Published online Dec 14, 2020. doi: 10.3748/wjg.v26.i46.7272
Peer-review started: October 11, 2020
First decision: November 3, 2020
Revised: November 6, 2020
Accepted: November 21, 2020
Article in press: November 21, 2020
Published online: December 14, 2020
The global incidence of coronavirus disease 2019 (COVID-19) continues to increase despite health care efforts. The disease is caused by coronavirus 2 with high transmission and mortality rates. Little is known about the management of COVID-19 in advanced liver disease. The aim of work was to propose a plan for management of this drastic disease in case of this specific population with review of medications that could be suitable for advanced liver disease. All the guidelines and medications available for treatment of COVID-19 were reviewed with selection of the less toxic medications that could be used in advanced liver disease. Drugs suitable to manage COVID-19 in patients with liver disease might include remdesivir intravenously, nitazoxanide + sofosbuvir, ivermectin, tocilizumab, convalescent plasma, and low molecular weight heparin in certain situations. Advanced liver disease is associated with portal hypertension and splenomegaly with reduction of blood elements and immune dysfunction and impaired T cell function. Thus, when confronted by cytokine storm as an immune response to COVID-19, there may be an increase in the mortality rate of these patients. Through this review, a plan to treat COVID-19 in this special group of patients with advanced cirrhosis is proposed.
Core Tip: The coronavirus disease 2019 (COVID-19) pandemic put the health care systems of many countries under great pressure, and it has been particularly challenging because of the lack of prediction parameters and effective pharmacotherapies for treating COVID-19 in advanced liver disease. Here, we provided a stepwise approach to treat COVID-19 in advanced liver disease, hoping for its application in a multicenter research trial.
- Citation: Hanafy AS, Abd-Elsalam S. Challenges in COVID-19 drug treatment in patients with advanced liver diseases: A hepatology perspective. World J Gastroenterol 2020; 26(46): 7272-7286
- URL: https://www.wjgnet.com/1007-9327/full/v26/i46/7272.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i46.7272
Serious viral infections are still evolving and are a significant health hazard, leading to unexpected and frequently occurring waves of viral epidemics in the previous decades. These include severe acute respiratory syndrome (SARS) by coronavirus (CoV) in 2002, H1N1 influenza in 2009, and the Middle East respiratory syndrome (MERS) CoV in Saudi Arabia in 2012.
In China, on December 31, 2019, an unexplained outbreak of pneumonia was attributed to a novel virus belonging to genus beta-coronavirus corona virus 2 (SARS-CoV-2); coronavirus disease 2019 (COVID-19). CoVs are single-stranded ribonucleic acid (RNA) viruses, crown-shaped due to the spiky appearance of their envelope induced by the arrangement of surface glycoproteins[1]. There are four subtypes of CoVs: Alpha-coronavirus, beta-coronavirus, delta-coronavirus, and gamma-coronavirus[2].
Viral subtypes that affect humans are 229E (alpha coronavirus), NL63 (alpha coronavirus), OC43 (beta coronavirus), and HKU1 (beta coronavirus); they can cause self-limited upper respiratory infections, and it was suggested that nearly 2% of the population are healthy carriers of a CoVs[3]. Other human CoVs, mainly beta CoVs, can cause epidemics with varying clinical severity including broncho-pulmonary and extra-pulmonary manifestations, with mortality rates approaching 35%. The newly isolated CoV showed more than 80% of nucleotide mimicry with bat SARS like CoV-ZXC21[4,5].
Patients with advanced cirrhosis have higher morbidity and mortality after exposure to such an aggressive form of viral infection due to inefficient immune function. The reported occurrence of acute hepatic insult with COVID-19 should be clarified and linked to the severity of COVID-19. In addition, it should be determined whether hepatic injury is due to overwhelming immune response, to direct viral invasion, or to the hepatotoxic effects of the utilized drugs in treatment.
Liver function can be evaluated by measuring biomarkers of liver cell injury mainly transaminases [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)], bile duct malfunction or bile stasis such as alkaline phosphatase, gamma-glutamyltransferase (GGT), and 5'-nucleotidase, hepatic secretion capacity by detecting serum bilirubin, and synthetic efficiency by measuring prothrombin time and serum albumin. Transaminases may be falsely elevated in COVID-19 due to complicating myositis, which is associated with increased creatinine kinase[6].
The cutoff values of ALT, AST, GGT, and serum total bilirubin that indicate abnormal liver function are > 40 U/L, > 40 U/L, > 49 U/L, and > 1 mg/dL respectively[7]. COVID-19 associated hepatic injury should be defined as a significant increase in hepatocellular and cholangiocytic markers mainly serum aminotransferases and bilirubin from the baseline level in healthy subjects or patients with a documented chronic liver disease during the active course of SARS-CoV-2.
The pattern of hepatic impairment in COVID-19 is hepatocellular when serum transaminases exceed more than three times the upper normal limit, cholangiocytic if alkaline phosphatase or GGT doubles the normal values, and mixed type if patients have both laboratory abnormalities[7]. Generally, liver function abnormalities are infrequent in mild cases of COVID, but it was noticed in severe cases and in cases with pre-existing liver disease.
There is a wide variation in reports of the incidence of abnormal levels of serum transaminases; ALT elevations ranged between 4%-33% in Chinese patients, and 39% in a large group from United States[8-10]. AST abnormalities were seen in 4%-53% of Chinese patients and up to 58% in a cohort of patients in the United States[8,11,12], and generally, AST and ALT elevations were mild, not exceeding five times the upper normal limit.
Abnormally elevated alkaline phosphatase was reported in up to 5% of patients[8,13], GGT was elevated in 13%-54% of patients with an average 23%[13,14], and total serum bilirubin elevations may be elevated in up to 18% of patients at admission[14,15]. The clinical significance of elevated liver function test biomarkers in COVID-19 still need to be clarified, as they may reflect the clinical severity of COVID-19[16,17]. However, some studies did not find any relation between these tests and mortality, intensive care unit admission, or duration of admission[18,19].
Advanced liver disease is associated with the upregulation of angiotensin-converting enzyme 2 (ACE2) as a physiological mechanism to counteract splanchnic vasodilatation, hepatic fibrosis, and portal hypertension mediated by angiotensin II. ACE2 catalyzes the conversion of angiotensin II to Ang-(1-7), which opposes angiotensin II through the Mas receptor[20]. At the same ACE2 also serves as a receptor for CoV, which is more numerous more in cholangiocytes than hepatocytes, which explains the bilopathic effect of COVID-19. This occurs with the help of trans-membrane serine protease 2, and as the oral and gastrointestinal mucosa express both ACE2 and transmembrane serine protease 2, they are commonly and early affected in COVID-19[21,22].
From the histopathological point of view, biopsies from deceased patients (n = 48) revealed no specific viral inclusion bodies. The main finding was endothelitis and fibrin microthrombi in liver sinusoids, massive dilation of portal venules with intraluminal thrombosis, and portal fibrosis; 15 samples out of 22 examined for SARS-CoV-2 RNA by RNA scope were positive, indicating that the liver effect was due to systemic inflammatory effect[23].
