The Role of Bioelectrical Impedance Analysis in Predicting COVID-19 Outcome
- 1Department of Internal Medicine, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia
- 2Cardiology Clinic, University Clinical Center Kragujevac, Kragujevac, Serbia
- 3Pulmonology Clinic, University Clinical Center Kragujevac, Kragujevac, Serbia
- 4Department of Pathophysiology, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia
- 5Department of Medical Statistics and Informatics, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia
- 6Ophthalmology Clinic, University Clinical Center Kragujevac, Kragujevac, Serbia
- 7Department of Ophthalmology, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia
- 8Department of Physical Medicine and Rehabilitation, University Clinical Center Kragujevac, Kragujevac, Serbia
- 9Department of Physical Medicine and Rehabilitation, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia
- 10Clinic of Endocrinology, Diabetes Mellitus and Metabolic Diseases, University Clinical Center Kragujevac, Kragujevac, Serbia
- 11Clinic of Rheumatology and Allergology, University Clinical Center Kragujevac, Kragujevac, Serbia
Background: Published data regarding the impact of obesity on COVID-19 outcomes are inconsistent. However, in most studies, body composition was assessed using body mass index (BMI) alone, thus neglecting the presence and distribution of adipose tissue. Therefore, we aimed to investigate the impact of body and visceral fat on COVID-19 outcomes.
Methods: Observational, prospective cohort study included 216 consecutive COVID-19 patients hospitalized at University Clinical Center Kragujevac (Serbia) from October to December 2021. Body composition was assessed using the BMI, body fat percentage (%BF), and visceral fat (VF) via bioelectrical impedance analysis (BIA). In addition to anthropometric measurements, variables in the research were socio-demographic and medical history data, as well as admission inflammatory biomarkers. Primary end-points were fatal outcomes and intensive care unit (ICU) admission.
Results: The overall prevalence of obesity was 39.3% according to BMI and 50.9% according to % BF, while 38.4% of patients had very high VF levels. After adjusting odds ratio values for cofounding variables and obesity-related conditions, all three anthropometric parameters were significant predictors of primary end-points. However, we note that % BF and VF, compared to BMI, were stronger predictors of both mortality (aOR 3.353, aOR 3.05, and aOR 2.387, respectively) and ICU admission [adjusted odds ratio (aOR) 7.141, aOR 3.424, and aOR 3.133, respectively].
Conclusion: Obesity is linked with COVID-19 mortality and ICU admission, with BIA measurements being stronger predictors of outcome compared to BMI use alone.
Introduction
Although most published studies refer to obesity as one of the independent predictors of disease severity and worse outcome in hospitalized COVID-19 patients (1–4), the results regarding mortality are still inconsistent. While some meta-analysis authors observed no significant relationship between obesity and COVID-19 mortality (5, 6), others emphasize such a relationship exists only in younger patients and those with fewer comorbidities (7, 8).
Potential mechanisms by which obesity adversely affects the course of SARS-CoV-2 infection are chronic inflammation and immune response dysregulation, endothelial dysfunction, increased thrombogenic potential, endocrine dysfunction, and the simultaneous presence of other known risk factors (such as cardiovascular disease, metabolic syndrome, and diabetes mellitus) (2, 4, 9).
Given that most of these pathophysiological mechanisms are the effect of adipose tissue (dominantly visceral), the main limitation of published studies is that body composition was assessed solely based on body mass index (BMI), without insight into the presence and distribution of adipose tissue. Moreover, several studies in which abdominal adipose tissue had been assessed using CT scan emphasized the importance of visceral adipose tissue, rather than subcutaneous, on COVID-19 severity and worse outcome (10).
For the reasons stated, it is valuable to examine the impact of body and visceral fat on the course and outcome of the novel coronavirus infection and their correlation with other significant predictors of disease severity, primarily inflammatory biomarkers. In addition, bioelectrical impedance analysis (BIA) measurements could be more precise than BMI in predicting the risk of mortality and worse outcome in hospitalized COVID-19 patients. To the best of the authors’ knowledge, this is the first study regarding BIA measurements and COVID-19 outcomes.
Materials and Methods
Study Population
The study was a part of the “COVID-19 admission PREDICTors of OUTCOME” (COVID-19 PREDICT OUTCOME) Registry, which was approved by the university’s Clinical Center Kragujevac (Serbia) Ethical Committee.
An observational, prospective cohort study included 216 consecutive COVID-19 patients hospitalized at University Clinical Center Kragujevac (Serbia) from October to December 2021. The patients were followed during the time of hospitalization. Inclusion criteria were adult age (>18 years old) and confirmed SARS-CoV-2 infection. Exclusion criteria were as follows: initial hospitalization at our Center for non-COVID pathology; pregnancy and the early postpartum period; impossibility to perform anthropometric measurements (i.e., poor general condition and severe deformities). In addition, for the reason that only one patient was underweight according to BMI and body fat percentage (%BF), that patient was excluded from further analysis.
