nucleocapsid (anti-N) IgG (Abbott Architect IgG). To explore the SARS-CoV-2-specific T-cell response, heparin-anticoagulated blood tubes were collected for interferon gamma (IFN-γ) enzyme-linked immunospot (ELISPOT) assay. Detailed methods of SARS-CoV-2 IFN-γ ELISPOT are described in Supplementary File 2. To investigate the presence of remnant SARS-CoV-2 RNA, nasopharyngeal and stool RT-PCR were conducted in patients who accepted to take the test (methods in Supplementary File 2). To complete the immunologic evaluation, interferon-α2 (IFN-α2), with Single Molecule Array (SIMOA, Quanterix [17]) and cytokines (interleukin 1β [IL-1β], IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-17A, IL18, IFN-γ, IP-10, G-CSF, GM-CSF, MCP-1, MIP-1α, MIP-1β and TNF-α, using a custom multiplex cytokine assay; Luminex Thermo Fisher) were measured from serum samples.
Psychologic Evaluation
A standardized interview was conducted by a clinical psychologist (R.A.-M.). The interview aimed at evaluating psychiatric history and treatments, presence of sleep issues and increase of anxiolytic use. The impact of the COVID-19 pandemic on quality of life was assessed by using the Medical Outcomes Study 36-item Short-form (SF-36) and Health Assessment Questionnaire (HAQ). Patients’ perspectives on the pandemic crisis were assessed. Finally, validated questionnaires, translated into French, were used to screen for anxiety/depression (Hospital Anxiety and Depression Scale [HADS-A/D]) and post-traumatic stress disorder (PTSD checklist [PCL-5] for the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition [DSM-5]). Positive screening for these mental disorders was confirmed with a score ≥ 10 for HADS-Anxiety, ≥ 7 for HADS-Depression [18] and ≥ 31 for PCL-5 [19].
Statistics
Quantitative data are reported as medians and interquartile range (IQR) and were compared by non-parametric Mann-Whitney tests. Categorical data are reported as numbers (%) and were compared by chi-square or Fisher exact tests, as appropriate. To compare initial and persistent clinical features, McNemar’s test was used with Bonferroni’s correction for multiple testing. Statistical analyses were carried out using GraphPad Prism 7.0. P < 0.05 was considered statistically significant.
Results
Patients
Thirty-four consecutive patients seeking help for persistent symptoms attributed to COVID-19 contacted our center between June and August 2020; 30 were included in the study. Reasons for non-inclusion were refusal of physical consultation because of geographical distance (n = 3) or spontaneous improvement of the condition (n = 1). In total, 60% were women (18/30) and the median age was 40 years (IQR 35–54). The cohort included two married couples, and other patients were unrelated. Before the initial presentation, none of the patient reported chronic pain or use of analgesics. Other characteristics are shown in Table 1.
Initial Presentation
All patients declared a viral illness compatible with COVID-19. Initial symptoms occurred between 1 February and 9 April 2020 and were mainly characterized by fever (60%), myalgia (76.7%), cough (73.3%) and anosmia (43.3%) (Fig. 1, Table 1). Among nine patients who underwent nasopharyngeal SARS-CoV-2 RT-PCR within 5 weeks of symptom onset, 55.6% (5/9) had a positive result, and all RT-PCR tests conducted after this time were negative (n = 11). Seven patients visited the emergency department, and one was hospitalized in a conventional hospital unit, receiving oxygen for 7 days, without any other specific treatment. All other patients were cared for at home. A total of 13 patients were prescribed treatments: 2 received hydroxychloroquine (400 mg/day), 4 prednisone (1 mg/kg for 5–7 days), 8 antibiotics (azithromycin, n = 6; amoxicillin, n = 2) and 2 low-dose aspirin. The other 17 patients received only symptomatic treatment (acetaminophen).