Mild COVID-19 is manifested by fever, malaise, cough, headache, myalgia, and diarrhea. Moderate COVID-19 is any symptom of mild disease and lung ultrasound features with hypoxemia on pulse oximetry. Severe COVID-19 is life threatening complications, mainly respiratory failure and the need for mechanical ventilation, denoted by presence of tachypnea > 30/min, oxygen saturation ≤ 93%, partial pressure of oxygen/fraction of inspired oxygen ≤ 300 mmHg, or evidence of other organ failure that needs intensive care unit admission[11].
Advanced liver disease is associated with portal hypertension and splenomegaly with reduction of blood elements and immune dysfunction and impaired T cell function. Thus, when the cytokine storm is confronted as an immune response to COVID-19, it may increase the mortality rate of these patients as well as the risk of hepatotoxicity that may occur by the utilized medications.
Treatment should be tailored according to the current hepatic status, whether non-cirrhotic liver or cirrhotic liver or liver transplantation, according to model for end-stage liver disease (MELD) score or Child Turcotte Pugh (CTP) score.
Remdesivir: A nucleoside analog that is changed into its active triphosphate metabolite that interferes with viral RNA polymerase and escapes proofreading by viral exoribonuclease without interfering with host RNA or deoxyribonucleic acid polymerases[24].
It has been used for the treatment of filoviruses such as Ebola and Marburg virus infections[25] with extended efficacy against other single-stranded RNA viruses such as respiratory syncytial virus, Lassa fever virus[26], pathogenic CoVs (MERS and SARS CoVs), and paramyxoviruses (Nipah and Hendra), with a promising effect on SARS-CoV-2[27].
Remdesivir significantly impacted the time to recovery of hospitalized patients[28]. Reversible grade 1 or 2 ALT or AST elevations were observed without abnormalities in other liver functions or renal function. Marked ALT/AST elevations were observed in 6% of patients and life-threatening elevations were present in 2%, mandating termination of treatment[29]. A report documented a sort of hepatocellular injury in the form of elevated transaminases that did not progress to severe liver damage or liver failure in patients without chronic liver disease, so it can be used with close monitoring of liver function[30].
Remdesivir was administered intravenously (IV, 200 mg) on the first day in adults within 30 min, followed by 100 mg within 1 h daily for 9-13 d depending on viral load[31].
Azithromycin: A semi-synthetic macrolide antibiotic that has a bacteriostatic effect against gram-positive and negative bacteria; it acts by inhibiting bacterial protein synthesis by binding to the 50S ribosomal subunit. It may have additional significant antiviral activity. In contrast to hydroxychloroquine, it has both in vitro and in vivo antiviral activity, mainly on respiratory syncytial virus and influenza viruses[32,33], with a report on significant antiviral effect on SARS-CoV-2[34]. The mechanism of the antiviral effect is by enhancing the immune response through stimulated production of interferon (IFN)-β and IFN-λ and their corresponding cytosolic genes involved in virus recognition, such as melanoma differentiation-associated protein 5 and retinoic acid-inducible gene I[35].
Regarding the liver, azithromycin can cause a benign type of cholestatic hepatitis, characterized by fever, fatigue, jaundice, pruritus, and eosinophilia. Rarely, the illness might be complicated with vanishing bile duct syndrome and liver cell failure requiring liver transplantation[36].
Doxycycline: A broad-spectrum tetracycline-class antibiotic that acts by chelating zinc on matrix metalloproteinases that are needed for survival, cell invasion, and replication of COVID-19. It inhibits viral serine protease; in addition, it has anti-inflammatory effects through down regulation of nuclear factor–kappa beta, decrease in levels of inflammatory cytokines such as tumor necrosis factor, interleukin (IL)-1b, and IL-6, and induction of apoptosis of mast cells[37]. Doxycycline is generally safe but on very rare occasions may be associated with vanishing bile duct syndrome[38].
Lopinavir/ritonavir (Kaletra 400/100 mg): It is a protease inhibitor, which is combined with ritonavir to increase its plasma half-life in the treatment of human immunodeficiency virus. With proven weak efficacy in SARS-CoV1, it should be started as early as possible after diagnosis[39].
High-dose of ritonavir (1200 mg/d) may cause severe hepatotoxicity, unless utilized in lower doses (200-400 mg) to boost other drugs[40]. A recent report showed increased levels of total bilirubin and GGT during hospitalization in patients treated with lopinavir/ritonavir (P < 0.004), so it should be used with great caution in patients with advanced liver disease.
In one study of patients admitted to hospital due to COVID-19, 1616 patients were randomly assigned to receive lopinavir/ritonavir and 3424 patients received usual treatment. Lopinavir–ritonavir treatment was not correlated with reductions in 28 d mortality, length of hospital stay, or reduced risk of progressing to mechanical ventilation or death[41].
Favipiravir (T-705) (Avigan): A broad-spectrum antiviral drug that was approved for the treatment of influenza in Japan. It is a pro-drug that is phosphorylated intracellularly to form the active metabolite favipiravir ibofuranosyl-5′-triphosphate and is then taken by viral RNA polymerase as a purine nucleotide, leading to effective inhibition of the RNA-dependent RNA polymerase. It has antiviral activity against Ebola, other RNA viruses such as West Nile virus, yellow fever virus, foot-and-mouth-disease virus, and Lassa virus[42].
A recent trial of Favipiravir (1600 mg twice in the first day then 600 mg twice daily on days 2-14) plus aerosolized IFN-α by inhalation (5 million U twice daily) and control arm patients who were treated with lopinavir/ritonavir 400 mg/100 mg twice daily for 14 d plus aerosolized IFN-α by inhalation (5 million U twice daily) demonstrated significantly shorter viral clearance time in the Favipiravir group (median 4 d vs 11 d) with fewer sides effects when compared to the control arm[43].
The identified sided effects of hyperuricemia and diarrhea were seen in 20% of the patients; neutropenia and transaminases elevation were in 2%, and rarely psychiatric symptoms. Overall, Favipiravir has a good safety profile[44].
It is recommended for mild to moderate COVID-19 at a dose of 1800 mg orally twice daily on day 1, followed by 800 mg orally twice daily, up to 14 d. It has a regional difference in pharmacokinetics; in patients from United States, it was 50% of that in Japanese patients. Therefore, the dose regimen should be judged by serum level if possible[45].
Nitazoxanide: Is an orally active nitrothiazolysalicylamide broad-spectrum antiparasitic and antiviral prodrug that is converted to the active metabolite tizoxanide that potentiates IFN-α and IFN-β production and interferes with the production of pro-inflammatory cytokines and IL-6, making it a potential candidate for COVID-19. At a dose of 600 mg twice daily[46], it has potential activity against hepatitis C virus (HCV)[47] and MERS CoV and other CoVs due to inhibition of the viral N protein expression[48]. It can be given safely in advanced liver disease; in addition, the metabolites are safe when compared to metronidazole. It may have a role in COVID with gastrointestinal symptoms, mainly diarrhea.