Data Collection
The socio-demographic and medical history data were obtained using the patient’s medical record (Health Informational System, ComTrade, Serbia). Patients were tracked during the hospitalization period, and primary end points were the following: (I) in-hospital mortality, (II) ICU admission, and (III) primary end-point (implying fatal outcome and/or ICU admission) (11).
Within 24 h of admission, a routine laboratory was sampled from peripheral venous blood (complete blood count, biomarkers of inflammation, coagulation parameters, and cardiac biomarkers).
Anthropometric measurements were obtained via the BIA method. Using the TANITA BC-543 apparatus (Tanita Corporation, Tokyo, Japan), patients were measured within the first 72 h of hospitalization, according to the manufacturer’s instructions (barefoot, in light clothing, after the morning toilette, and before eating or drinking).
Anthropometric parameters of interest were:
(A) BMI, calculated using the formula: BMI [kg/m2] = BM [kg]/BH [m2], where BM is body mass expressed in kilograms (with0.1-kg precision), and BH is body height expressed in meters (with0.01 m precision). According to BMI values, patients were categorized as follows: (12). (I) underweight < 18.5 kg/m2; (II) normal weight 18.6–24.9 kg/m2; (III) overweight 25–29.9 kg/m2; (IV) Class 1 obesity 30–34.9 kg/m2; (V) Class 2 obesity 35–39.9 kg/m2; (VI) Class 3 obesity > 40 kg/m2.
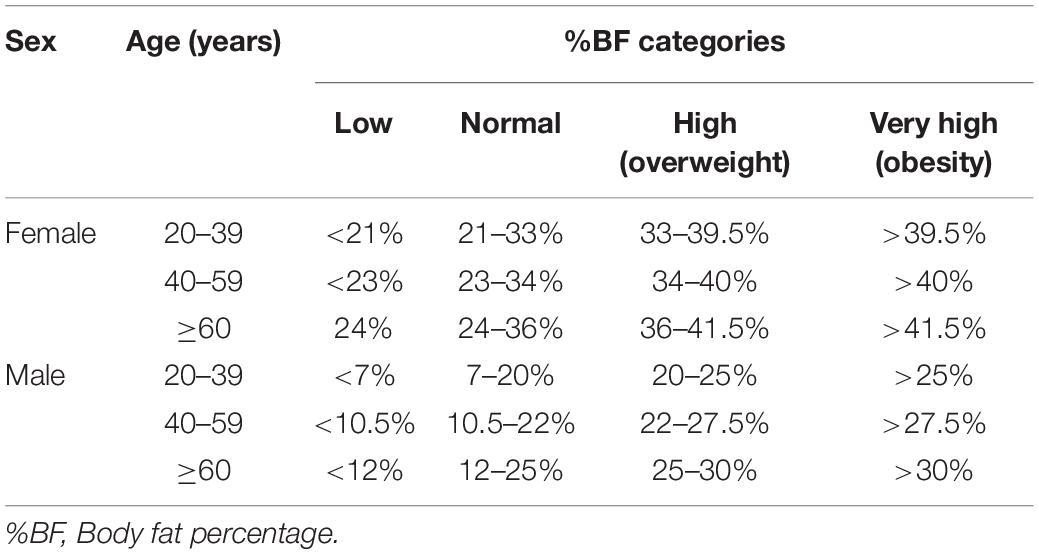
(B) % BF, expressed as a percentage of the total mass (with0.1% precision). According to % BF values, regarding age and sex, patients were categorized as follows (13): (I) Low % BF; (II) Normal % BF; (III) High % BF, (IV) Very high % BF (age and sex adjusted cut-off values are presented in Table 1).
(C) Visceral fat (VF) levels, according to which patients were categorized as follows (14): (I) Normal (1–9); (II) High (10–14); (III) Very high (≥15)
Statistical Analysis
Statistical analysis was performed using the IBM SPSS statistical package version 23 (IBM Corporation, Armonk, NY, United States). The relationship between continuous variables was tested using Spearman’s correlation. Cohen’s kappa coefficient was used in order to measure the level of agreement between different anthropometric measurements in terms of defining obesity. Univariate analysis separately compared anthropometric parameters and other variables with primary end-points. Categorical variables were compared using the χ2-test and continuous variables using the Mann-Whitney U test. After identifying the variables associated with end-points, uni- and multivariable binary logistic regression was performed. The strength of the relationship between examined variables and outcome was expressed as odds ratio (OR) belonging to 95% CI for univariate, and as adjusted OR (aOR) belonging to 95% CI for multivariate analysis. P-values < 0.05 were considered significant.
