-
PDF
- Split View
-
Views
-
Cite
Cite
Ghady Haidar, Ashley Ayres, Wendy C King, Mackenzie McDonald, Alan Wells, Stephanie L Mitchell, Andrew L Bilderback, Tami Minnier, John W Mellors, Preprocedural SARS-CoV-2 Testing to Sustain Medically Needed Health Care Delivery During the COVID-19 Pandemic: A Prospective Observational Study, Open Forum Infectious Diseases, Volume 8, Issue 2, February 2021, ofab022, https://doi.org/10.1093/ofid/ofab022
Close - Share Icon Share
Abstract
We implemented a preprocedural severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) screening initiative designed to sustain health care during a time when the extent of SARS-CoV-2 infection was unknown.
This was a prospective study of patients undergoing procedures at 3 academic hospitals in Pittsburgh, Pennsylvania (April 21–June 11), and 19 community hospitals across Middle/Western Pennsylvania and Southwestern New York (May 1–June 11). Patients at academic hospitals underwent symptom screening ≤7 days preprocedure, then SARS-CoV-2 nasopharyngeal polymerase chain reaction (PCR) testing 1–4 days preprocedure. A subset also underwent day-of-procedure testing. Community hospital patients underwent testing per local protocols. We report SARS-CoV-2 PCR positivity rates, impact, and barriers to testing encountered through June 11. PCR positivity rates of optional preprocedural SARS-CoV-2 testing for 2 consecutive periods following the screening initiative are also reported.
Of 5881 eligible academic hospital patients, 2415 (41.1%) were tested (April 21–June 11). Lack of interest, distance, self-isolation, and nursing home/incarceration status were barriers. There were 11 PCR-positive patients (10 asymptomatic) among 10 539 patients tested (0.10%; 95% CI, 0.05%–0.19%): 3/2415 (0.12%; 95% CI, 0.02%–0.36%) and 8/8124 (0.10%; 95% CI, 0.04%–0.19%) at academic and community hospitals, respectively. Procedures were performed as scheduled in 40% (4/10) of asymptomatic PCR-positive patients. Positivity increased during subsequent coronavirus disease 2019 (COVID-19) surges: 54/34 948 (0.15%; 95% CI, 0.12%–0.20%) and 101/24 741 (0.41%; 95% CI, 0.33%–0.50%) PCR-positive patients from June 12–September 10 and September 11–December 15, respectively (P < .0001).
Implementing preprocedural PCR testing was complex and revealed low infection rates (0.24% overall), which increased during COVID-19 surges. Additional studies are needed to define the COVID-19 prevalence threshold at which universal preprocedural screening is warranted.
Preprocedural testing of patients for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has several theoretical advantages [1], such as preventing exposure to healthcare workers and reducing the risk of complications, particularly among patients undergoing complex procedures. However, published and practical experience remains limited. The American College of Surgeons does not recommend universal preprocedural SARS-CoV-2 testing [2], instead encouraging institutions to develop local policies based on testing availability, bed capacity, and community prevalence of coronavirus disease 2019 (COVID-19). Additionally, the Infectious Diseases Society of America (IDSA) provides nuanced and conditional guidelines for and against asymptomatic SARS-CoV-2 testing of patients undergoing procedures [3] based on community prevalence, the nature, complexity, and urgency of the procedure, and availability of testing and personal protective equipment (PPE). However, the authors of the IDSA guidelines acknowledge the dearth of studies addressing this issue [3].
The first COVID-19 cases in Allegheny County, where UPMC’s academic hub is located, were reported on March 11, 2020 [4]. UPMC operates 40 academic and community hospitals and >700 outpatient sites across Pennsylvania and neighboring states, where local public health guidance has discouraged the delivery of “elective” health care, defined as procedures that are not emergent/urgent or necessary to prevent death, preserve organ function, or avoid imminent harm from an underlying condition [5–7]. This guidance was conveyed on March 19, relaxed on April 27, and then re-enforced on November 23 in response to COVID-19 surges [5–7]. However, throughout 2020, UPMC Health System leadership recognized that most “elective” procedures are medically needed and are necessary to preserve health, improve quality of life, avoid delays in preventive care, and sustain health care systems and economies [1]. UPMC leadership also recognized the need to maintain health care delivery during a time when the frequency of asymptomatic SARS-CoV-2 infection was unknown.
Accordingly, UPMC leadership formed the COVID-19 Pre-procedural Task Force in April 2020 to implement SARS-CoV-2 testing of patients undergoing medically needed procedures, which were defined as procedures that physicians deemed necessary to be performed, regardless of whether they were “elective” or time-sensitive. The Task Force created a protocol for SARS-CoV-2 testing, oversaw the project, and collected data through June 11, after which the screening initiative was delegated to individual hospitals. On September 10, based on 5-month data showing consistently low polymerase chain reaction (PCR) positivity rates despite regional COVID-19 surges in the summer of 2020 [4, 8], hospital leadership discontinued universal preprocedural testing protocols, although physicians were still permitted to order preprocedural SARS-CoV-2 testing. Our objectives here are to describe the implementation of the screening initiative, address the extent to which it met our objectives, describe barriers encountered, and report temporal trends of preprocedural SARS-CoV-2 PCR positivity rates over an 8-month period during changes in COVID-19 incidence.
METHODS
Overview of Testing Time Periods
We report preprocedural SARS-CoV-2 PCR positivity rates across 3 periods: (1) April 21–June 11 (when the Task Force was overseeing the project), (2) June 12–September 10 (when individual hospitals were overseeing testing), and (3) September 11–December 15 (after universal testing was no longer recommended). These 3 periods also correspond to progressive increases in COVID-19 cases in Allegheny County (1056, 10 014, and 30 524 new cases, respectively) [4] and Pennsylvania (43 471, 63 415, and 366 435 new cases, respectively) [9].