Initial and residual clinical features of patients with persistent symptoms self-attributed to COVID-19. a Residual symptoms collected at a median of 152 days (IQR 102–164) after initial presentation (n = 30 patients). Horizontal rectangles indicate symptom prevalence, and bars are standard error measure. P-values were calculated with McNemar’s test with Bonferroni correction for multiple comparisons. b Comparison of initial/residual symptoms between immunized (red rectangles; positive for SARS-CoV-2 IFN-γ ELISPOT and at least one serologic assay) and non-immunized (blue rectangles) patients. Horizontal rectangles and black bars are mean ± standard error measure. *p < 0.05 by chi-square or Fisher’s exact test (if appropriate)
Persistent Clinical Features
Patients were clinically evaluated after a median of 152 days (IQR 102–164) following the reported onset of initial symptoms. Seventeen (56.7%) reported a resolution of initial symptoms after a median of 21 days (IQR 15–33), followed by a resurgence at a median of 21 days later (IQR 15–44). Conversely, the 13 other patients had no symptom-free intervals (Figure S1). Persistent symptoms had a cyclical pattern in 28 (93.3%) patients and were mostly represented by fatigue, myalgia and thoracic oppression (Fig. 1a). Fever, shivering and cough were significantly less frequent compared with the initial presentation (p < 0.005 for all; Fig. 1a). Fatigue was severe for most patients and rated at a median of 7 (IQR 5–8) on a 10-point scale, with pain rated at 5 (IQR 2–6).
Overall, 60% and 43.4% of patients exhibited diffuse paresthesia and burning pain later after the initial presentation (Fig. 1a). The DN4 questionnaire screening neuropathic pain was positive (≥ 4/10) for 50% (15/30) of patients, and the FiRST questionnaire screening for fibromyalgia-like symptoms was positive (≥ 5/6) for 56.7% (17/30; Figure S2).
The clinical examination, including neurologic examination, was unremarkable. At this point, no patients received corticosteroids, non-steroidal antiinflammatory drugs or opioids. Finally, 16 (53.3%) patients reported a trend toward a decrease of symptoms over time.
Virologic and Specific Immunologic Evaluation
Specific analyses related to SARS-CoV-2 infection were conducted at a median of 174 (IQR 144–215) days after symptom onset. At this time, 18 (60%) and 6 (20%) patients provided nasopharyngeal and stool samples for SARS-CoV-2 RT-PCR; all tests were negative.
Exploration of the cellular immune response by SARS-CoV-2 IFN-γ ELISPOT assay revealed that 15 (50%) patients had a positive response to at least SARS-CoV-2 nucleocapsid and spike proteins (considered ELISPOT-positive, Fig. 2A). Among ELISPOT-positive patients, 73.3% (11/15) also had a cellular response against non-structural SARS-CoV-2 protein compared with only 1 (6.7%) of ELISPOT-negative patients (p < 0.0001). Cellular responses to the spike protein of human coronavirus 229E and OC43 were similar in both SARS-CoV-2 ELISPOT groups (100% in ELISPOT-positive vs. 86.7% in ELISPOT-negative and 80% in ELISPOT-positive vs. 73.3% in ELISPOT-negative, respectively).
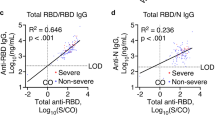
Specific immunologic responses to SARS-CoV-2 in 30 patients reporting persistent symptoms self-attributed to long-COVID. a Results of SARS-CoV-2 serologic assays, according to the result of the SARS-CoV-2 interferon-γ (IFN-γ) ELISPOT (n = 15/group). Results for the following assays are shown: anti-RBD total antibody (Wantai total antibody); anti-RBD IgG/IgM (Biosynex BSS IgM/IgG assay); anti-S IgG (Euroimmun); anti-N IgG (Abbott Architect). Columns show the prevalence of test positivity, and black bars represent SEM. b Two patterns of patients were identified: those with objective signs of SARS-CoV-2 immunity (cellular AND humoral response, n = 15) and those without (n = 15). Positive IgG was defined as a positive result against spike, receptor binding domain or nucleocapsid protein. *One patient with virologically unproven initial presentation had an isolated anti-S IgG–positive assay result. IFN-γ ELISPOT and 3 other serologic assays were negative
Among patients with a negative IFN-γ ELISPOT result, all but one had negative serology results (Fig. 2a, Table 2). This patient had an isolated positive anti-S IgG assay. Among the 15 patients with a positive IFN-γ ELISPOT result, all had at least one positive serologic assay. In detail, 14 (93.3%) had a positive result for anti-RBD total antibodies, 11 (73.3%) anti-RBD IgG/IgM, 12 (80%) anti-S IgG and 11 (73.3%) anti-N IgG (Fig. 2a). We dichotomized the patients into an immunized group (ELISPOT-positive and at least one positive serologic assay, n = 15) and a non-immunized group (ELISPOT-negative, n = 15; Fig. 2b), who exhibited little difference in terms of baseline characteristics and clinical features (Fig. 1b, Tables 1 and 2). Additionally, we compared patients with persistent symptoms to age-matched COVID-19 convalescent individuals without persistent symptoms (Table 1). Clinical features of these patients were similar to those with persistent symptoms (Table 2).