Results
Cohort Characteristics
Our cohort consisted of 216 adult patients with COVID-19 hospitalized at University Clinical Center Kragujevac (Serbia) from October to December 2021. The patient’s characteristics are presented in Table 2. The median age was 67 years, and the most frequent comorbidities were arterial hypertension, diabetes mellitus, and chronic kidney disease. In our cohort, 16.7% of patients had a fatal outcome, 33.8% required ICU admission, and 35.6% had experienced primary end-point (implying fatal outcome and/or ICU admission).

Table 2. Cohort characteristics regarding socio-demographic data, comorbidities, and data concerning disease course and outcome.
Anthropometric Measurements
In our cohort, 39.3% of patients were obese according to BMI, 50.9% had a very high level of % VF, and 57.9% had an excessive level of VF (Figure 1). We noted that older patients had significantly higher values of VF compared to those younger than 65 years, although older patients were less frequently obese according to both BMI and % BF. Regarding sex differences, we observe that women were more frequently obese according to BMI and % BF, whereas men had higher VF levels.
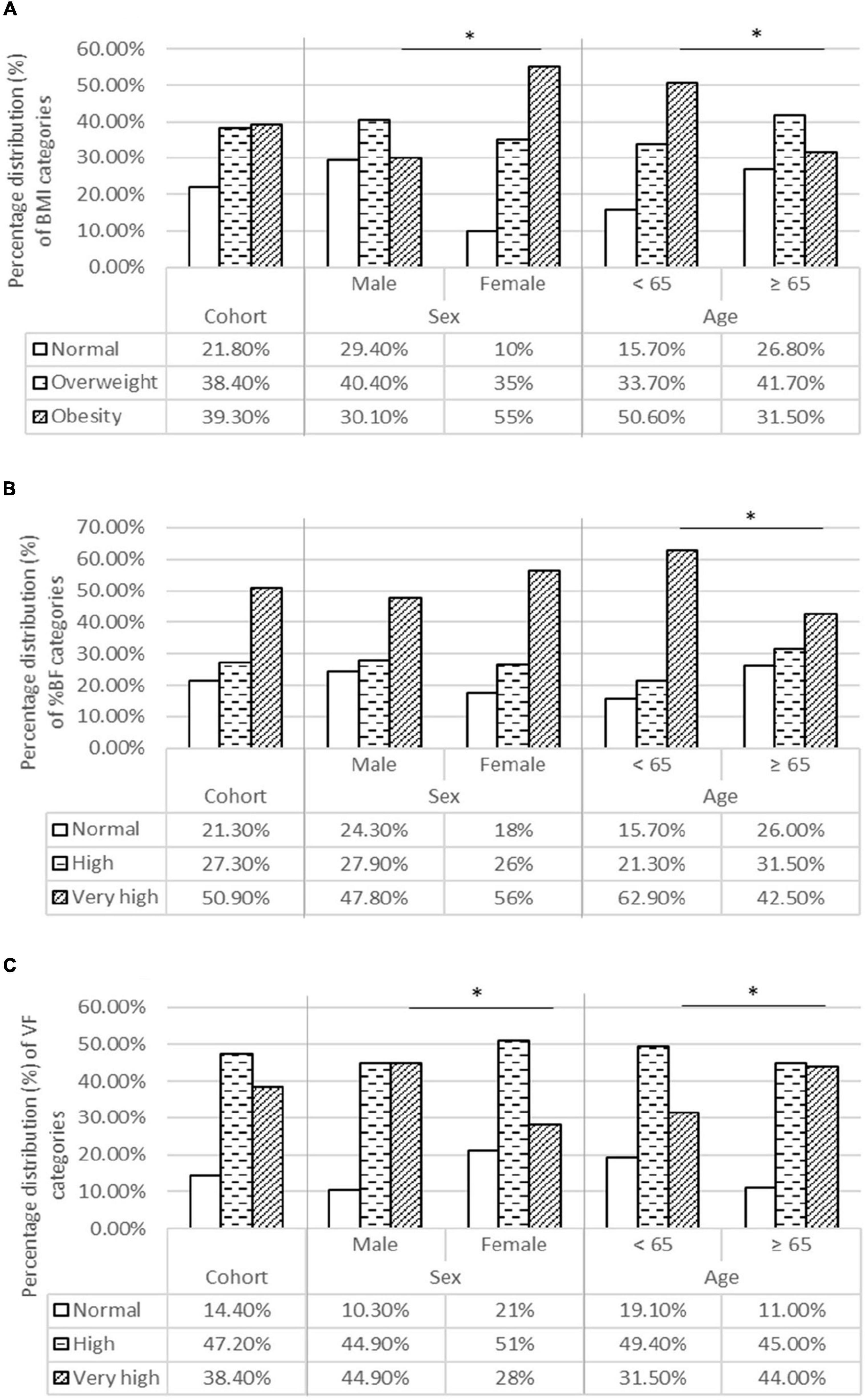
Figure 1. Body composition categories for BMI (A), BF% (B), and VF (C) in the entire cohort and for age and sex categories. (A) Percentage distribution of BMI categories. (B) Percentage distribution of % BF categories. (C) Percentage distribution of VF categories. BF %, Body fat percentage; BMI, Body mass index; VF, Visceral fat. *Statistical significance level is taken for “p” values below 0,05, using the χ2-test.