Sofosbuvir and daclatasvir: One of the non-structural proteins of human CoV is RNA dependent RNA polymerase (RdRp), which is an important enzyme for survival of RNA viruses, such as CoVs and HCV. Sofosbuvir and ribavirin are nucleotide derivatives that compete with the RdRp active site; so, when sofosbuvir is combined with ribavirin, it may have possible efficacy in treating COVID-19[49]. Daclatasvir has a binding strength to SARS-CoV-2 RdRp similar to that of remdesivir[50].
Patients who were diagnosed with moderate to severe COVID-19 (n = 66) were treated with hydroxychloroquine 200 mg twice daily with or without the combination of lopinavir plus ritonavir, 250 mg twice daily. Thirty-three patients randomized to the treatment group received the combination of sofosbuvir plus daclatasvir 460 mg once daily for 14 d, and the recovery at 14 d was higher in the sofosbuvir group (88% vs 67%) Median time to clinical recovery was significantly faster in the sofosbuvir group (6 vs 11 d; P = 0.041); SARS-CoV-2 viral loads, however, were not measured[51].
In another study, one group received ribavirin and the other received sofosbuvir /daclatasvir, and all had received the recommended standard treatment at that time (lopinavir/ritonavir and single-dose hydroxychloroquine). Median duration of hospital stay was 5 d for the sofosbuvir/daclatasvir group and 9 d for the ribavirin group. The mortality in the sofosbuvir/daclatasvir group was 6% and 33% for the ribavirin group, with encouraging preliminary results with this combination[52].
Ivermectin: A broad spectrum antihelmenthic commonly prescribed for oncocerciasis, lymphatic filariasis, and strongyloidiasis[53]. Recently, a proven antiviral effect was demonstrated against certain dengue fever, Japanese encephalitis, and tick-borne encephalitis virus. It was shown to reduce the SARS-CoV-2 viral load significantly in 48 h[54]. It acts by sequestering SARS-COV-2 viral nucleocapsid protein into the host nucleus through the nuclear-pore-complex, inhibiting host importin α/β transporter protein, which decreases translocation of SARS COV nucleocapsid protein from the cytoplasm to the nucleus, thus interfering with viral propagation and survival[54].
Ivermectin at a dose of 200 mcg/kg body weight maximum 12 mg BID, for 3-7 d, showed promising results both in terms of symptom index as well as viremia reduction[55]. Ivermectin is usually well tolerated, with mild and self-limited hepatic injury if rarely happening, acute or chronic liver dysfunction are not linked with ivermectin[56].
Interleukin inhibitors: They lessen the damage to lung tissue caused by cytokine release in serious COVID-19. The extensive release of IL-6, IL-1, IL-12, and tumor necrosis factor-α enhance pulmonary inflammatory response with increased alveolar-capillary gas permeability. The amount released is dose dependent. Still, there is insufficient data to recommend for or against use of IL-6 inhibitors[57], and the proper time of its use is not yet determined as some patients may develop worsening of symptoms. It should be given with marked elevation of C-reactive protein (CRP), IL-6, and ferritin levels with worsening respiratory symptoms.
Three recombinant monoclonal antibodies against IL-6 have been studied in COVID-19; tocilizumab (TOCI), sarilumab (SARI), and siltuximab. TOCI is a humanized monoclonal antibody (mAB) that binds to soluble and membrane-bound IL-6 receptors; SARI is a fully human anti-IL-6 receptor mAB. Siltuximab is a chimeric, human-murine mAB that binds to and neutralizes the human IL-6 cytokine not the receptor. The half-life of these agents can extend up to 30 d, with no noted dose adjustments for renal or hepatic disease. Toxicities include neutropenia, thrombocytopenia, elevated liver enzymes, rash, pruritus, septic shock, and intestinal perforation[58]. TOCI 8 mg/kg IV maximum 800 mg in two divided doses 12 h apart improved clinical outcome, decreased need for mechanical ventilation due to acute respiratory distress syndrome (ARDS), and lowered rate of death[59-63].
The global phase 3 trial investigating IV administered Kevzara® (SARI) at a dose of 200 mg or 400 mg in severely or critically ill patients hospitalized with COVID-19 did not meet its primary endpoint and key secondary endpoint when Kevzara was compared to placebo added to usual hospital care[64].
TOCI has minimal hepatic metabolism; liver injury may be due to effect on IL-6 pathway, which is important in liver regeneration. There are rare cases of jaundice and activation of hepatitis B virus, HCV, and cytomegalovirus, which should be tested before its use[65].
Convalescent plasma transfusion: Its use had been tried in previous viral outbreaks and pandemics, as convalescent plasma antibodies can limit the virus reproduction and help clear the virus. Convalescent plasma transfusion (CPT) may be considered for critically sick COVID-19 patients with better outcome; the optimal dose of CPT for COVID-19 is still to be determined, but it needs an antibody titer > 1/640. In addition, it may be of additional benefit in patients with advanced liver disease and hypoalbuminemia or ascites[66].
Five studies had included a total of 27 patients who received CPT therapy for COVID-19. The doses of CPT used as minimal as a single dose of 200 mL convalescent plasma with neutralizing antibody titers > 1:640 up to a maximum of 2400 mL in divided eight doses. Almost all the patients showed improvements of symptoms, including fever, size of lung lesions, ARDS, and weaning from mechanical ventilation but certain drawbacks were met, including the variable dose. CPT was not given alone but with other agents with possible synergistic effect[67-71].
Severe COVID-19 may be complicated with coagulopathy and disseminated intravascular coagulation, and the respiratory dysfunction may be due to microthrombosis of small pulmonary blood vessels. Inn nearly 30%, it may be due to disseminated intravascular coagulation, secondary antiphospholipid syndrome, complement activation, and endothelial dysfunction[72]. Patients who have the great benefit from anticoagulation are those with higher sepsis induced coagulopathy score ≥ 4 based on prothrombin time (1.2-1.4: score 1, > 1.4: score 2), platelet count (100-150: score1, < 100: score 2), sequential organ failure assessment (SOFA 1: score 1, ≥ 2: score 2)[73], or by another evaluation combining respiratory rate > 24, oxygen saturation < 90%, elevated C reactive protein, rising D-dimer levels, and elevated fibrinogen levels. Anticoagulation should be started[74].
Echinacea extract: They are rich in alkyl amides that activate macrophage activity and support healthy immune responses[75,76], modulate cytokine secretion, and support respiratory tract function and health[77]. Echinacea extract may have a potential role if supplied early in the course of the disease in a dose of 60 mg.
Zinc: Its role was shown by the inhibition of RNA synthesis by SARS-CoV infected cells with 2 µmol/L zinc. This inhibition was blocked when magnesium edetate was added as a zinc chelator. High zinc concentration inactivates p38 mitogen activated protein kinase, which is required for viral replication[78,79].