One ELISPOT-positive patient showed a decreasing signal for anti-N (equivocal to negative) and anti-S signal (positive to equivocal) between day 133 and day 251. One patient with negative results for both IFN-γ ELISPOT and serology assays (conducted 140 days after symptom onset) previously had a positive nasopharyngeal RT-PCR SARS-CoV-2 result early after the first symptoms (Fig. 2b). This patient also had two negative IgG serology tests for SARS-CoV-2 at 37 and 85 days after symptom onset (Biosynex ICT and CLIA Cobas Roche, respectively).
IFN-α2 levels were similar for patients with persistent symptoms (regardless of their immunity status) and for convalescent COVID-19 patients without persistent symptoms (sampled > 12 weeks after infection; Fig. 3). Other cytokine levels were low or non-detectable and were similar in immunized and non-immunized post-acute COVID-19 syndrome patients. However, we found that the level of monocyte chemotactic protein 1 (MCP-1) was significantly lower in immunized and non-immunized groups of post-acute COVID-19 syndrome patients (23.0 [11.6–33.4] and 14.1 [10.0–15.6] ng/ml, respectively) compared to the group of convalescent COVID-19 patients without persistent symptoms (33.3 [24.5–60.5] ng/ml, p < 0.05 and p < 0.0001, respectively; Figure S3).
Normal levels of IFN-α2 for patients with persistent symptoms attributed to COVID-19 compared to convalescent COVID-19 individuals. Ultra-sensitive IFN-α2 levels were measured by using Single Molecule Array (SIMOA) in patients with persistent symptoms self-attributed to COVID-19 (post-acute COVID-19 syndrome) whether they were immunized against SARS-CoV-2 (positive for SARS-CoV-2 IFN-γ ELISPOT and at least one serologic assay, n = 15) or non-immunized (n = 15). As a comparison, results from individuals with confirmed COVID-19 (serology and IFN-γ positive) without persistent symptoms (sampled at least 12 weeks after infection; n = 17) and patients with active systemic lupus erythematosus (n = 18) are shown. Each point corresponds to a single patient; the central bar shows the median with interquartile ranges. The red dotted line shows the lower limit of detection. Ns, non-significant; ***p < 0.001 versus all other groups by non-parametric Kruskal-Wallis test with Dunn’s correction for multiple testing
Biologic Evaluation
Biologic analyses (Supplementary File 1) were conducted at the same time as the clinical evaluation (152 days [IQR 102–164] after initial presentation). Routine biologic test results were within normal limits for all but one patient (an iron-deficiency anemia that was further investigated and corrected). Values for markers of cardiac and muscle injury (troponin and creatine phosphokinase) and coagulopathy (D-dimers, fibrinogen) were normal. Serology for HIV, hepatitis C virus and Lyme disease were negative for all patients.
Screening for autoimmunity revealed low (1/160) and medium (1/320 to 1/640) titers of anti-nuclear antibodies in 12 and 3 patients, respectively. Low to medium anti-nuclear antibody titers were numerically more prevalent in SARS-CoV-2 immunized than non-immunized patients (66.7% vs. 33.3%, p = 0.067). Screening for anti-extractable nuclear antigens, anti-double-stranded DNA, anti-citrullinated protein and anti-neutrophil cytoplasmic antibodies as well as rheumatoid factor was negative for all patients. Eight patients (4 in each immunization group) showed isolated low titers (< 3 times normal range) of anti-cardiolipin antibodies, with no history of thrombosis (IgM for 6, IgG for 2), and one patient was positive for lupus anticoagulant. After 12 weeks, the repeat antiphospholipid antibodies testing was negative for all patients.