When comparing an agreement between BMI and % BF in terms of defining obesity, we found moderate agreement (kappa coefficient 0.543; p = 0.045) for three anthropometric categories (eutrophic/overweight/obesity) and good agreement (kappa coefficient 0.733; p = 0.045) when comparing two groups (obesity/no obesity). However, despite a good agreement between BMI and % BF in defining obesity, 24.5% (n = 27) of patients with very high % BF values were categorized as normal-/overweight according to BMI. It is important to point out the high incidence of mortality and ICU admission in this group of patients (24.8 and 55.6%, respectively).
Obesity and Inflammatory Biomarkers
Upon interpreting associations between predictive laboratory biomarkers on admission [including C-reactive protein (CRP), procalcitonin, interleukin-6 (IL-6), ferritin, lactate-dehydrogenase (LDH), and fibrinogen] and anthropometric parameters, no significant relationship was found regarding BMI. However, patients obese according to BF % had significantly higher serum levels of LDH (median values: 793.5 and 701, respectively; p = 0.024) compared to non-obese (including normal and overweight). More interestingly, patients with very high VF levels had significantly higher serum values of CRP (median values: 116.2 and 88.8, respectively; p = 0.014) and IL-6 (median values: 88 and 50.4, respectively; p = 0.028) compared to those with normal/high VF levels. Statistical significance for other biomarkers was not found (Supplementary Table 1).
Body Composition and Primary End-Points
Table 3 shows crude and adjusted OR for different anthropometric measurements in regard to predicting primary end-points. Initially, % BF and VF levels were significant predictors of mortality, while BMI, although borderline, lacked statistical significance. However, after adjusting OR for age, sex, days from symptom onset, and obesity-related comorbidities (diabetes mellitus), all three anthropometric measurements were statistically significant predictors of mortality, with % BF and VF being stronger predictors compared to BMI.
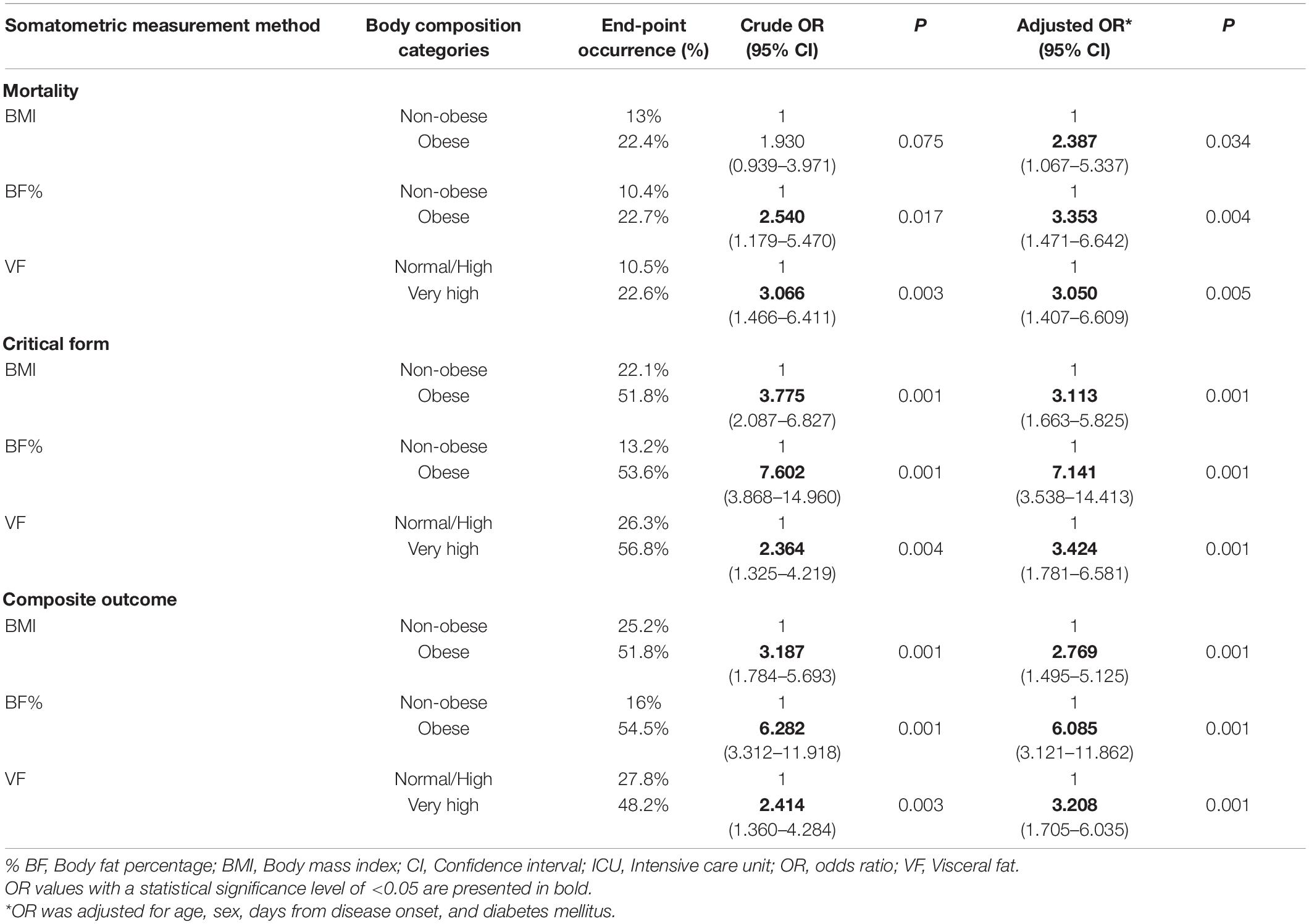
Table 3. Crude and adjusted OR (with 95% CI and “p” values) for different somatometric measurement methods in regard to primary end-points occurrence (mortality, ICU admission, and either primary end-point).
In predicting ICU admission and the development of either primary end-point, all three anthropometric measurements were significant predictors before and after adjustment. Similar to mortality prediction, % BF and VF had higher aOR compared to BMI.
The impact of socio-demographic characteristics and comorbidities on primary end-point occurrences are presented in Supplementary Table 2.
Discussion