Overview of Testing
April 21–June 11 (Academic Hospitals)
The initiative was launched at UPMC’s 3 academic hospitals on April 21. All academic hospital patients undergoing a procedure that the performing physician deemed medically necessary were contacted (or an attempt to contact was made) for testing through this protocol. Preprocedural PCR testing was voluntary, unless required by the physician performing the procedure; testing was mandatory for all patients undergoing organ transplant or cellular therapies for hematological malignancies. Testing was performed at UPMC’s Clinical Laboratory Improvement Amendments (CLIA)–certified lab with the following US Food and Drug Agency Emergency Use Authorization–approved assays for detection of SARS-CoV-2 RNA in nasopharyngeal swabs by reverse transcription PCR (RT-PCR): Cepheid Xpert Xpress SARS-Cov-2, CDC 2019-nCoV real-time RT-PCR Diagnostic Panel, and Abbott RealTime SARS-CoV-2 [10–12]. Specificity for all assays (per the manufacturers) is 100%. All assays are highly sensitive and comparable. The limit of detection (LOD) for the Cepheid Xpert Xpress SARS-CoV-2 is 0.0050 and 0.0200 PFU/mL for the nucleocapsid (N)-2 and envelope (E) targets, respectively. The LOD for CDC 2019-nCoV is 10 RNA copies/mL. The LOD for Abbott SARS-CoV-2 is 100 virus copies/mL (3.1 genomic equivalents/reaction).
We developed an algorithm for PCR testing 1–4 days preprocedure (with results in ≤24 hours), with a subset of PCR-negative patients randomly selected for repeat testing using the Cepheid platform on the day of the procedure (with results in ≤1 hour) to identify cases that converted from negative to positive (Table 1). We were unable to retest all patients on the day of the procedure with the Cepheid platform (our only “rapid” test) because of supply limitations. On a case-by-case basis, we permitted patients who had not been tested 1–4 days preprocedure to undergo day-of-procedure testing only.
Preprocedural SARS-CoV-2 Testing Workflow for Academic Hospitalsa (April 21–June 11, 2020)
| . | Testing Platform . | ≤7 Days Before Procedure . | 1–4 Days Before Procedure . | Day of Procedureb . | After Procedure . |
|---|---|---|---|---|---|
| Contact patient | X (phone) | ||||
| Symptom screenc | X (phone) | X (in-person) | X (in-person) | ||
| Assess for barriers causing patients to decline testingd | X (phone) | ||||
| NP swab PCR, (results in ≤24 h) | UPMC laboratory-developed assay adapted from the CDC assay after EUA [10] or Abbott RealTime SARS-CoV-2 assay [11] | X | Phone survey every 4 d for 14 d if PCR positive, starting on the date of the positive PCR | ||
| 2nd PCR for randomly selected subset (results in ≤1 h) | Cepheid Xpert Xpress SARS-CoV-2 test [12] | X |
| . | Testing Platform . | ≤7 Days Before Procedure . | 1–4 Days Before Procedure . | Day of Procedureb . | After Procedure . |
|---|---|---|---|---|---|
| Contact patient | X (phone) | ||||
| Symptom screenc | X (phone) | X (in-person) | X (in-person) | ||
| Assess for barriers causing patients to decline testingd | X (phone) | ||||
| NP swab PCR, (results in ≤24 h) | UPMC laboratory-developed assay adapted from the CDC assay after EUA [10] or Abbott RealTime SARS-CoV-2 assay [11] | X | Phone survey every 4 d for 14 d if PCR positive, starting on the date of the positive PCR | ||
| 2nd PCR for randomly selected subset (results in ≤1 h) | Cepheid Xpert Xpress SARS-CoV-2 test [12] | X |
Abbreviations: CDC; Centers for Disease Control; d, days; EUA, emergency use authorization; h, hours; NP, nasopharyngeal; PCR, polymerase chain reaction; UPMC, University of Pittsburgh Medical Center.
aCommunity hospitals developed their own symptom screening and PCR testing workflows. After June 11, academic hospitals adjusted the PCR testing algorithms based on their own infrastructure.
bAs the process evolved, we developed the capacity to test patients only on the day of procedure on a case-by-case basis. Same-day testing ability was limited.
cPatients were assessed for fever, cough, and shortness of breath and were asked to self-report other symptoms during preprocedural phone calls, at the PCR testing visit, and on the procedure day. Hospital staff were asked to record symptoms in a central database.
dFor patients who declined, hospital staff were instructed to ask open-ended questions about why testing was declined. The responses were then grouped into broad categories.
Preprocedural SARS-CoV-2 Testing Workflow for Academic Hospitalsa (April 21–June 11, 2020)
| . | Testing Platform . | ≤7 Days Before Procedure . | 1–4 Days Before Procedure . | Day of Procedureb . | After Procedure . |
|---|---|---|---|---|---|
| Contact patient | X (phone) | ||||
| Symptom screenc | X (phone) | X (in-person) | X (in-person) | ||
| Assess for barriers causing patients to decline testingd | X (phone) | ||||
| NP swab PCR, (results in ≤24 h) | UPMC laboratory-developed assay adapted from the CDC assay after EUA [10] or Abbott RealTime SARS-CoV-2 assay [11] | X | Phone survey every 4 d for 14 d if PCR positive, starting on the date of the positive PCR | ||
| 2nd PCR for randomly selected subset (results in ≤1 h) | Cepheid Xpert Xpress SARS-CoV-2 test [12] | X |
| . | Testing Platform . | ≤7 Days Before Procedure . | 1–4 Days Before Procedure . | Day of Procedureb . | After Procedure . |
|---|---|---|---|---|---|
| Contact patient | X (phone) | ||||
| Symptom screenc | X (phone) | X (in-person) | X (in-person) | ||
| Assess for barriers causing patients to decline testingd | X (phone) | ||||
| NP swab PCR, (results in ≤24 h) | UPMC laboratory-developed assay adapted from the CDC assay after EUA [10] or Abbott RealTime SARS-CoV-2 assay [11] | X | Phone survey every 4 d for 14 d if PCR positive, starting on the date of the positive PCR | ||
| 2nd PCR for randomly selected subset (results in ≤1 h) | Cepheid Xpert Xpress SARS-CoV-2 test [12] | X |
Abbreviations: CDC; Centers for Disease Control; d, days; EUA, emergency use authorization; h, hours; NP, nasopharyngeal; PCR, polymerase chain reaction; UPMC, University of Pittsburgh Medical Center.
aCommunity hospitals developed their own symptom screening and PCR testing workflows. After June 11, academic hospitals adjusted the PCR testing algorithms based on their own infrastructure.
bAs the process evolved, we developed the capacity to test patients only on the day of procedure on a case-by-case basis. Same-day testing ability was limited.
cPatients were assessed for fever, cough, and shortness of breath and were asked to self-report other symptoms during preprocedural phone calls, at the PCR testing visit, and on the procedure day. Hospital staff were asked to record symptoms in a central database.
dFor patients who declined, hospital staff were instructed to ask open-ended questions about why testing was declined. The responses were then grouped into broad categories.