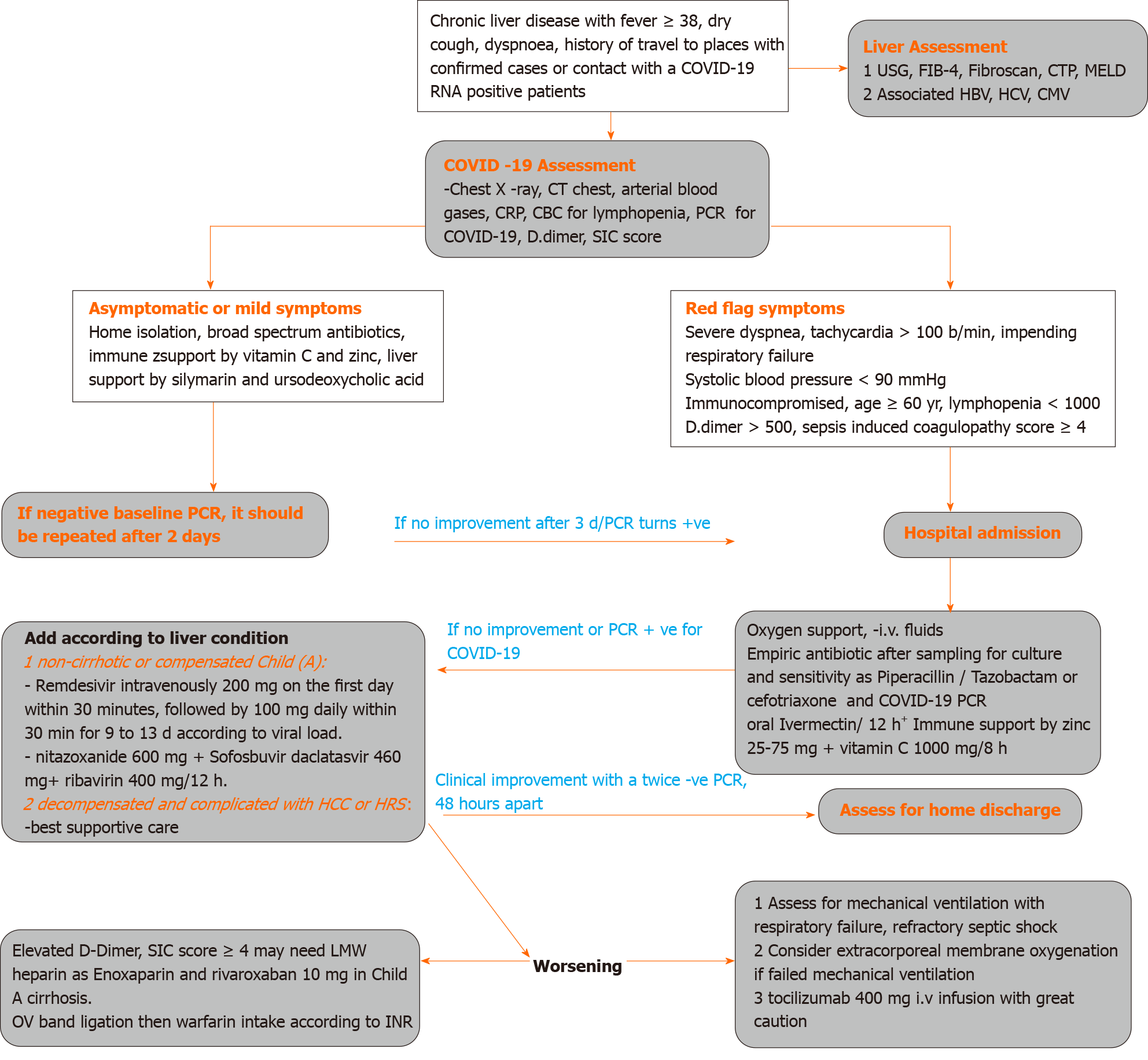
Based on our experience, guidelines for managing COVID-19, and the current knowledge of medications suitable for patients with advanced liver disease; we propose an approach to treat COVID-19 in patients with liver disease (Figure 1).
Any patient with suspected liver disease and COVID 19 infection should be evaluated regarding the severity of COVID-19 (mild-moderate-severe), presence of liver cirrhosis by Fibrosis 4, abdominal ultrasonography criteria, and fibroscan reading > 12.5 kPa, and severity of cirrhosis by CTP score and MELD score to evaluate the prognosis.
Severity of COVID should be evaluated as mild if manifested by acute onset of fever, malaise, cough, headache, myalgia, and diarrhea; moderate COVID-19 if any symptom of mild disease and lung ultrasound features with hypoxemia on pulse oximetry, and severe COVID-19 if life threatening complications, mainly respiratory failure and the need for mechanical ventilation.
COVID should be suspected if there is unsatisfactory response to antibiotics or there is a history of travel to places with confirmed cases, contact with a COVID-19 RNA positive patient, or even if there is unexplained deterioration of the patient’s general condition, including unexplained disturbed conscious level. Lung ultrasound may show thickened irregular pleural lines sub-pleural pulmonary consolidations, and high resolution computed tomography (CT) chest may show ground-glass opacities, with a rounded morphology and peripheral lung affection[80,81].
Non-cirrhotic patients, after judging by platelet count > 150/cm, ultrasonography, and fibroscan value < 12.5 kPa or patient with compensated cirrhosis Child Pugh class A and MELD score < 9 should be treated as normal subjects.
Patients with cirrhosis Child Pugh class B, fibroscan < 20 kPa, ultrasound documented absence of ascites, and MELD score < 10-29 should be given COVID therapy with very close monitoring of liver function.
Cirrhotic patients with Child Pugh class C, fibroscan > 20 kPa, ultrasound documented presence of ascites, MELD score > 30 with added additional morbidity such as hepatocellular carcinoma or hepatic encephalopathy should be given supportive therapy and medications with very minimal hepatic effect.
Favipiravir is changed into the hydroxylated form by aldehyde oxidase and xanthine oxidase, and in patients with Child Pugh C, there is a marked accumulation of theses metabolites. Thus, it should be reduced or avoided[82].
Lopinavir is degraded mainly by CYP3A, and ritonavir is an inhibitor of CYP3A. Therefore, hepatic impairment led to a 30% accumulation of lopinavir, leading to elevated liver enzymes and bilirubin with worsening of liver function[83].
Aerosolized IFN-α is by nature contraindicated in patients with autoimmune hepatitis or decompensated liver disease[84].
Remdesivir has a short plasma half-life with a faster systemic elimination, and it has low hepatocytotoxicity in vitro. It should be given with caution in hepatic impairment if benefit outweighs the potential risk. It should not be initiated in patients with ALT ≥ 5 times the upper limit of normal at baseline[85].
Patients with chronic liver disease due to active HCV with positive viremia and acute COVID can get benefit from treatment with sofosbuvir, daclatasvir, and ribavirin.
Patients with liver transplantation should have certain precautions. Special consideration should be taken into account when there was travel to a residence in a high-risk area, contact with a suspected case of COVID-19 ≤ 21 d, or contact with a confirmed case of COVID-19 ≤ 28 d before acute onset of fever > 38 °C, malaise or flu-like symptoms, new cough, shortness of breath, unexplained abdominal pain, nausea or diarrhea, or loss of sense of taste or smell.