The research was conducted on 216 patients with COVID-19 consecutively hospitalized at our Center between October and December 2021, in a period of the presumed predominance of SARS-CoV-2 delta variant in our country. The majority of hospitalized COVID-19 patients in our cohort had disturbed body composition, with only two out of ten hospitalized patients having normal BMI and % BF, and 14.4% of patients having normal VF levels. The shown disturbances of body composition in hospitalized COVID-19 patients are not unexpected. First, obesity is a globally raging pandemic whose consequences are also noticeable in Serbia. According to a WHO report from 2013, 58.6% of the adult population in Serbia were overweight or obese, and, according to a model at the time, the predicament was that the obesity prevalence in 2020 would be 44% in adult men and 31% in adult women (15). Second, several studies have demonstrated that obesity is a significant risk factor for hospital admission (3, 6, 16), therefore it is somewhat expected to have a high prevalence of obesity among hospitalized COVID-19 patients. We must note that only one patient (man, 70 years old) was underweight according to BMI and % BF; therefore, he was neglected in further analysis. Although some studies have shown an increased risk for death and the need for mechanical ventilation in underweight patients (17).
Regarding age categories, patients younger than 65 years had a higher prevalence of obesity according to BMI and % BF measurements. In contrast, older patients had significantly higher VF levels. We accentuate that the high VF levels are associated with numerous health disorders and general mortality (18, 19), along with worse outcomes and death in patients with COVID-19 (10, 20–23). Therefore, excessive VF levels could be one of the links associated with increased mortality and severity in older patients with COVID-19, among others (7, 16, 24, 25). Moreover, some studies advocate obesity as a risk factor for COVID-19 mortality and severity dominantly for younger patients, with weaker or no impact at all in older patients (5, 26, 27). Perhaps this could be a misconclusion, for cited studies have used BMI alone as a tool for accessing obesity, neglecting the significance of VF (7, 16, 24, 25).
In the initial analysis, only % BF and VF level were significant predictors of mortality, while BMI, although borderline, lacked statistical significance (Table 3). However, after adjusting OR for age, sex, days from disease onset, and obesity-related comorbidities (diabetes mellitus), all three anthropometric methods were significant predictors of mortality, with both % BF and VF having higher aOR values compared to BMI (aOR 3.353, aOR 3.05, and aOR 2.387, respectively). Results regarding ICU admission and experiencing either primary end-point were more concordant, where all three anthropometric measurement methods had significant predictive importance, with % BF (aOR 7.411 and 6.085, respectively) and VF (aOR 3.424 and 3.208, respectively) again having higher aOR values compared to BMI (aOR 3.113 and 2.769, respectively).