At academic hospitals, staff contacting patients were instructed to ask about fever, cough, and dyspnea, to ask open-ended questions about other symptoms and why testing was declined, and to record these answers in a central database. Testing barriers were then grouped into categories. Table 1 and Figure 1 show full workflows. Procedure cancellation for PCR positivity or symptoms was left to the discretion of the physician performing the procedure. PCR-positive patients underwent a phone survey 4 times over 14 days from the date of the positive PCR test to assess 16 COVID-19 symptoms, exposures, and travel (Supplementary Table 1). We estimated that a sample size of ~2000 participants would provide 80% power to detect SARS-CoV-2 prevalence (95% CI) of 0.50% ± 0.29%, 0.25% ± 0.21%, or 0.125% ± 0.15% in a population of 1.2 million people [13]. Our sample size for academic hospitals was achieved in the second week of June 2020. Thus, as of June 12, we delegated the process to individual hospitals.
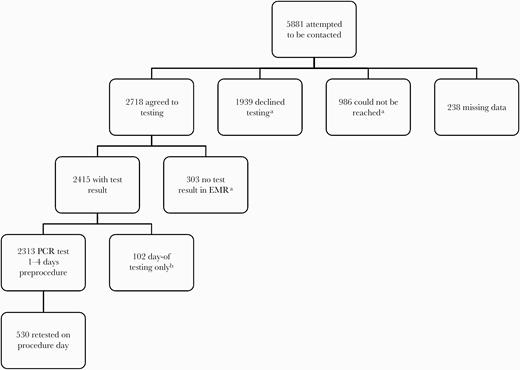
Participant flow in in academic hospitals (April 21–June 11, 2020). aThese groups make up the “not tested group” in Table 2. bThis group agreed to undergo testing 1–4 days preprocedure, but testing was not done (reason for lack of test not recorded). Thus, they underwent same-day testing only instead. Abbreviations: EMR, electronic medical record; PCR, polymerase chain reaction.
May 1—June 11 (Community Hospitals)
On May 1, 2020, PCR surveillance was expanded to 19 adult community hospitals (Western Pennsylvania [73.7%; n = 14], Central Pennsylvania [21%; n = 4], Southwestern New York [5.3%; n = 1]). Community hospitals created their own screening protocols and testing schedules and utilized their own PCR testing platforms based on local infrastructure and testing capacity. No repeat testing was done. Surveys for PCR-positive patients and recording of symptoms and barriers were not performed at community hospitals.
June 12–September 10 and September 11–December 15 (Summer and Fall Surge Periods)
Routine, universal PCR testing was continued across all 21 academic and community hospitals for 3 additional months (June 12–September 10), a period that included the July/August COVID-19 surge [4, 9]. After September 11, hospital leadership discontinued the recommendation to perform universal preprocedural PCR testing, but physicians were still allowed to order preprocedural SARS-CoV-2 PCR testing. Thus, we also report data for 3 months after the recommendation to perform universal testing was discontinued (September 11–December 15), which includes the November/December COVID-19 surge [4, 9]. No repeat testing was done during these periods.
The initiative was approved by UPMC’s Quality Review Committee. The percent PCR positive with 95% Clopper-Pearson exact confidence intervals was calculated. Kruskal-Wallis and χ 2 tests were used for comparisons by testing status among academic hospital patients during the initiative. The Cochran-Armitage Trend test was used to evaluate the positivity rate across the 3 time periods. Median income data by ZIP code were obtained from the US Census [14]. Statistical significance was set at a 2-sided P value <.05. Analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC, USA), and PASS, version 13.0.1 (NCSS LLC, Kaysville, UT, USA).
RESULTS
Detection of SARS-CoV-2 RNA
From April 21 to June 11, of 5881 eligible patients at academic hospitals, 2415 (41.1%) were tested at least once (Figure 1). Symptoms were recorded for 38.0% (817/2145) of academic hospital patients. Academic hospital patient characteristics by testing status, procedure types, and barriers are reported in Table 2. Colonoscopies/endoscopies and orthopedic, gynecological, and neurosurgical procedures were the most common procedure types for tested and nontested patients.
