To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
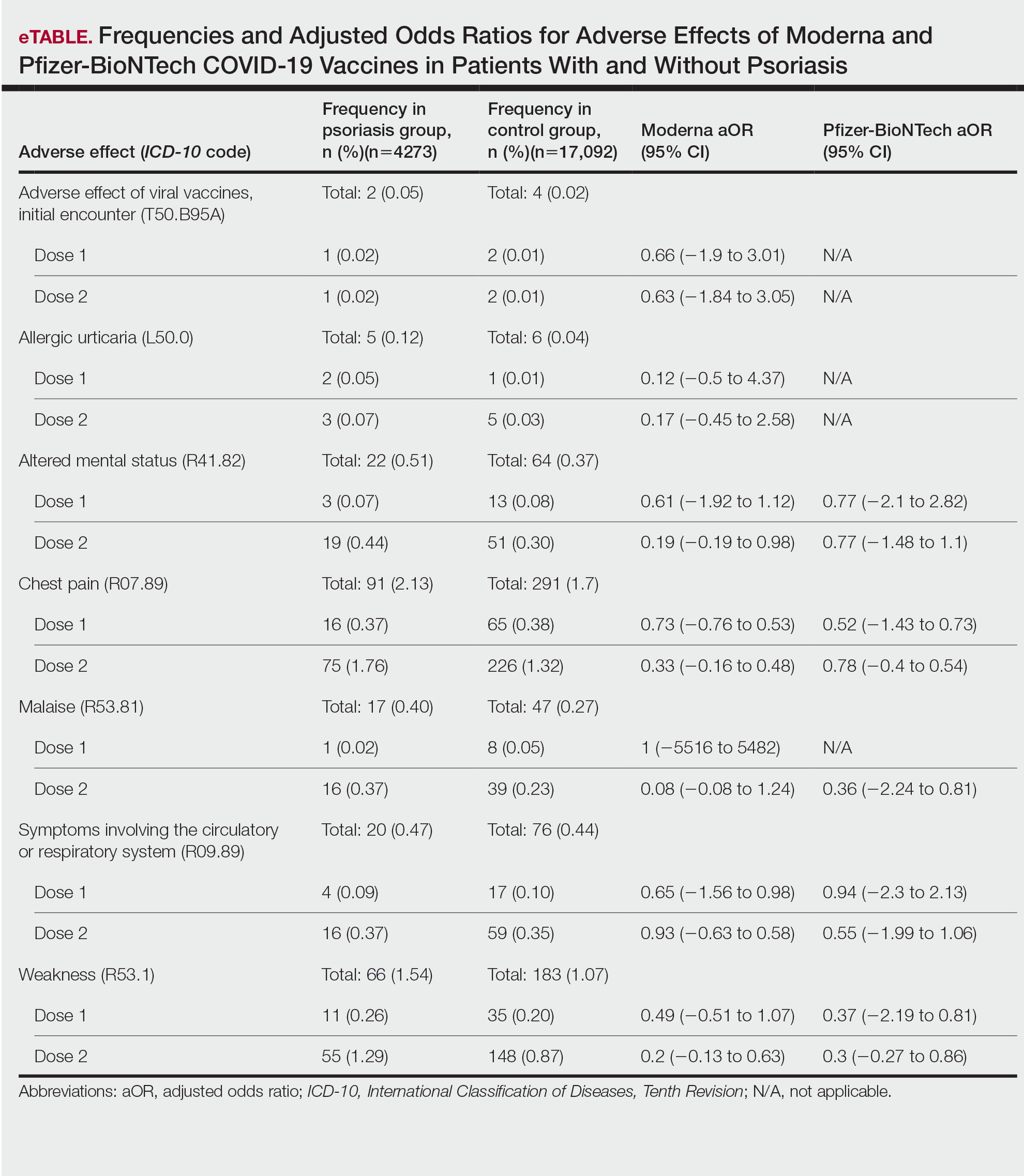
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.
This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.
The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.