We must note that comorbidities selection for OR adjustment was arbitrary, and diabetes mellitus was chosen as a known obesity-related condition. Furthermore, a different selection of “adjusting” variables in a model (in addition to age and gender) did not significantly alter the aOR and “p” values of either anthropometric measurement. In a sensitivity analysis (Supplementary Table 3), conducted by implementing all socio-demographic and medical history data in a model, all three anthropometric measurements remained significant predictors of primary end-points, with % BF having an increase of aOR at the expense of a wider confidence interval range. In addition to anthropometric parameters, the model showed a significant predictive value of the Charlson comorbidity index for mortality and female sex for ICU admission.
Literature data agree that obesity, defined by BMI, is a significant predictor of disease severity (OR 1.47–5.47) (3–6, 28, 29), need for intensive care unit (OR 1.29–5.49) (3, 5, 6), and invasive mechanical ventilation (OR 1.2–6.01) (3, 5, 6, 17). However, the results regarding mortality are still inconsistent. Although some studies advocate obesity, defined by BMI, as a significant predictor of mortality, with OR ranging from 1.04 to 4.4, or even higher (1, 3, 4, 26, 30–32), others failed to show statistical significance or even showed negative predictive values (3, 5, 6, 16, 33, 34).
A relatively wide range of OR values in these studies, as well as lack of statistical significance for mortality, could be explained by different BMI cut-offs, sample size, diversity of study population (regarding diverse socio-demographic and comorbidity characteristics of the cohort, as well as different COVID-19 severity among patients), the predominance of different SARS-CoV-2 mutation variants, and other. Also, it is important to point out that all cited studies used BMI as the only measurement for defining obesity, possibly leading to misinterpretation of body composition by neglecting total body and visceral fat, especially in older and more comorbid patients (2). This is important because the majority of mechanisms by which obesity adversely affects the course of SARS-CoV-2 infection (chronic inflammation and immune response dysregulation, endothelial dysfunction and increased thrombogenic potential, endocrine dysfunction, etc.) are mostly effects of the adipose tissue (2, 4, 9). In addition, several studies in which abdominal adipose tissue had been evaluated using CT scan emphasized the importance of visceral adipose tissue on COVID-19 severity and mortality (10, 20–23). One of the pathophysiological explanations of this phenomenon lies in the fact that visceral adipose tissue, compared to subcutaneous, secretes 2–3 times higher concentrations of interleukin 6 (35), which is associated with the development of severe forms and fatal outcomes for patients with COVID-19 (1, 7). In our cohort, patients with excessive VF had significantly higher serum levels of CRP (p = 0.014) and IL-6 (p = 0.028) on admission compared to those with normal VF levels, possibly suggesting higher inflammation grade. We also note that patients with very high % BF had significantly higher values of LDH (p = 0.024), another notable COVID-19 predictor (1, 7), while no statistically significant relationship was found between BMI and any proinflammatory marker on admission. Finally, despite a good agreement between BMI and % BF in defining obesity, 24.5% of patients with very high % BF values were categorized as non-obese according to BMI. We accentuate the high incidence of mortality and ICU admission in this group of patients (24.8 and 55.6%, respectively).
All stated mechanisms could explain, at least partially, why BMI lacked statistical significance in terms of predicting mortality of patients with COVID-19 in cited studies. Also, stated mechanisms could explain why BIA measurements, both % BF and VF, had higher OR in predicting each primary end-point compared to BMI. Due to the relatively small sample size and other study limitations, perhaps the exact OR values for anthropometric measurements could not be generalized, particularly in terms of mortality. However, the results are suggestive of a link between obesity and COVID-19 severity and mortality.
Conclusion
Obesity is a globally raging pandemic that is, in addition to many other comorbidities and all-cause mortality, a significant predictor of COVID-19 severity and death. For that reason, intensive obesity prevention campaigns and programs should be one of the main focuses of healthcare systems worldwide.
Bioelectrical impedance analysis measurements could be a helpful tool in predicting COVID-19 severity and mortality on admission.
By having insight into the total body and visceral fat distribution, BIA measurements (both % BF and VF) were stronger predictors of each primary end-point (mortality and ICU admission) compared to BMI.
Study Limitations
Our study had several limitations. First, COVID-19, like infection and inflammation, can impact body composition. To minimize that effect, we have measured patients in the initial days of hospitalization. Second, a substantial number of patients were excluded from the study, because of their inability to undergo BIA assessment (such as poor general condition, dementia, and lack of limbs). Third, we have included a relatively small number of patients for the generalization of the results.