Demographic, Procedure, and Testing Information for Academic Hospitals (April 21–June 11, 2020) by Testing Status
| . | Tested . | Not tested . | P Value . |
|---|---|---|---|
| . | n = 2415a . | n = 3228a . | . |
| Age, median (IQR, range), y | 61 (47–69, 15–93) | 58 (43–68, 13–96) | <.0001 |
| Age group, No. (%) | <.0001 | ||
| <18 y | 6 (0.3) | 22 (0.7) | |
| 18–39 y | 388 (16.1) | 669 (20.7) | |
| 40–59 y | 730 (30.2) | 1049 (32.5) | |
| 60–79 y | 1164 (48.2) | 1305 (40.4) | |
| ≥80 y | 127 (5.3) | 183 (5.7) | |
| Sex, No. (%) | n = 3225 | ||
| Female | 1343 (55.6) | 1802 (55.8) | .84 |
| Race, No. (%)b | n = 2332 | n = 3041 | <.001 |
| White | 2066 (88.6) | 2579 (84.8) | |
| Black | 240 (10.3) | 432 (14.2) | |
| Asian | 22 (0.9) | 22 (0.7) | |
| Other | 4 (0.2) | 8 (0.3) | |
| Ethnicity, No. (%)b | n = 1896 | n = 2665 | |
| Hispanic | 15 (0.8) | 23 (0.9) | .78 |
| Nursing home resident or incarceration | 2 (0.08) | 27 (0.8) | <.0001 |
| Procedure, No. (%)c | <.0001 | ||
| Colonoscopy/endoscopy | 451 (18.7) | 627 (21.2) | |
| Orthopedic | 401 (16.6) | 309 (10.4) | |
| General surgery | 230 (9.5) | 411 (13.9) | |
| Gynecological | 166 (6.1) | 215 (7.3) | |
| Neurosurgical | 133 (5.5) | 164 (5.5) | |
| Urological | 122 (4.6) | 140 (4.7) | |
| Cardiac | 122 (4.6) | 131 (4.4) | |
| Breast surgery | 86 (3.6) | 31 (1.1) | |
| Ophthalmology | 78 (3.2) | 50 (1.7) | |
| Otolaryngology | 74 (3.1) | 80 (2.7) | |
| Obstetric | 53 (2.2) | 134 (4.5) | |
| Vascular | 35 (1.4) | 101 (3.4) | |
| Other (<3% each in both groups)d | 524 (21.7) | 548 (18.6) | |
| Symptoms 1–4 d before procedure, No. (%)e | n = 817 | Not available | Not applicable |
| No symptoms | 799 (97.8) | ||
| Shortness of breath | 9 (1.1) | ||
| Cough | 7 (0.9) | ||
| Headache | 2 (0.2) | ||
| Allergies | 1 (0.1) | ||
| Fatigue | 1 (0.1) | ||
| Reason preprocedural testing was not performed, No. (%)f | Not applicable | Not applicable | |
| Unable to reach patient | 986 (30.5) | ||
| Patient provided no reason | 462 (14.3) | ||
| Unknown; patient agreed to testing but not tested | 303 (9.4) | ||
| Patient not interested | 430 (13.3) | ||
| Distance from testing location | 427 (13.2) | ||
| Patient self-isolating | 220 (6.8) | ||
| Patient believed they were previously testedg | 97 (3.0) | ||
| Other commitments | 96 (3.0) | ||
| Lack of transport | 91 (2.8) | ||
| Patient believed they were not at risk for COVID-19 | 62 (1.9) | ||
| Patient was already hospitalized | 48 (1.5) | ||
| Nursing home residence or incarceration | 27 (0.8) | ||
| Fear of going to a testing center | 25 (0.8) | ||
| Currently symptomatic | 5 (0.2) | ||
| Other | 93 (2.9) |
| . | Tested . | Not tested . | P Value . |
|---|---|---|---|
| . | n = 2415a . | n = 3228a . | . |
| Age, median (IQR, range), y | 61 (47–69, 15–93) | 58 (43–68, 13–96) | <.0001 |
| Age group, No. (%) | <.0001 | ||
| <18 y | 6 (0.3) | 22 (0.7) | |
| 18–39 y | 388 (16.1) | 669 (20.7) | |
| 40–59 y | 730 (30.2) | 1049 (32.5) | |
| 60–79 y | 1164 (48.2) | 1305 (40.4) | |
| ≥80 y | 127 (5.3) | 183 (5.7) | |
| Sex, No. (%) | n = 3225 | ||
| Female | 1343 (55.6) | 1802 (55.8) | .84 |
| Race, No. (%)b | n = 2332 | n = 3041 | <.001 |
| White | 2066 (88.6) | 2579 (84.8) | |
| Black | 240 (10.3) | 432 (14.2) | |
| Asian | 22 (0.9) | 22 (0.7) | |
| Other | 4 (0.2) | 8 (0.3) | |
| Ethnicity, No. (%)b | n = 1896 | n = 2665 | |
| Hispanic | 15 (0.8) | 23 (0.9) | .78 |
| Nursing home resident or incarceration | 2 (0.08) | 27 (0.8) | <.0001 |
| Procedure, No. (%)c | <.0001 | ||
| Colonoscopy/endoscopy | 451 (18.7) | 627 (21.2) | |
| Orthopedic | 401 (16.6) | 309 (10.4) | |
| General surgery | 230 (9.5) | 411 (13.9) | |
| Gynecological | 166 (6.1) | 215 (7.3) | |
| Neurosurgical | 133 (5.5) | 164 (5.5) | |
| Urological | 122 (4.6) | 140 (4.7) | |
| Cardiac | 122 (4.6) | 131 (4.4) | |
| Breast surgery | 86 (3.6) | 31 (1.1) | |
| Ophthalmology | 78 (3.2) | 50 (1.7) | |
| Otolaryngology | 74 (3.1) | 80 (2.7) | |
| Obstetric | 53 (2.2) | 134 (4.5) | |
| Vascular | 35 (1.4) | 101 (3.4) | |
| Other (<3% each in both groups)d | 524 (21.7) | 548 (18.6) | |
| Symptoms 1–4 d before procedure, No. (%)e | n = 817 | Not available | Not applicable |
| No symptoms | 799 (97.8) | ||
| Shortness of breath | 9 (1.1) | ||
| Cough | 7 (0.9) | ||
| Headache | 2 (0.2) | ||
| Allergies | 1 (0.1) | ||
| Fatigue | 1 (0.1) | ||
| Reason preprocedural testing was not performed, No. (%)f | Not applicable | Not applicable | |
| Unable to reach patient | 986 (30.5) | ||
| Patient provided no reason | 462 (14.3) | ||
| Unknown; patient agreed to testing but not tested | 303 (9.4) | ||
| Patient not interested | 430 (13.3) | ||
| Distance from testing location | 427 (13.2) | ||
| Patient self-isolating | 220 (6.8) | ||
| Patient believed they were previously testedg | 97 (3.0) | ||
| Other commitments | 96 (3.0) | ||
| Lack of transport | 91 (2.8) | ||
| Patient believed they were not at risk for COVID-19 | 62 (1.9) | ||
| Patient was already hospitalized | 48 (1.5) | ||
| Nursing home residence or incarceration | 27 (0.8) | ||
| Fear of going to a testing center | 25 (0.8) | ||
| Currently symptomatic | 5 (0.2) | ||
| Other | 93 (2.9) |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; PCR, polymerase chain reaction.
aTest by nasopharyngeal swab PCR. n = 2145, unless a lower value is specified within the table due to missing data. The “not tested” group includes 986 patients who could not be contacted, 1939 who declined testing, and 303 who did not attend a scheduled test visit or for whom no test result was found in the electronic medical records.