Liver grafts recovered from COVID-19-positive deceased donors 21–28 d after symptom resolution and two negative swabs 24 h apart are considered safe for transplantation. High-risk donors with clinical symptoms within 28 d before organ procurement should not be considered, even if they test negative.
For living donors, 14-21 d self or hospital-based quarantining has been recommended. COVID-19-positive or high-risk living donors should postpone donation until at least 21-28 d after symptom resolution and until two negative polymerase chain reaction (PCR) tests have been observed. The recipient should undergo a real-time PCR test within 24 h of transplantation. Recipients with clinical suspicion or active SARS-CoV-2 infection should have transplantation deferred until 28 d after symptom resolution and after two negative tests at least 24 h apart[86].
A large Italian liver transplantation survey (n = 640) showed COVID-19 in only 1.25%, with the majority having mild disease[87]. However, the report showed bad outcome in long-term LT recipients, with higher risk of severe disease, denoting that immunosuppression did not increase the risk of severe COVID-19 and that metabolic complications that occurred on the long term run were responsible for the increased risk of severe COVID-19 in this population. In addition, immunosuppression may cause longer shedding time of the virus[87].
There is a postulated protective role of immunosuppression against systemic inflammation in COVID-19 and lung injury. In mild COVID, immunosuppression modifications are discouraged. On the other hand, reduction or discontinuation of antiproliferative agents and lymphocyte-depleting agents is indicated in severe lymphopenia and progressing pneumonia with secondary bacterial or fungal infection (e.g., by the American Association for the Study of Liver Diseases; see Related links). The interaction between COVID-19 treatments interaction with immunosuppressive drugs needs further studies[88].
Patients with liver cirrhosis and in need of upper endoscopy or endoscopic retrograde cholangiopancreatography should be left for emergency cases, mainly gastrointestinal bleeding or sepsis due to bacterial cholangitis, as the risk of infection is high due to droplet infection[89]. Endoscopy should be done with great caution and after complete protection of the medical staff, better in negative pressure chambers, and the medical staff should receive ivermectin for 3 d after the procedure.
The patients should be managed according to CTP score, MELD score, and fibroscan value and whether the liver is decompensated or not. Regarding anticoagulation, patients with ARDS or severe lung affection with significantly increased D-dimer should receive anticoagulation after securing variceal bleeding by band ligation and according to CTP score. Non-cirrhotic and cirrhotic CTP class A can be given enoxaparin then rivaroxaban 10 mg; however, decompensated cirrhosis with ascites may be given low dose enoxaparin and warfarin guided by prothrombin time and international normalized ratio values.
Fortunately, most pediatric patients develop mild COVID; however, rare cases may develop severe symptoms with multisystem affection[90]. Obesity is considered a risk factor for severe COVID in pediatric patients[91]. Red flag signs of disease progression are worsening tachypnea, disturbed conscious level, increasing serum lactate, bilateral lung infiltration, pleural effusion or rapid spread of the lesions, and associated morbidity as congenital heart disease, anemia, and severe malnutrition.
Multisystem inflammatory syndrome is characterized by rise of temperature more than 3 d and skin rash, mucocutaneous inflammatory affection, shock, coronary vasculitis, coagulopathy, diarrhea and abdominal pain, elevated CRP, or procalcitonin[92].
The treatment approach is tailored according to severity. In asymptomatic and mild cases, only antipyretics if needed (paracetamol 10-15 mg/kg), and non-steroidal anti-inflammatory drugs should be avoided. In severe or critical cases remdesivir (dose in children < 40 kg, in the first day, 5 mg/kg IV infused in 30 min) followed by 2.5 mg/kg IV day for 9 d, together with ivermectin, dexamethasone or methyl-prednisolone, oxygen therapy, adequate hydration, and caloric intake. Regarding anticoagulation, pediatric population have a much lower risk of thrombosis than adults, and prophylaxis is not supported except in neonates and adolescents, as the risk is higher especially in multisystem inflammatory syndrome or ARDS[93]. Empirical antibiotics are indicated with increased procalcitonin, CRP, and neutrophilic leucocytosis.
Patients should be isolated at home or hospitalized according to the severity of the clinical condition with great attention to the red flag signs as severe dyspnea, tachycardia > 100 beat/min, impending respiratory failure, systolic blood pressure < 90 mmHg, immunocompromised, and age ≥ 60 years.
Medications should be empirically given according to the clinical situation, drug availability, and till investigations are revealed, which should include chest X-ray, CT of the chest, arterial blood gases, CRP, complete blood count for lymphopenia, PCR for COVID-19, influenza RNA in respiratory secretions, rapid antigen tests for influenza viruses A and B, adenovirus, respiratory syncytial virus, para influenza virus, and varicella-zoster virus according to clinical circumstances.
Initial therapy is supportive by fluids and oxygen, broad spectrum IV antibiotic such as levofloxacin 750 once daily or cefotriaxone 2 g daily or piperacillin/ tazobactam 4.5 gm if bacterial superinfections were suspected + oral acyclovir 400/6 h + ivermectin 200 µg/kg and immune support by zinc gluconate 25-75 mg daily + vitamin C 1000 mg/12 h.
Liver function can be supported by adding silymarin 420 mg and ursodeoxycholic acid 500 mg daily, especially if viral infection is associated with rising transaminases and serum total bilirubin. Supportive treatment of ascites should be initiated if associated with signs of impending hepatorenal syndrome denoted by rising serum creatinine > 1.5 mg, serum cystatin c, prolonged international normalized ratio, and hypoalbuminemia. In suspected critically ill patients with hepatorenal syndrome, supportive therapy can be given as nor-epinephrine IV as a continuous infusion (0.5 to 3 mg/h) aiming to raise the mean arterial pressure by 10 mmHg; albumin is given for the first 2 d as an IV bolus (1 g/kg per day, 100 g maximum then 25-50 mg daily) or terlipressin given as an IV bolus (1 to 2 mg every 4-6 h) and albumin, as mentioned above, in addition to COVID medications as ivermectin, daclatasvir, and CPT.
Clinical improvement with negative PCR for COVID-19 RNA should be confirmed twice with a time interval of 48 h. However, if COVID-19 positivity was confirmed, patients should be hospitalized and with cautious selection of medications that will not worsen liver function, as mentioned above.
Monitoring with serum ferritin and CRP is important as higher values are associated with exacerbated COVID-19 or secondary bacterial infection, cytokine storm with multi-organ failure, and poor prognosis[94].
Patients should be transferred to intensive care unit if oxyhemoglobin saturation < 93%, respiratory rate > 30/min, heart rate > 120/min, or signs of organ failure were documented. CALL score was postulated to predict deterioration in COVID-19 pneumonia, which included associated co-morbidity. Older age > 60 years, lower lymphocyte count < 1000, and higher lactate dehydrogenase > 500 at presentation were independent high-risk factors for COVID-19 progression[95].