Taken together, these extensive biologic studies were unremarkable, and multiplex cytokine and ultra-sensitive interferon-α2 measurements were similar between COVID-19 long-haulers and convalescent COVID-19 individuals without persistent symptoms. Using SARS-CoV-2 serology tests and IFN-γ ELISPOT, we found evidence of a previous SARS-CoV-2 infection in 50% (15/30) of post-acute COVID-19 syndrome patients, with evidence of a lack of immune response, or a waning immune response, in two patients.
Psychologic Evaluation
Psychologic evaluation was carried out by a phone interview with a clinical psychologist, at median 224 [202–238] days after initial symptom onset. In all, 10% (3/30) and 26.7% (8/30) of patients had a history of depression and anxiety disorders, respectively. Sleep issues were reported by 23 (73.3%) patients, and 4 (13.3%) had started an anxiolytic prescription (Table S1); 5 (16.7%) and 7 (23.3%) reported loss of employment and financial difficulties.
HADS screening for anxiety and depression was positive for 11 (36.7%) and 13 (43.3%) of patients, respectively (Table S1). Using the PCL-5 questionnaire, nine (30%) patients had scores compatible with PTSD (Fig. 4). These values did not differ between immunized and non-imunized groups of post-acute COVID-19 syndrome patients. Several components of the SF-36 scale, physical limitations, energy and pain, were severely affected, equally with no significant difference between patients immunized, or not, for SARS-CoV-2 (Figure S4). Family, friends and colleagues were a major source of support for 20 (66.7%), 18 (60%) and 11/25 (44%) patients, respectively. Conversely, 7 (23.3%) patients felt that physicians provided a significant level of support.
Prevalence of anxiety/depression disorders and post-traumatic stress syndrome in patients seeking medical help for persistent symptoms self-attributed to COVID-19. Total population (n = 30, gray bar), patients immunized to SARS-CoV-2 (positive for SARS-CoV-2 IFN-γ ELISPOT and at least one serologic assay, n = 15, red bar) and non-immunized (n = 15, blue bar) are shown. Data are mean and black bars show SEM
Discussion
In this study, we included 30 consecutive patients seeking medical help for persistent symptoms (median of 6 months) self-attributed to COVID-19. We identified two clinically comparable groups of post-acute COVID-19 syndrome patients: those with and those without SARS-CoV-2 immunity. In 93% of patients, persistent symptoms had a cyclical pattern and were mostly represented by fatigue, thoracic oppression, myalgia, paresthesia and burning pain, which agrees with the literature [20, 21]. We failed to find a significant difference in persistent clinical features between anti-SARS-CoV-2 immunized and non-immunized patients (Fig. 1b), and both groups were characterized by similar pain/fatigue indexes, quality of life, cytokines levels and psychologic burden. Additionally, patients with post-acute COVID-19 syndrome had similar initial clinical features and interferon-a2 levels compared to COVID-19-convalescent individuals who had experienced no persistent symptoms.
Unexpectedly, only half of post-acute COVID-19 syndrome patients had cellular (IFN-γ ELISPOT-based) and humoral immunity for SARS-CoV-2 (Fig. 2b). There are three possible (and non-exclusive) explanations for this result. First, some patients may have been infected with SARS-CoV-2, but not developed detectable immunity. As already described [22], one of our patients with RT-PCR–proven SARS-CoV-2 infection had a negative SARS-CoV-2 serology result on several occasions (36, 85 and 140 days after initial symptoms) as well as a negative IFN-γ ELISPOT (after 140 days). Second, immunity may have developed in some patients but subsequently waned over time, although such reports are discordant in the literature [23, 24]. In our cohort, one patient with RT-PCR/ELISPOT-confirmed infection presented waning IgG serology levels between days 133 and 251, both on anti-N (from equivocal to negative) and anti-S (from positive to equivocal) ELISA. Additionally, patients with cellular immunity might present negative SARS-CoV-2 serology tests, particularly with regard to anti-N IgG (Fig. 2a) [25]. Finally, some patients may have presented a non-specific viral illness and subsequent symptoms, which were falsely attributed to SARS-CoV-2. The first pandemic wave was a period of high anxiety for many people and may have exacerbated pre-existing psychologic conditions and induced a nocebo effect in some patients [26]. Although it could be argued that some of these patients may not, in fact, have initially developed COVID-19, we believe it is important to study these patients without any preconceived ideas, since they may represent a significant proportion of post-acute COVID-19 syndrome patients, at least in our cohort.