bRace and ethnicity were self-reported. Race was set to missing for participants who did not self-report their race as 1 or more of the investigator-defined categories (ie, White, Black, Asian, American Indian/Alaska Native, Native Hawaiian/other Pacific Islander, mixed race). Race categories other than White, Black, and Asian were combined as other race due to low representation.
cProcedure types were available for all tested patients and 2950 not-tested patients.
dThe following procedures were performed in <3% of patients in both groups; numbers in parentheses indicate tested vs not tested, respectively: cardiothoracic (n = 69 vs n = 56), endocrine surgery (n = 65 vs n = 40), bronchoscopy (n = 56 vs n = 47), interventional radiology (n = 54 vs n = 83), living donor kidney or liver transplant (n = 44 vs n = 0), hematopoietic cell transplant or chimeric antigen modified T-cell therapy (n = 31 vs n = 0), biopsy (n = 26 vs n = 39), tumor resection (n = 13 vs n = 9), other (n = 166 vs n = 314).
eOf 2415 patients, symptoms were recorded for only 817 patients during their preprocedural testing visits on days 1–4 before the procedure. Two patients reported >1 symptom.
f125 patients reported >1 reason for declining testing.
gNo test results could be found for these patients.
Demographic, Procedure, and Testing Information for Academic Hospitals (April 21–June 11, 2020) by Testing Status
| . | Tested . | Not tested . | P Value . |
|---|---|---|---|
| . | n = 2415a . | n = 3228a . | . |
| Age, median (IQR, range), y | 61 (47–69, 15–93) | 58 (43–68, 13–96) | <.0001 |
| Age group, No. (%) | <.0001 | ||
| <18 y | 6 (0.3) | 22 (0.7) | |
| 18–39 y | 388 (16.1) | 669 (20.7) | |
| 40–59 y | 730 (30.2) | 1049 (32.5) | |
| 60–79 y | 1164 (48.2) | 1305 (40.4) | |
| ≥80 y | 127 (5.3) | 183 (5.7) | |
| Sex, No. (%) | n = 3225 | ||
| Female | 1343 (55.6) | 1802 (55.8) | .84 |
| Race, No. (%)b | n = 2332 | n = 3041 | <.001 |
| White | 2066 (88.6) | 2579 (84.8) | |
| Black | 240 (10.3) | 432 (14.2) | |
| Asian | 22 (0.9) | 22 (0.7) | |
| Other | 4 (0.2) | 8 (0.3) | |
| Ethnicity, No. (%)b | n = 1896 | n = 2665 | |
| Hispanic | 15 (0.8) | 23 (0.9) | .78 |
| Nursing home resident or incarceration | 2 (0.08) | 27 (0.8) | <.0001 |
| Procedure, No. (%)c | <.0001 | ||
| Colonoscopy/endoscopy | 451 (18.7) | 627 (21.2) | |
| Orthopedic | 401 (16.6) | 309 (10.4) | |
| General surgery | 230 (9.5) | 411 (13.9) | |
| Gynecological | 166 (6.1) | 215 (7.3) | |
| Neurosurgical | 133 (5.5) | 164 (5.5) | |
| Urological | 122 (4.6) | 140 (4.7) | |
| Cardiac | 122 (4.6) | 131 (4.4) | |
| Breast surgery | 86 (3.6) | 31 (1.1) | |
| Ophthalmology | 78 (3.2) | 50 (1.7) | |
| Otolaryngology | 74 (3.1) | 80 (2.7) | |
| Obstetric | 53 (2.2) | 134 (4.5) | |
| Vascular | 35 (1.4) | 101 (3.4) | |
| Other (<3% each in both groups)d | 524 (21.7) | 548 (18.6) | |
| Symptoms 1–4 d before procedure, No. (%)e | n = 817 | Not available | Not applicable |
| No symptoms | 799 (97.8) | ||
| Shortness of breath | 9 (1.1) | ||
| Cough | 7 (0.9) | ||
| Headache | 2 (0.2) | ||
| Allergies | 1 (0.1) | ||
| Fatigue | 1 (0.1) | ||
| Reason preprocedural testing was not performed, No. (%)f | Not applicable | Not applicable | |
| Unable to reach patient | 986 (30.5) | ||
| Patient provided no reason | 462 (14.3) | ||
| Unknown; patient agreed to testing but not tested | 303 (9.4) | ||
| Patient not interested | 430 (13.3) | ||
| Distance from testing location | 427 (13.2) | ||
| Patient self-isolating | 220 (6.8) | ||
| Patient believed they were previously testedg | 97 (3.0) | ||
| Other commitments | 96 (3.0) | ||
| Lack of transport | 91 (2.8) | ||
| Patient believed they were not at risk for COVID-19 | 62 (1.9) | ||
| Patient was already hospitalized | 48 (1.5) | ||
| Nursing home residence or incarceration | 27 (0.8) | ||
| Fear of going to a testing center | 25 (0.8) | ||
| Currently symptomatic | 5 (0.2) | ||
| Other | 93 (2.9) |
| . | Tested . | Not tested . | P Value . |
|---|---|---|---|
| . | n = 2415a . | n = 3228a . | . |
| Age, median (IQR, range), y | 61 (47–69, 15–93) | 58 (43–68, 13–96) | <.0001 |
| Age group, No. (%) | <.0001 | ||
| <18 y | 6 (0.3) | 22 (0.7) | |
| 18–39 y | 388 (16.1) | 669 (20.7) | |
| 40–59 y | 730 (30.2) | 1049 (32.5) | |
| 60–79 y | 1164 (48.2) | 1305 (40.4) | |
| ≥80 y | 127 (5.3) | 183 (5.7) | |
| Sex, No. (%) | n = 3225 | ||
| Female | 1343 (55.6) | 1802 (55.8) | .84 |
| Race, No. (%)b | n = 2332 | n = 3041 | <.001 |
| White | 2066 (88.6) | 2579 (84.8) | |
| Black | 240 (10.3) | 432 (14.2) | |
| Asian | 22 (0.9) | 22 (0.7) | |
| Other | 4 (0.2) | 8 (0.3) | |
| Ethnicity, No. (%)b | n = 1896 | n = 2665 | |
| Hispanic | 15 (0.8) | 23 (0.9) | .78 |
| Nursing home resident or incarceration | 2 (0.08) | 27 (0.8) | <.0001 |
| Procedure, No. (%)c | <.0001 | ||
| Colonoscopy/endoscopy | 451 (18.7) | 627 (21.2) | |
| Orthopedic | 401 (16.6) | 309 (10.4) | |
| General surgery | 230 (9.5) | 411 (13.9) | |
| Gynecological | 166 (6.1) | 215 (7.3) | |
| Neurosurgical | 133 (5.5) | 164 (5.5) | |
| Urological | 122 (4.6) | 140 (4.7) | |
| Cardiac | 122 (4.6) | 131 (4.4) | |
| Breast surgery | 86 (3.6) | 31 (1.1) | |
| Ophthalmology | 78 (3.2) | 50 (1.7) | |
| Otolaryngology | 74 (3.1) | 80 (2.7) | |
| Obstetric | 53 (2.2) | 134 (4.5) | |
| Vascular | 35 (1.4) | 101 (3.4) | |