For patients with severe respiratory distress or significant pleural effusion on CT scan, a high-flow nasal oxygen or non-invasive mechanical ventilation would be provided to maintain positive end-expiratory pressure to guard against alveolar collapse and avoidance of volume overload by fluids. Clinical worsening despite these efforts necessitates invasive mechanical ventilation using lower tidal volume, lower inspiratory pressures, and higher positive end-expiratory pressure.
Indications of extracorporeal membrane oxygenation in COVID-19 are hypoxemic respiratory failure with partial pressure of oxygen/fraction of inspired oxygen < 100 mmHg despite optimization of mechanical ventilation, hypercapnic respiratory failure with an arterial pH < 7.20, cardiogenic shock refractory to treatment, and refractory septic shock with cardiac arrest.
The use of corticosteroid in patients with advanced liver disease should be avoided, but in case of a serious respiratory viral infection as COVID-19, methylprednisolone in a low dose of 40 mg/8 h may be considered, especially with increasing lung infiltrates and worsening clinical status even under invasive mechanical ventilation. However, its use still controversial and should be left to real-life clinical circumstances[96].
The use of IL-6 inhibitors as TOCI in advanced liver diseases should be judged according to clinical circumstances with great precaution. The use of anticoagulation in advanced liver disease should be based on the evaluation of risk factors as D-Dimer and sepsis induced coagulopathy score ≥ 4 with the use of low molecular weight heparin as enoxaparin or oral rivaroxaban 10 mg daily in non-cirrhotic or Child A cirrhosis. In decompensated cirrhosis, when there is a need of anticoagulation, endoscopy should be done to treat esophageal band ligation before commencing warfarin.
In summary, COVID-19 put the health care systems of many countries under great pressure, and it has been particularly challenging because of the lack of prediction parameters and effective pharmacotherapies for treating COVID-19 in advanced liver disease. We provided herein a stepwise approach to treat COVID-19 in advanced liver disease, hoping for its application in a multicenter research trial.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giordano P, Rawat K, Trifan A S-Editor: Zhang L L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1079] [Cited by in F6Publishing: 1107] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 2. | Chan JF, To KK, Tse H, Jin DY, Yuen KY. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21:544-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 3. | Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1873] [Cited by in F6Publishing: 1741] [Article Influence: 435.3] [Reference Citation Analysis (1)] |
| 4. | Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1813] [Cited by in F6Publishing: 1851] [Article Influence: 462.8] [Reference Citation Analysis (0)] |
| 5. | Bauch CT, Lloyd-Smith JO, Coffee MP, Galvani AP. Dynamically modeling SARS and other newly emerging respiratory illnesses: past, present, and future. Epidemiology. 2005;16:791-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19202] [Cited by in F6Publishing: 17990] [Article Influence: 4497.5] [Reference Citation Analysis (5)] |
| 7. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 623] [Cited by in F6Publishing: 580] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 317] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 9. | Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 676] [Article Influence: 169.0] [Reference Citation Analysis (0)] |
| 10. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium; Barnaby DP; Becker LB; Chelico JD; Cohen SL; Cookingham J; Coppa K; Diefenbach MA; Dominello AJ; Duer-Hefele J; Falzon L; Gitlin J; Hajizadeh N; Harvin TG; Hirschwerk DA; Kim EJ; Kozel ZM; Marrast LM; Mogavero JN; Osorio GA; Qiu M; Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6024] [Cited by in F6Publishing: 6161] [Article Influence: 1540.3] [Reference Citation Analysis (0)] |
| 11. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13871] [Cited by in F6Publishing: 12365] [Article Influence: 3091.3] [Reference Citation Analysis (1)] |
| 12. | Qian GQ, Yang NB, Ding F, Ma AHY, Wang ZY, Shen YF, Shi CW, Lian X, Chu JG, Chen L, Wang ZY, Ren DW, Li GX, Chen XQ, Shen HJ, Chen XM. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 2020;113:474-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 13. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1184] [Cited by in F6Publishing: 1219] [Article Influence: 304.8] [Reference Citation Analysis (4)] |
| 14. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 741] [Cited by in F6Publishing: 692] [Article Influence: 173.0] [Reference Citation Analysis (0)] |
| 15. | Huang Y, Tu M, Wang S, Chen S, Zhou W, Chen D, Zhou L, Wang M, Zhao Y, Zeng W, Huang Q, Xu H, Liu Z, Guo L. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: A retrospective single center analysis. Travel Med Infect Dis. 2020;36:101606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 16. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large US Cohort. Hepatology. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 211] [Article Influence: 52.8] [Reference Citation Analysis (2)] |
| 17. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Zhou J, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu L, Chen G, Li H, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 288] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 18. | Vespa E, Pugliese N, Piovani D, Capogreco A, Danese S, Aghemo A; Humanitas Covid-19 Task Force. Liver tests abnormalities in COVID-19: trick or treat? J Hepatol. 2020;73:1275-1276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, Li F, Xu Q, Zhang Y, Xu S, Song Z, Zeng Y, Shen Y, Shi Y, Zhu T, Lu H. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80:e1-e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 536] [Cited by in F6Publishing: 505] [Article Influence: 126.3] [Reference Citation Analysis (0)] |
| 20. | Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281-H2290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 294] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038-2040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 22. | Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518-1519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 954] [Cited by in F6Publishing: 908] [Article Influence: 227.0] [Reference Citation Analysis (1)] |
| 23. | Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-2116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 189] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 24. | Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 920] [Cited by in F6Publishing: 945] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 25. | Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, Van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, McMullan LK, Chen SS, Fearns R, Swaminathan S, Mayers DL, Spiropoulou CF, Lee WA, Nichol ST, Cihlar T, Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 965] [Cited by in F6Publishing: 1012] [Article Influence: 126.5] [Reference Citation Analysis (0)] |
| 26. | Lo MK, Jordan R, Arvey A, Sudhamsu J, Shrivastava-Ranjan P, Hotard AL, Flint M, McMullan LK, Siegel D, Clarke MO, Mackman RL, Hui HC, Perron M, Ray AS, Cihlar T, Nichol ST, Spiropoulou CF. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci Rep. 2017;7:43395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 322] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 27. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4155] [Cited by in F6Publishing: 3698] [Article Influence: 924.5] [Reference Citation Analysis (1)] |
| 28. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5013] [Cited by in F6Publishing: 4695] [Article Influence: 1173.8] [Reference Citation Analysis (0)] |
| 29. | Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 987] [Cited by in F6Publishing: 915] [Article Influence: 228.8] [Reference Citation Analysis (0)] |