The high prevalence of probable anxiety and depressive disorders (36.7% and 43.3% respectively) is striking. A cohort study in the UK revealed that the incidence of probable anxiety and depressive disorders in the general population was 24% and 18.1%, respectively, during the pandemic [27]. A history of reported COVID-19 was associated with increased risk anxiety and depression. We also highlighted a high rate of probable PTSD (33.3%), which is similar to the 30.8% prevalence observed in a US study [28]. PTSD reflects the hardship of the patient’s experience with COVID-19. The persistence of physical symptoms (related or not to COVID-19) is likely associated with the psychologic burden of the pandemic, synergistically contributing to the emergence of the post-acute COVID-19 syndrome and may explain the high prevalence of psychologic disorders in our cohort.
Post-acute COVID-19 syndrome is an ill-defined condition characterized by symptoms persisting for at least 4 to 12 weeks after SARS-CoV-2 infection, depending on the study [11, 13]. It is crucial to exclude any alternative diagnoses before diagnosis of post-acute COVID-19 syndrome, especially as this latter does not necessarily require evidence of SARS-CoV-2 immunity [13, 20]. Deep phenotyping of our patients did not lead to alternative diagnoses for any patient. Although most authors studying post-acute COVID-19 syndrome focused on patients hospitalized with COVID-19 [29], a recent study including 958 patients with mild COVID-19 found persistent symptoms after 4 months in 27.8% of cases. This study, together with ours, suggests that post-acute COVID-19 syndrome may follow a mild initial infection in a high number of cases. The pathophysiologic mechanisms underlying post-acute COVID-19 syndrome are likely to be multifactorial in such patients, and physical deconditioning [30], psychologic disorders [31], viral encephalitis [7], dysautonomia [32] and autoimmunity are plausible suspects which could co-exist. Indeed, autoimmunity has been documented following COVID-19 [10, 33]. However, viral-induced autoimmunity is likely transient, as demonstrated by the negative repeat screening for antiphospholipid antibodies in eight initially positive patients. We did not find a significant increase in IFN-α2 or other proinflammatory cytokines between post-acute COVID-19 syndrome patients and convalescent individuals without persistent symptoms. The only difference was a significantly lower level of MCP-1 in post-acute COVID-19 syndrome patients, for which higher levels have been associated with severe disease and blood-brain barrier permeability [34, 35]. Further studies will be needed to explore other potential mechanisms at play in such patients (e.g., dysautonomia, neurologic studies).
This study has several limitations. First, our recruitment was limited to 30 consecutive patients, which may not allow capturing the complexity of patients with post-acute COVID-19 syndrome and thus limits its generalizability. However, this relatively small cohort allowed for an extensive multimodal evaluation without significant missing data. Additionally, the consecutive recruitment method limited potential selection bias. Given our study design, however, we cannot infer the prevalence of post-acute COVID-19 syndrome, which has been evaluated at 10% in the UK [11]. Second, only nine patients had a RT-PCR test in our cohort in the 5 weeks after symptom onset because RT-PCR tests were not readily available for mild disease during the first epidemic wave in France. To investigate for viral persistence at the time of clinical evaluation, we asked patients to make an appointment for nasopharyngeal and stool RT-PCR tests, which were only carried out by 60% and 20% of patients, respectively. These tests were negative and argue against viral persistence in clinically accessible sites. Finally, we cannot exclude that our design implied a selection bias of patients with psychologic distress. However, our study did allow to accurately and thoroughly evaluate multiple biologic and clinical characteristics of patients seeking medical advice for persistent symptoms self-attributed to post-acute COVID-19 syndrome and will therefore be of use for clinicians who may be under-prepared to answer these patients’ questions. Although no treatment has been approved for post-acute COVID-19 syndrome, more than half of our patients reported a spontaneous and gradual improvement of symptoms over time, which provides a window of hope for patients suffering from this disorder.
Conclusion
To conclude, our study sheds light on the burden experienced by patients reporting long-term symptoms self-attributed to COVID-19 as well as some of the mechanisms at play. A better recognition and understanding of post-acute COVID-19 syndrome will help healthcare providers to better care for these patients.
References
Carfì A, Bernabei R, Landi F, for the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603.