| Other (<3% each in both groups)d | 524 (21.7) | 548 (18.6) | |
| Symptoms 1–4 d before procedure, No. (%)e | n = 817 | Not available | Not applicable |
| No symptoms | 799 (97.8) | ||
| Shortness of breath | 9 (1.1) | ||
| Cough | 7 (0.9) | ||
| Headache | 2 (0.2) | ||
| Allergies | 1 (0.1) | ||
| Fatigue | 1 (0.1) | ||
| Reason preprocedural testing was not performed, No. (%)f | Not applicable | Not applicable | |
| Unable to reach patient | 986 (30.5) | ||
| Patient provided no reason | 462 (14.3) | ||
| Unknown; patient agreed to testing but not tested | 303 (9.4) | ||
| Patient not interested | 430 (13.3) | ||
| Distance from testing location | 427 (13.2) | ||
| Patient self-isolating | 220 (6.8) | ||
| Patient believed they were previously testedg | 97 (3.0) | ||
| Other commitments | 96 (3.0) | ||
| Lack of transport | 91 (2.8) | ||
| Patient believed they were not at risk for COVID-19 | 62 (1.9) | ||
| Patient was already hospitalized | 48 (1.5) | ||
| Nursing home residence or incarceration | 27 (0.8) | ||
| Fear of going to a testing center | 25 (0.8) | ||
| Currently symptomatic | 5 (0.2) | ||
| Other | 93 (2.9) |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; PCR, polymerase chain reaction.
aTest by nasopharyngeal swab PCR. n = 2145, unless a lower value is specified within the table due to missing data. The “not tested” group includes 986 patients who could not be contacted, 1939 who declined testing, and 303 who did not attend a scheduled test visit or for whom no test result was found in the electronic medical records.
bRace and ethnicity were self-reported. Race was set to missing for participants who did not self-report their race as 1 or more of the investigator-defined categories (ie, White, Black, Asian, American Indian/Alaska Native, Native Hawaiian/other Pacific Islander, mixed race). Race categories other than White, Black, and Asian were combined as other race due to low representation.
cProcedure types were available for all tested patients and 2950 not-tested patients.
dThe following procedures were performed in <3% of patients in both groups; numbers in parentheses indicate tested vs not tested, respectively: cardiothoracic (n = 69 vs n = 56), endocrine surgery (n = 65 vs n = 40), bronchoscopy (n = 56 vs n = 47), interventional radiology (n = 54 vs n = 83), living donor kidney or liver transplant (n = 44 vs n = 0), hematopoietic cell transplant or chimeric antigen modified T-cell therapy (n = 31 vs n = 0), biopsy (n = 26 vs n = 39), tumor resection (n = 13 vs n = 9), other (n = 166 vs n = 314).
eOf 2415 patients, symptoms were recorded for only 817 patients during their preprocedural testing visits on days 1–4 before the procedure. Two patients reported >1 symptom.
f125 patients reported >1 reason for declining testing.
gNo test results could be found for these patients.
Out of 2313 academic hospital patients tested 1–4 days preprocedure, only 1 (0.04%; 95% CI, 0.00%–0.24%) was PCR positive. Of 530 initially PCR-negative patients retested on the day of the procedure, 2 (0.38%; 95% CI, 0.05%–1.36%) were PCR positive. All 3 PCR-positive academic hospital patients were asymptomatic at the time of testing. No patients (0%; 95% CI, 0.00%–3.55%) tested only on the day of the procedure (n = 102) were PCR positive. For community hospitals, 8 of 8124 (0.10%; 95 CI%, 0.04%–0.19%) patients were PCR positive (Supplementary Table 2); 1 was symptomatic and subsequently developed COVID-19 pneumonia. This was the sole patient who underwent D-dimer and C-reactive protein testing (both elevated). The other 7 community hospital patients did not develop symptoms of COVID-19 within 2 weeks after their first positive PCR test. Because of electronic medical record (EMR) differences, demographics from community hospitals are unavailable.
In total, we identified 11 PCR-positive patients (10 asymptomatic) (Supplementary Table 2) among 10 539 tested (0.10%; 95% CI, 0.05%–0.19%) across academic and community hospitals between April 21 and June 11. As the only patients who underwent repeat testing on the day of the procedure were academic hospital patients, we recalculated the PCR positivity rate after excluding the 2 PCR-positive cases that were only detected by repeat testing. The revised “single-test” preprocedural PCR positivity for academic and community hospitals across this time period remained low: 0.09% (9 PCR-positive patients out of 10 347 tested; 95% CI, 0.04%–0.17%).
Impact of PCR Testing and Symptom Assessment on Procedures
Procedures were canceled or deferred in 7 of 10 539 (0.07%) PCR-tested patients: all 3 PCR-positive academic hospital patients (3 of 2145 PCR-tested patients, 0.12%) and 4 of 8 PCR-positive community hospital patients (4 of 8124 PCR-tested patients, 0.05%) (Supplementary Table 2). Four asymptomatic PCR-positive community hospital patients underwent their procedures (dilation and curettage, C-section, dialysis catheter removal, and hip surgery) as scheduled due to the time-sensitive nature of the procedures (Supplementary Table 2). None of the PCR-positive patients in this study had a COVID-19-related emergency room visit, hospitalization, or death within 2 weeks after the positive test. Four of 11 (36.4%) PCR-positive patients were required by their physicians to undergo repeat PCR testing to document a negative result before undergoing their procedure.