| 30. | Zampino R, Mele F, Florio LL, Bertolino L, Andini R, Galdo M, De Rosa R, Corcione A, Durante-Mangoni E. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;14:881-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 31. | Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1216] [Cited by in F6Publishing: 1109] [Article Influence: 277.3] [Reference Citation Analysis (0)] |
| 32. | Tran DH, Sugamata R, Hirose T, Suzuki S, Noguchi Y, Sugawara A, Ito F, Yamamoto T, Kawachi S, Akagawa KS, Ōmura S, Sunazuka T, Ito N, Mimaki M, Suzuki K. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. J Antibiot (Tokyo). 2019;72:759-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 33. | Beigelman A, Isaacson-Schmid M, Sajol G, Baty J, Rodriguez OM, Leege E, Lyons K, Schweiger TL, Zheng J, Schechtman KB, Castro M, Bacharier LB. Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 2015; 135: 1171-8. e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 34. | Bleyzac N, Goutelle S, Bourguignon L, Tod M. Azithromycin for COVID-19: More Than Just an Antimicrobial? Clin Drug Investig. 2020;40:683-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 35. | Menzel M, Akbarshahi H, Tufvesson E, Persson C, Bjermer L, Uller L. Azithromycin augments rhinovirus-induced IFNβ via cytosolic MDA5 in experimental models of asthma exacerbation. Oncotarget. 2017;8:31601-31611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Moseley RH. Macrolide antibiotics. Hepatotoxicity of antimicrobials and antifungal agents. In: Kaplowitz N, DeLeve LD. Drug-induced Liver Disease. 3rd ed. Amsterdam: Elsevier, 2013: 466-467. [Cited in This Article: ] |
| 37. | Sodhi M, Etminan M. Therapeutic Potential for Tetracyclines in the Treatment of COVID-19. Pharmacotherapy. 2020;40:487-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 38. | Heaton PC, Fenwick SR, Brewer DE. Association between tetracycline or doxycycline and hepatotoxicity: a population based case-control study. J Clin Pharm Ther. 2007;32:483-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3386] [Cited by in F6Publishing: 3503] [Article Influence: 875.8] [Reference Citation Analysis (0)] |
| 40. | Sulkowski MS, Mehta SH, Chaisson RE, Thomas DL, Moore RD. Hepatotoxicity associated with protease inhibitor-based antiretroviral regimens with or without concurrent ritonavir. AIDS. 2004;18:2277-2284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 41. | RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 432] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 42. | Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 649] [Cited by in F6Publishing: 686] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 43. | Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y, Shen C, Li X, Peng L, Huang D, Zhang J, Zhang S, Wang F, Liu J, Chen L, Chen S, Wang Z, Zhang Z, Cao R, Zhong W, Liu Y, Liu L. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing). 2020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 702] [Cited by in F6Publishing: 738] [Article Influence: 184.5] [Reference Citation Analysis (0)] |
| 44. | Pilkington V, Pepperrell T, Hill A. A review of the safety of favipiravir - a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020;6:45-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 45. | Du YX, Chen XP. Favipiravir: Pharmacokinetics and Concerns about Clinical Trials for 2019-nCoV Infection. Clin Pharmacol Ther. 2020;108:242-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 242] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 46. | Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020;19:149-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1082] [Cited by in F6Publishing: 1058] [Article Influence: 264.5] [Reference Citation Analysis (0)] |
| 47. | Nikolova K, Gluud C, Grevstad B, Jakobsen JC. Nitazoxanide for chronic hepatitis C. Cochrane Database Syst Rev. 2014;CD009182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Rossignol JF. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health. 2016;9:227-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 49. | Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 443] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 50. | Beck BR, Shin B, Choi Y, Park S, Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Struct Biotechnol J. 2020;18:784-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 377] [Cited by in F6Publishing: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 51. | Sadeghi A, Ali Asgari A, Norouzi A, Kheiri Z, Anushirvani A, Montazeri M, Hosamirudsai H, Afhami S, Akbarpour E, Aliannejad R, Radmard AR, Davarpanah AH, Levi J, Wentzel H, Qavi A, Garratt A, Simmons B, Hill A, Merat S. Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial. J Antimicrob Chemother. 2020;75:3379-3385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 52. | Eslami G, Mousaviasl S, Radmanesh E, Jelvay S, Bitaraf S, Simmons B, Wentzel H, Hill A, Sadeghi A, Freeman J, Salmanzadeh S, Esmaeilian H, Mobarak M, Tabibi R, Jafari Kashi AH, Lotfi Z, Talebzadeh SM, Wickramatillake A, Momtazan M, Hajizadeh Farsani M, Marjani S, Mobarak S. The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19. J Antimicrob Chemother. 2020;75:3366-3372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 53. | Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376:1175-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 411] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 54. | Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1171] [Cited by in F6Publishing: 1218] [Article Influence: 304.5] [Reference Citation Analysis (0)] |
| 55. | Clinical Trials Registry- India Randomised controlled trial of ivermectin in hospitalised patients with COVID19. Available from: https://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=44196&EncHid=&userName=ivermectinCTRIUnique ID:CTRI/2020/06/026001. [Cited in This Article: ] |
| 56. | Veit O, Beck B, Steuerwald M, Hatz C. First case of ivermectin-induced severe hepatitis. Trans R Soc Trop Med Hyg. 2006;100:795-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 57. | Zhang X, Wu K, Wang D, Yue X, Song D, Zhu Y, Wu J. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-kappaB. Virology. 2007;365:324-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Bae SC, Lee YH. Comparison of the efficacy and tolerability of tocilizumab, sarilumab, and sirukumab in patients with active rheumatoid arthritis: a Bayesian network meta-analysis of randomized controlled trials. Clin Rheumatol. 2018;37:1471-1479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Michot JM, Albiges L, Chaput N, Saada V, Pommeret F, Griscelli F, Balleyguier C, Besse B, Marabelle A, Netzer F, Merad M, Robert C, Barlesi F, Gachot B, Stoclin A. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol. 2020;31:961-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 236] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 60. | Zhang X, Song K, Tong F, Fei M, Guo H, Lu Z, Wang J, Zheng C. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4:1307-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 61. | Cellina M, Orsi M, Bombaci F, Sala M, Marino P, Oliva G. Favorable changes of CT findings in a patient with COVID-19 pneumonia after treatment with tocilizumab. Diagn Interv Imaging. 2020;101:323-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 62. | Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92:814-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 834] [Cited by in F6Publishing: 854] [Article Influence: 213.5] [Reference Citation Analysis (0)] |
| 63. | Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117:10970-10975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1811] [Cited by in F6Publishing: 1695] [Article Influence: 423.8] [Reference Citation Analysis (0)] |
| 64. | Regeneron and Sanofi. (2020) Sanofi and Regeneron provide update on Kevzara® (sarilumab) Phase 3 U.S. trial in COVID-19 patients [Press release]. 1 September 2020. Available from: https://www.sanofi.com/en/media-room/press-releases/2020/2020-07-02-22-30-00. [Cited in This Article: ] |
| 65. | Hiura M, Abe S, Tabaru A, Shimajiri S, Hanami K, Saito K, Tanaka Y, Harada M. Case of severe liver damage after the induction of tocilizumab therapy for rheumatoid vasculitis. Hepatol Res. 2011;41:492-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020;92:479-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 674] [Cited by in F6Publishing: 699] [Article Influence: 174.8] [Reference Citation Analysis (1)] |