Living with Covid19 [Internet]. National Institute for Health Research; 2020 Oct [cited 2020 Nov 13]. Available from: https://evidence.nihr.ac.uk/themedreview/living-with-covid19/
Covid Long Haulers Describe the Devastating Aftereffects of the Disease. Bloomberg.com [Internet]. 2020 Nov 9 [cited 2020 Dec 5]; Available from: https://www.bloomberg.com/news/features/2020-11-09/coronavirus-long-haulers-tell-us-their-symptoms-and-the-aftereffects-of-disease
Long Covid: ‘Is this now me forever?’ [Internet]. the Guardian. 2020 [cited 2020 Dec 5]. Available from: http://www.theguardian.com/world/2020/nov/29/eleanor-morgan-is-still-struggling-with-long-covid-months-after-catching-the-virus
Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, et al. Prevalence of Inflammatory Heart Disease Among Professional Athletes With Prior COVID-19 Infection Who Received Systematic Return-to-Play Cardiac Screening. JAMA Cardiol [Internet]. 2021 Mar 4 [cited 2021 Jun 1]; Available from: https://jamanetwork.com/journals/jamacardiology/fullarticle/2777308
Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med [Internet]. 2021 May 5 [cited 2021 Jun 1];0(0). Available from: https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(21)00174-0/abstract
Pero A, Ng S, Cai D. COVID-19: a perspective from clinical neurology and neuroscience. Neuroscientist. 2020;26(5–6):387–91.
Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2020;174(4):576–8.
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021;397(10270):220–32.
Ramos-Casals M, Brito-Zerón P, Mariette X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat Rev Rheumatol. 2021;17(6):315–32.
Prevalence of long COVID symptoms and COVID-19 complications - Office for National Statistics [Internet]. [cited 2020 Dec 16]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/datasets/prevalenceoflongcovidsymptomsandcovid19complications
Yelin D, Wirtheim E, Vetter P, Kalil AC, Bruchfeld J, Runold M, et al. Long-term consequences of COVID-19: research needs. Lancet Infect Dis. 2020;20(10):1115–7.
Raveendran AV. Long COVID-19: Challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab Syndr Clin Res Rev. 2021;15(1):145–6.
Salje H, Kiem CT, Lefrancq N, Courtejoie N, Bosetti P, Paireau J, et al. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369(6500):208–11.
Cruccu G, Sommer C, Anand P, Attal N, Baron R, Garcia-Larrea L, et al. EFNS guidelines on neuropathic pain assessment: revised 2009: Neuropathic pain assessment. Eur J Neurol. 2010;17(8):1010–8.
Perrot S, Bouhassira D, Fermanian J. Development and validation of the Fibromyalgia Rapid Screening Tool (FiRST): Pain. 2010;150(2):250–6.
Rodero MP, Decalf J, Bondet V, Hunt D, Rice GI, Werneke S, et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J Exp Med. 2017;214(5):1547–55.
Roberge P. A psychometric evaluation of the French Canadian version of the Hospital Anxiety and Depression Scale in a large primary care population. J Affect Disord. 2013;9.
Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, et al. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychol Assess. 2016;28(11):1379–91.
Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;m3026.
Ladds E, Rushforth A, Wieringa S, Taylor S, Rayner C, Husain L, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020;20(1):1144.
Schwarzkopf S, Krawczyk A, Knop D, Klump H, Heinold A, Heinemann FM, et al. Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2–Specific IgG - Volume 27, Number 1—January 2021 - Emerging Infectious Diseases journal - CDC. [cited 2021 Jun 1]; Available from: https://wwwnc.cdc.gov/eid/article/27/1/20-3772_article
Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, et al. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383(11):1085–7.
Tonn T, Corman VM, Johnsen M, Richter A, Rodionov RN, Drosten C, et al. Stability and neutralising capacity of SARS-CoV-2-specific antibodies in convalescent plasma. Lancet Microbe. 2020;1(2):e63.
Gallais F, Velay A, Nazon C, Wendling M-J, Partisani M, Sibilia J, et al. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg Infect Dis. 2021;27(1):113–21.
Amanzio M, Howick J, Bartoli M, Cipriani GE, Kong J. How do nocebo phenomena provide a theoretical framework for the COVID-19 pandemic? Front Psychol. 2020;11:589884.
Kwong ASF, Pearson RM, Adams MJ, Northstone K, Tilling K, Smith D, et al. Mental health before and during COVID-19 in two longitudinal UK population cohorts. Br J Psychiatry. 2020;24:1–27.