Of 817 academic hospital patients whose symptoms 1–4 days preprocedure were recorded, 1.9% (n = 16) reported respiratory symptoms (Table 1). All were PCR negative, and none had procedures canceled.
Interpretation of Positive PCR Results in Asymptomatic Academic Hospital Patients
Cycle threshold (Ct) values (which were not reported in the EMR) and longitudinal telephone assessments were available for the 3 PCR-positive academic hospital patients (Supplementary Table2). We therefore examined the clinical and laboratory characteristics of patients 1–3 more closely to determine the significance of a positive PCR test in these 3 asymptomatic individuals.
Patient 1 recalled no prior COVID-19 symptoms, did not develop symptoms 14 days after her positive test, and was SARS-CoV-2 IgG and IgA seronegative (tested on the day of surgery). She had undergone PCR testing using the Cepheid platform; the E gene target was not detected, and the Ct value for the N2 gene was 42.2. Patient 2 recalled no prior COVID-19 symptoms but developed fatigue and myalgias 10 days after the positive test. The Ct value of his PCR test was 30.39 (1 value reported by the Abbott RealTime SARS-CoV-2 assay). He also underwent repeat PCR testing 14 and 27 days later (both negative), but antibody testing was not performed. Patient 3 recalled experiencing multiple COVID-19 symptoms (including cough, coryza, anosmia, and ageusia) 3 months prior but was not tested for COVID-19 at the time. She was SARS-CoV-2 IgG and IgA seropositive (day of surgery). She had undergone PCR testing using the Cepheid platform; the E gene target was not detected, and the Ct value for the N2 gene was 42.2. She underwent repeat testing on the same day and 6 days later and was PCR negative on both occasions.
Taken together, these data demonstrate weakly positive Ct values for all these patients and suggest that patient 1 had a false-positive PCR result, patient 2 had possible presymptomatic infection with atypical symptoms or false-positive PCR test [15], and patient 3 had chronic intermittent shedding of inert viral RNA [16, 17].
Barriers to Testing
In total, 54.9% (3228/5881) of academic hospital patients were not tested: 986 (16.8%) could not be reached due to inaccurate EMR contact information, and 1939 (33.0%) declined. Tested patients were older than nontested patients (median age, 61 vs 58 years, respectively; P < .0001), and a higher proportion were white (88.6% vs 84.8%; P < .001). Lack of interest in testing, distance from testing facility or transport issues, and perception of not being at risk for COVID-19 due to self-isolation were common reasons for declining (13.3%, 13.2%, 2.8%, and 1.9%, respectively). A minority of patients (0.8%) reported fear of acquiring SARS-CoV-2 infection at our testing sites as a reason for declining testing. Additionally, nontested patients were more likely to be nursing home residents or incarcerated compared with tested patients (0.8% vs 0.08%, respectively; P < .0001) (Table 2). Finally, we analyzed median annual income by ZIP code across the 2 groups and found that the median income was slightly higher in tested vs nontested patients ($51 900 vs $48 600, respectively; P < .0001).
PCR Positivity Rates During COVID-19 Surge Periods
Preprocedural PCR positivity rates progressively increased during the modest July/August surge and the more severe November/December COVID-19 surge [4, 8]: 54 of 34 948 patients were PCR positive between June 12 and September 10 (0.15%; 95% CI, 0.12%–0.20%), and 101 of 24 741 patients were PCR positive between September 11 and December 15 (0.41%; 95% CI, 0.33%–0.50%) (P < .0001 for comparison across the 3 time periods). The increase across these periods was unchanged even if the PCR results of patients who underwent repeat testing at academic hospitals between April 21 and June 11 were excluded. Overall, the preprocedural PCR positivity rates remained low throughout the entire 8-month study period: 166 PCR-positive patients out of 70 228 tested (0.24%; 95% CI, 0.20%–0.28%).
DISCUSSION
During a period of concern about an expanding pandemic, we successfully implemented universal SARS-CoV-2 PCR testing among patients undergoing medically necessary procedures during 3 consecutive phases of the COVID-19 pandemic. Even though infection during the entire study period was uncommon (0.24%), we observed increases in SARS-CoV-2 PCR positivity corresponding to regional increases in COVID-19 cases. Preprocedural PCR positivity was lowest between April and June (0.10%), mirroring low regional COVID-19 activity [4, 8, 9, 18], with 1056 and 43 471 new cases in Allegheny County and Pennsylvania, respectively. Preprocedural PCR positivity remained low but increased to 0.15% between June and September in the setting of modest regional COVID-19 surges in July–August 2020 (10 014 and 63 415 new cases in Allegheny County and Pennsylvania, respectively) [4, 9]. Finally, PCR positivity between September and December was greatest (0.41%), corresponding to the severe surges in November/December (30 524 and 366 435 new cases in Allegheny County and Pennsylvania, respectively) [4, 9]. Nonetheless, preprocedural PCR positivity for the entire 8-month period remained low (0.24%), which may be a result of patients taking precautions to avoid exposure to COVID-19 due to comorbidities or planned procedures, or of adherence to statewide stay-at-home orders or mandates for the donning of masks and face covers in public spaces, which were implemented on April 1 and 15, respectively [19]. A previous smaller study in pediatric presurgical patients also demonstrated low PCR positivity rates (<1%), but the clinical impact, complexities of implementation, barriers encountered, and longitudinal characterization of prevalence were not reported [20].