Finally, although BIA measurements have satisfactory insight into total body fat and fat-free mass and are widely used for body composition assessment in the general population, this method has difficulty distinguishing visceral from abdominal fat, for which CT and MRI remain the gold standard (36, 37). Due to stated study limitations and presented results, the authors suggest and encourage continuing research on this issue.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the University Clinical Center Kragujevac, Kragujevac, Serbia. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DS, VZ, MiP, VM, and IC contributed to conception and design of the study. VZ, MaP, IC, and VM ensured quality of the research and performed final review of the manuscript. DS and MiP organized the database. SM and NZ performed the statistical analysis and contributed to presentation of results. DT, AD, MM, DB, AA, and AJ contributed to data collection and helped in writing chapters of the manuscript. DS and MiP wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.906659/full#supplementary-material
References
1. Manolis AS, Manolis AA, Manolis TA, Apostolaki NE, Melita H. COVID-19 infection and body weight: a deleterious liaison in a J-curve relationship. Obes Res Clin Pract. (2021) 15:523–35. doi: 10.1016/j.orcp.2021.10.006
2. Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. (2020) 142:4–6. doi: 10.1161/CIRCULATIONAHA.120.047659
3. Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. (2020) 21:e13128. doi: 10.1111/obr.13128
4. Pranata R, Lim MA, Yonas E, Vania R, Lukito AA, Siswanto BB, et al. Body mass index and outcome in patients with COVID-19: a dose-response meta-analysis. Diabetes Metab. (2021) 47:101178. doi: 10.1016/j.diabet.2020.07.005
5. Chu Y, Yang J, Shi J, Zhang P, Wang X. Obesity is associated with increased severity of disease in COVID-19 pneumonia: a systematic review and meta-analysis. Eur J Med Res. (2020) 25:64. doi: 10.1186/s40001-020-00464-9
6. Zhang X, Lewis AM, Moley JR, Brestoff JR. A systematic review and meta-analysis of obesity and COVID-19 outcomes. Sci Rep. (2021) 11:7193. doi: 10.1038/s41598-021-86694-1
7. Mesas AE, Cavero-Redondo I, Álvarez-Bueno C, Sarriá Cabrera MA, Maffei de Andrade S, Sequí-Dominguez I, et al. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS One. (2020) 15:e0241742. doi: 10.1371/journal.pone.0241742
8. Jimenez-Solem E, Petersen TS, Hansen C, Hansen C, Lioma C, Igel C, et al. Developing and validating COVID-19 adverse outcome risk prediction models from a bi-national European cohort of 5594 patients. Sci Rep. (2021) 11:3246. doi: 10.1038/s41598-021-81844-x
9. Kwok S, Adam S, Ho JH, Iqbal Z, Turkington P, Razvi S, et al. Obesity: a critical risk factor in the COVID-19 pandemic. Clin Obes. (2020) 10:e12403. doi: 10.1111/cob.12403
10. Watanabe M, Caruso D, Tuccinardi D, Risi R, Zerunian M, Polici M, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. (2020) 111:154319. doi: 10.1016/j.metabol.2020.154319
11. World Healt Organisation.COVID-19 Clinical Management: Living Guidance. (2021). Available online at: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (accessed March 28, 2022).
12. World Health Organ Tech Rep Ser. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. (2000) 894:i–xii, 1–253.
13. Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. (2000) 72:694–701. doi: 10.1093/ajcn/72.3.694
14. TANITA.Medical Product Guiden. (2021). Available online at: https://tanita.eu/uploads/2021/10/EN-Medical-Product-Guide-36pp-September-2021-ONLINE.pdf (accessed March 28, 2022).
15. World Health Organization.Nutrition, Physical Activity and Obesity – Serbia. (2013). Available online at: https://www.euro.who.int/__data/assets/pdf_file/0017/243323/Serbia-WHO-Country-Profile.pdf (accessed March 28, 2022).
16. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. (2020) 369:m1966. doi: 10.1136/bmj.m1966
17. Kim TS, Roslin M, Wang JJ, Kane J, Hirsch JS, Kim EJ, et al. BMI as a risk factor for clinical outcomes in patients hospitalized with COVID-19 in New York. Obesity (Silver Spring). (2021) 29:279–84. doi: 10.1002/oby.23076
18. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham heart study. Circulation. (2007) 116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355
19. Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring). (2006) 14:336–41. doi: 10.1038/oby.2006.43
20. Ogata H, Mori M, Jingushi Y, Matsuzaki H, Katahira K, Ishimatsu A, et al. Impact of visceral fat on the prognosis of coronavirus disease 2019: an observational cohort study. BMC Infect Dis. (2021) 21:1240. doi: 10.1186/s12879-021-06958-z
21. Favre G, Legueult K, Pradier C, Raffaelli C, Ichai C, Iannelli A, et al. Visceral fat is associated to the severity of COVID-19. Metabolism. (2021) 115:154440. doi: 10.1016/j.metabol.2020.154440
22. Goehler A, Hsu TH, Seiglie JA, Siedner MJ, Lo J, Triant V, et al. Visceral adiposity and severe COVID-19 disease: application of an artificial intelligence algorithm to improve clinical risk prediction. Open Forum Infect Dis. (2021) 8:ofab275. doi: 10.1093/ofid/ofab275
23. Bunnell KM, Thaweethai T, Buckless C, Shinnick DJ, Torriani M, Foulkes AS, et al. Body composition predictors of outcome in patients with COVID-19. Int J Obes (Lond). (2021) 45:2238–43. doi: 10.1038/s41366-021-00907-1
24. Ruscica M, Macchi C, Iodice S, Tersalvi G, Rota I, Ghidini S, et al. Prognostic parameters of in-hospital mortality in COVID-19 patients-an Italian experience. Eur J Clin Invest. (2021) 51:e13629. doi: 10.1111/eci.13629
25. Gallo Marin B, Aghagoli G, Lavine K, Yang L, Siff EJ, Chiang SS, et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol. (2021) 31:1–10. doi: 10.1002/rmv.2146
26. Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring). (2020) 28:1595–9. doi: 10.1002/oby.22913
27. Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. (2020) 71:896–7. doi: 10.1093/cid/ciaa415
28. Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: a systematic review and meta-analysis. J Med Virol. (2021) 93:257–61. doi: 10.1002/jmv.26237
29. Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. (2020) 43:1392–8. doi: 10.2337/dc20-0576
30. Page-Wilson G, Arakawa R, Nemeth S, Bell F, Girvin Z, Touchy M, et al. Obesity is independently associated with septic shock, renal complications, and mortality in a multiracial patient cohort hospitalized with COVID-19. PLoS One. (2021) 16:e0255811. doi: 10.1371/journal.pone.0255811
31. Guerson-Gil A, Palaiodimos L, Assa A, Karamanis D, Kokkinidis D, Chamorro-Pareja N, et al. Sex-specific impact of severe obesity in the outcomes of hospitalized patients with COVID-19: a large retrospective study from the Bronx, New York. Eur J Clin Microbiol Infect Dis. (2021) 40:1963–74. doi: 10.1007/s10096-021-04260-z
32. Czernichow S, Beeker N, Rives-Lange C, Guerot E, Diehl JL, Katsahian S, et al. Obesity doubles mortality in patients hospitalized for severe acute respiratory syndrome coronavirus 2 in Paris hospitals, France: a cohort study on 5,795 patients. Obesity (Silver Spring). (2020) 28:2282–9. doi: 10.1002/oby.23014
33. Intensive Care Natioanal Adult and Research Center (Icnarc).ICNARC Report on COVID-19 in Critical Care. (2020). Available online at: https://www.icnarc.org/DataServices/Attachments/Download/8419d345-c7a1-ea11-9126-00505601089b (accessed March 28, 2022).
34. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of covid-19 in New York city. N Engl J Med. (2020) 382:2372–4. doi: 10.1056/NEJMc2010419
35. Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. (2020) 7:22. doi: 10.3389/fcvm.2020.00022
36. Chaudry O, Grimm A, Friedberger A, Kemmler W, Uder M, Jakob F, et al. Magnetic resonance imaging and bioelectrical impedance analysis to assess visceral and abdominal adipose tissue. Obesity. (2020) 28:277–83. doi: 10.1002/oby.22712
Keywords: body fat percentage, body mass index, COVID-19, obesity, visceral fat
Citation: Stevanovic D, Zdravkovic V, Poskurica M, Petrovic M, Cekerevac I, Zdravkovic N, Mijailovic S, Todorovic D, Divjak A, Bozic D, Marinkovic M, Jestrovic A, Azanjac A and Miloradovic V (2022) The Role of Bioelectrical Impedance Analysis in Predicting COVID-19 Outcome. Front. Nutr. 9:906659. doi: 10.3389/fnut.2022.906659
Received: 28 March 2022; Accepted: 23 May 2022;
Published: 11 July 2022.
Edited by:
Timotius Ivan Hariyanto, University of Pelita Harapan, IndonesiaReviewed by:
Daiva Nielsen, McGill University, CanadaLeigh C. Ward, The University of Queensland, Australia
Copyright © 2022 Stevanovic, Zdravkovic, Poskurica, Petrovic, Cekerevac, Zdravkovic, Mijailovic, Todorovic, Divjak, Bozic, Marinkovic, Jestrovic, Azanjac and Miloradovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vladimir Zdravkovic, vladazdrav@gmail.com
 Djordje Stevanovic
Djordje Stevanovic Vladimir Zdravkovic1,2*
Vladimir Zdravkovic1,2*  Nemanja Zdravkovic
Nemanja Zdravkovic Sara Mijailovic
Sara Mijailovic