Liu CH, Zhang E, Wong GTF, Hyun S, Hahm H “Chris”. Factors associated with depression, anxiety, and PTSD symptomatology during the COVID-19 pandemic: Clinical implications for U.S. young adult mental health. Psychiatry Res. 2020;290:113172.
Ghosn J, Piroth L, Epaulard O, Turnier PL, Mentré F, Bachelet D, et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect [Internet]. 2021 May 10 [cited 2021 May 20];0(0). Available from: https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(21)00147-6/abstract
Covid-19 will be followed by a deconditioning pandemic [Internet]. The BMJ. 2020 [cited 2021 Jan 3]. Available from: https://blogs.bmj.com/bmj/2020/06/15/covid-19-will-be-followed-by-a-deconditioning-pandemic/
Butler M, Pollak TA, Rooney AG, Michael BD, Nicholson TR. Neuropsychiatric complications of covid-19. BMJ. 2020 Oct 13;371:m3871.
Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med Lond Engl. 2021;21(1):e63–7.
Wang EY, Mao T, Klein J, Dai Y, Huck JD, Jaycox JR, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;19:1–9.
Jøntvedt Jørgensen M, Holter JC, Christensen EE, Schjalm C, Tonby K, Pischke SE, et al. Increased interleukin-6 and macrophage chemoattractant protein-1 are associated with respiratory failure in COVID-19. Sci Rep. 2020;10(1):21697.
Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, et al. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2005;25(5):593–606.
Acknowledgements
We thank all patients who agreed to take part in the study. We thank Valle Meyer for her precious help in planning the study and patient communication. We also thank COVID-HUS and Strasbourg University Hospital for their support.
Funding
No funding or sponsorship was received for this study or publication of this article. The Journal’s Rapid Service Fee was funded by the authors.
Editorial Assistance
Editorial assistance in the production of this manuscript was provided by Dr. Kate Dunning (Hôpitaux Universitaires de Strasbourg).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Marc Scherlinger, Renaud Felten, Jean Sibilia, Emmanuel Chatelus and Luc Pijnenburg recruited and evaluated clinically the patients. Floriane Gallais, Charlotte Nazon and Samira Fafi-Kremer conducted the virologic evaluations. Amaury Mengin, Adrien Gas and Pierre Vidailhet participated in the psychiatric evaluation. Rachel Arnould-Michel conducted the psychologic evaluation. Sabrina Bibi-Triki, Raphael Carapito, Sophie Trouillet-Assant, Magali Perre, Alexandre Belot and Seiamak Bahram conducted the immunologic assays (cytokines). Laurant Arnaud, Jacques Eric-Gottenberg helped in the design of the study and critically reviewed the manuscript. Marc Scherlinger, Renaud Felten, Floriane Gallais, Samira Fafi-Kramer and Jean Sibilia wrote the manuscript. Marc Scherlinger, Renaud Felten and Floriane Gallais are co-first authors. Samira Fafi-Kramer and Jean Sibilia are co-last authors.
Disclosures
Samira Fafi-Kremer has received speaking fees from BMS and Roche.
Compliance with Ethics Guidelines
Patients recruited in the present study provided written consent to participate. The study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. This study is part of the “COVID-HUS” study, which was approved by the Ethics Committee of the University Hospital of Strasbourg (NCE–2020–51). Individuals with a history of COVID-19 without persistent symptoms, were recruited as part of the Study “SeroCOVHUS”, ClinicalTrials.gov Identifier: NCT04441684, Ethical Committee authorization from SUD MEDITERRANEE III, Number 2020.04.15 bis_ 20.04.10.66856).
Data Availability
Data are available upon request to the corresponding authors.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Marc Scherlinger, Renaud Felten, Floriane Gallais, Samira Fafi-Kremer and Jean Sibilia participated equally in the study.
Supplementary Information
Below is the link to the electronic supplementary material.




Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Scherlinger, M., Felten, R., Gallais, F. et al. Refining “Long-COVID” by a Prospective Multimodal Evaluation of Patients with Long-Term Symptoms Attributed to SARS-CoV-2 Infection. Infect Dis Ther 10, 1747–1763 (2021). https://doi.org/10.1007/s40121-021-00484-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-021-00484-w