While testing through our initiative provided an estimate of asymptomatic SARS-CoV-2 shedding in this patient population, its impact on clinical care remains uncertain and it highlighted the challenges of large-scale PCR screening efforts of asymptomatic persons. Testing did provide physicians with the reassurance to perform procedures on PCR-negative patients irrespective of symptoms. However, while presymptomatic patients in particular may have high nasopharyngeal viral burdens [15], the performance of SARS-CoV-2 PCR testing in asymptomatic individuals is unknown [3, 21], and we are in agreement with the IDSA guidelines that confidence in negative PCR test results should be tempered by the possibility of false-negative results, both in asymptomatic and symptomatic persons [3]. Furthermore, despite the intuitive appeals of utilizing a targeted approach for asymptomatic preprocedural PCR testing based on the complexity, time sensitivity, and aerosol-generating potential of the procedure as recommended by the IDSA [3], the actual performance of PCR testing in asymptomatic patients is not expected to change with the nature of the procedure. Thus, institutions following a tiered approach for preprocedural SARS-CoV-2 PCR testing, in which (a) testing is only performed on patients undergoing major and time-sensitive procedures, (b) PPE alone (without testing) is used for other procedures [3], and (c) no PPE is used if a patient is PCR negative, must consider the risk of false-negative PCR results and develop plans for PPE allocation whenever possible, as recommended by the IDSA. Indeed, at our center, despite the fact that universal preprocedural SARS-CoV-2 PCR testing was discontinued in September because of overall low prevalence rates, hospital leadership continues to recommend universal PPE for all physicians performing procedures irrespective of PCR results or whether PCR testing was ordered, out of concern for false-negative results.
Health care workers must also be cognizant of the challenges of interpreting positive PCR results among individuals who are asymptomatic at the time of testing. These patients may have presymptomatic or asymptomatic SARS-CoV-2 infection that is capable of transmission [15], chronic RNA shedding due to resolved COVID-19 (that is not thought to be infectious) [16, 17], or even false-positive tests. Indeed, our symptom, Ct, and antibody data suggest that the positive PCR results of patients 1, 2, and 3 (all from academic hospitals) represented 1 false-positive PCR test, 1 presymptomatic infection or false-positive PCR test, and 1 case of chronic intermittent RNA shedding, respectively. If correct, procedures need not have been canceled for patients 1 and 3, and possibly not for patient 2. Unfortunately, ascertaining which category a patient fell under by conducting a careful interview and interpreting Ct values (which are not available clinically) was impractical in the busy preoperative setting, leading to deferral of procedures in these 3 patients plus 3/7 asymptomatic PCR-positive patients from community hospitals out of an abundance of caution. It should be noted that procedures were successfully performed on 4 of 10 asymptomatic PCR-positive patients with time-sensitive procedures, none of whom experienced any complications related to COVID-19 (Supplementary Table 2). However, these procedures were low risk for complications. Whether deferral of procedures for all asymptomatic SARS-CoV-2 PCR-positive patients is necessary or whether deferral should be restricted to subgroups of patients (such as those expected to undergo immunosuppression or procedures resulting in long lengths of stay, who may paradoxically experience more harm if their procedures are delayed) warrants further study. Additionally, while a Ct of >30 generally implies the presence of residual RNA or false-positive results [22], Ct values have not been validated for clinical decision-making, and thresholds for transmissibility have not been defined and may vary by platform. Preprocedural testing resulted in 4 patients being retested to document a negative result before rescheduling their procedures (Supplementary Table 2), but this practice may lead to long delays due to persistent RNA shedding [16, 17]. Finally, the positivity rate that included the results of retesting on the procedure day, which was performed to identify new cases of asymptomatic infection between the initial test and the procedure, while slightly higher than the positivity rate that only included 1-time testing, was also low, only detecting 2 PCR-positive cases out of 530 retested (Supplementary Table 2).
We encountered several challenges during implementation of this initiative. Only 41.4% of academic hospital patients were tested, primarily because of inaccurate EMR contact information that prevented us from contacting them. Distance from and lack of transport to our testing facilities were common barriers, which we eventually addressed by creating community-based testing sites. Lack of interest in testing, perception of not being at risk for COVID-19 due to self-isolation, and fear of being exposed to persons with COVID-19 at testing sites were also barriers. Black patients were less likely to be tested than White patients, which may be due to economic and/or health disparities [23]. Median income (based on ZIP codes) was slightly higher among patients who underwent testing, suggesting that socioeconomic status may be associated with willingness to be tested. Additionally, most nursing home residents and incarcerated patients, individuals known to have high rates of COVID-19 infection [15, 24], were not tested but underwent their procedures regardless.
The limitations of our study include a small PCR-positive cohort and missing symptom EMR, comorbidity, and PPE use data. We were unable to retest more patients on the day of the procedure due to testing capacity limits; our PCR positivity rate may have been higher had all patients undergone repeat testing on the day of the procedure. Nevertheless, preprocedural SARS-CoV-2 testing revealed low PCR positivity rates (0.24%) among asymptomatic patients undergoing medically necessary procedures, rates that increased in parallel with regional and nationwide COVID-19 surges [4, 8, 9]. Even though our study period captured the severe November/December COVID-19 surges [4, 9], our highest PCR positivity rate during the study period remained relatively low at 0.41%. Our findings may therefore not be applicable to “hotspots” experiencing even higher volumes of COVID-19 cases. Guidance from the IDSA suggests that a community prevalence threshold of 2% may be considered for asymptomatic screening of hospitalized (but not preprocedural) patients. However, the precise COVID-19 community prevalence threshold above which the benefits of broad-scale preprocedural testing outweigh any potential disadvantages related to logistics and test interpretation need to be defined.
The challenges of large-scale PCR testing should be considered by hospitals and organizations seeking to leverage the intuitive benefits of testing as they seek to “reopen.” Future studies should focus on defining community prevalence thresholds at which universal preprocedural testing is beneficial and determining whether Cts can be used for risk-stratifying PCR-positive preprocedural patients.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We would like to thank UPMC’s Executive Leadership and VPs of operation, unit directors, nurses, hospital staff, IT personnel, data analysts, UPMC communications, infection preventionists, and laboratory leadership and personnel, who were instrumental in operationalizing this initiative.
Financial support. The research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR001856, awarded to G.H.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interests. G.H. receives research funds from Karius. W.C.K. receives research funds from Abbott. J.W.M. is a consultant to Gilead Sciences and Merck and owns shares in Co-Crystal Pharmaceuticals, Infectious Disease Connect, and Abound Bio. The rest of the authors have no disclosures. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. The study does not include factors necessitating written patient consent. The design of the work was approved by the local ethical committee (UPMC Quality Review Committee, project # 2593) and conforms to standards currently applied in the United States.





Comments