| 67. | Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, Peng C, Yuan M, Huang J, Wang Z, Yu J, Gao X, Wang D, Yu X, Li L, Zhang J, Wu X, Li B, Xu Y, Chen W, Peng Y, Hu Y, Lin L, Liu X, Huang S, Zhou Z, Zhang L, Wang Y, Zhang Z, Deng K, Xia Z, Gong Q, Zhang W, Zheng X, Liu Y, Yang H, Zhou D, Yu D, Hou J, Shi Z, Chen S, Chen Z, Zhang X, Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020;117:9490-9496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1251] [Cited by in F6Publishing: 1283] [Article Influence: 320.8] [Reference Citation Analysis (0)] |
| 68. | Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, Wei J, Xiao H, Yang Y, Qu J, Qing L, Chen L, Xu Z, Peng L, Li Y, Zheng H, Chen F, Huang K, Jiang Y, Liu D, Zhang Z, Liu Y, Liu L. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020;323:1582-1589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1580] [Cited by in F6Publishing: 1549] [Article Influence: 387.3] [Reference Citation Analysis (0)] |
| 69. | Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, Chen Q, Zhang L, Zhong Q, Zhang X, Zou Y, Zhang S. Treatment With Convalescent Plasma for Critically Ill Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Chest. 2020;158:e9-e13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 285] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 70. | Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, Jeong SJ, Kim JH, Ku NS, Yeom JS, Roh J, Ahn MY, Chin BS, Kim YS, Lee H, Yong D, Kim HO, Kim S, Choi JY. Use of Convalescent Plasma Therapy in Two COVID-19 Patients with Acute Respiratory Distress Syndrome in Korea. J Korean Med Sci. 2020;35:e149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 71. | Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, Xia X, Lv T. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 261] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 72. | Li T, Lu H, Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9:687-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 195] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 73. | Iba T, Levy JH, Warkentin TE, Thachil J, van der Poll T, Levi M; Scientific and Standardization Committee on DIC; and the Scientific and Standardization Committee on Perioperative and Critical Care of the International Society on Thrombosis and Haemostasis. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17:1989-1994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 322] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 74. | Atallah B, Mallah SI, AlMahmeed W. Anticoagulation in COVID-19. Eur Heart J Cardiovasc Pharmacother. 2020;6:260-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 75. | Cech NB, Kandhi V, Davis JM, Hamilton A, Eads D, Laster SM. Echinacea and its alkylamides: effects on the influenza A-induced secretion of cytokines, chemokines, and PGE₂ from RAW 264.7 macrophage-like cells. Int Immunopharmacol. 2010;10:1268-1278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Hudson JB. Applications of the phytomedicine Echinacea purpurea (Purple Coneflower) in infectious diseases. J Biomed Biotechnol. 2012;2012:769896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 77. | Šutovská M, Capek P, Kazimierová I, Pappová L, Jošková M, Matulová M, Fraňová S, Pawlaczyk I, Gancarz R. Echinacea complex--chemical view and anti-asthmatic profile. J Ethnopharmacol. 2015;175:163-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 543] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 79. | Kumar A, Kubota Y, Chernov M, Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med Hypotheses. 2020;144:109848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 80. | Fiala MJ. Ultrasound in COVID-19: a timeline of ultrasound findings in relation to CT. Clin Radiol. 2020;75:553-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 81. | Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology. 2020;295:202-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1827] [Cited by in F6Publishing: 1641] [Article Influence: 410.3] [Reference Citation Analysis (0)] |
| 82. | Madelain V, Nguyen TH, Olivo A, de Lamballerie X, Guedj J, Taburet AM, Mentré F. Ebola Virus Infection: Review of the Pharmacokinetic and Pharmacodynamic Properties of Drugs Considered for Testing in Human Efficacy Trials. Clin Pharmacokinet. 2016;55:907-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 83. | Peng JZ, Pulido F, Causemaker SJ, Li J, Lorenzo A, Cepeda C, García Cabanillas JA, DaSilva B, Brun SC, Arribas J. Pharmacokinetics of lopinavir/ritonavir in HIV/hepatitis C virus-coinfected subjects with hepatic impairment. J Clin Pharmacol. 2006;46:265-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Li L, Wang X, Wang R, Hu Y, Jiang S, Lu X. Antiviral Agent Therapy Optimization in Special Populations of COVID-19 Patients. Drug Des Devel Ther. 2020;14:3001-3013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 85. | Fact sheet for health care providers: emergency use authorization (EUA) of Remdesivir (GS-5734™). Available from: https://www.fda.gov/media/137566/download. [Cited in This Article: ] |
| 86. | Kumar D, Manuel O, Natori Y, Egawa H, Grossi P, Han SH, Fernández-Ruiz M, Humar A. COVID-19: A global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20:1773-1779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 162] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 87. | Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 88. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration; UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6366] [Cited by in F6Publishing: 6354] [Article Influence: 1588.5] [Reference Citation Analysis (0)] |
| 89. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 317] [Article Influence: 79.3] [Reference Citation Analysis (1)] |
| 90. | Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, Debray A, Basmaci R, Salvador E, Biscardi S, Frange P, Chalumeau M, Casanova JL, Cohen JF, Allali S. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 645] [Cited by in F6Publishing: 639] [Article Influence: 159.8] [Reference Citation Analysis (0)] |
| 91. | Nogueira-de-Almeida CA, Del Ciampo LA, Ferraz IS, Del Ciampo IRL, Contini AA, Ued FDV. COVID-19 and obesity in childhood and adolescence: a clinical review. J Pediatr (Rio J). 2020;96:546-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 92. | Venturini E, Montagnani C, Garazzino S, Donà D, Pierantoni L, Lo Vecchio A, Nicolini G, Bianchini S, Krzysztofiak A, Galli L, Villani A, Castelli-Gattinara G; Italian SITIP-SIP SARS-Cov-2 pediatric infection study group. Treatment of children with COVID-19: position paper of the Italian Society of Pediatric Infectious Disease. Ital J Pediatr. 2020;46:139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 93. | Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1289] [Cited by in F6Publishing: 1256] [Article Influence: 314.0] [Reference Citation Analysis (0)] |
| 94. | Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P, Chen G, Cheng G, Wang Y, Bi J, Tan L, Lau G, Qin E. Prediction for Progression Risk in Patients With COVID-19 Pneumonia: The CALL Score. Clin Infect Dis. 2020;71:1393-1399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 390] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 95. | Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46:837-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 355] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 96. | Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, Han Y, Hu B, Hu F, Li BH, Li YR, Liang K, Lin LK, Luo LS, Ma J, Ma LL, Peng ZY, Pan YB, Pan ZY, Ren XQ, Sun HM, Wang Y, Wang YY, Weng H, Wei CJ, Wu DF, Xia J, Xiong Y, Xu HB, Yao XM, Yuan YF, Ye TS, Zhang XC, Zhang YW, Zhang YG, Zhang HM, Zhao Y, Zhao MJ, Zi H, Zeng XT, Wang YY, Wang XH; for the Zhongnan Hospital of Wuhan University Novel Coronavirus Management and Research Team; Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 881] [Cited by in F6Publishing: 1109] [Article Influence: 277.3] [Reference Citation Analysis (0)] |