- 1School of Pharmacy, Monash University Malaysia, Subang Jaya, Malaysia
- 2School of Biosciences, Faculty of Health & Medical Sciences, Taylor’s University, Subang Jaya, Malaysia
- 3School of Medicine, Faculty of Health & Medical Sciences, Taylor’s University, Subang Jaya, Malaysia
- 4Asian Centre for Evidence Synthesis in Population, Implementation and Clinical Outcomes (PICO) Health and Well-being Cluster, Global Asia in the 21st Century (GA21) Platform, Monash University Malaysia, Subang Jaya, Malaysia
- 5School of Pharmacy, Faculty of Health & Medical Sciences, Taylor’s University, Subang Jaya, Malaysia
The outbreak of a novel coronavirus (SARS-CoV-2) in Wuhan, China in December 2019 has now become a pandemic with no approved therapeutic agent. At the moment, the genomic structure, characteristics, and pathogenic mechanisms of SARS-CoV-2 have been reported. Based upon this information, several drugs including the directly acting antivirals have been proposed to treat people with coronavirus disease 2019 (COVID-19). This rapid review aims to describe the directly acting antivirals that have been examined for use in the management of COVID-19. Searches were conducted in three electronic databases, supplemented with a search on arXiv, bioRxiv, medRxiv, ChinaXiv, ClinicalTrials.gov, and Chinese Clinical Trial Registry for studies examining the use of antivirals in COVID-19 to identify for case reports, case series, observational studies, and randomized controlled studies describing the use of antivirals in COVID-19. Data were extracted independently and presented narratively. A total of 98 studies were included, comprising of 38 published studies and 60 registered clinical trials. These drugs include the broad spectrum antivirals such as umifenovir, protease inhibitors such as lopinavir/ritonavir as well as the RNA-dependent RNA polymerase inhibitors, remdesivir, and favipiravir. Other drugs that have been used include the nucleosidase inhibitors and polymerase acidic endonuclease inhibitors which are currently approved for prevention of influenza infections. While some of the drugs appear promising in small case series and reports, more clinical trials currently in progress are required to provide higher quality evidence.
Introduction
In December 2019, an outbreak caused by a novel coronavirus was reported in Wuhan city, in Hubei province, China. The outbreak was found to be caused by a novel virus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Cheng and Shan, 2020; Wu and McGoogan, 2020). Since then, the cases of SARS-CoV-2 have been reported in every single continent around the world. With over nine million individuals infected with coronavirus disease 2019 (COVID-19) and over 450 thousand death as of mid-June 2020, COVID-19 is now a public health emergency. In many individuals with COVID-19, they often present with a decrease in both CD4+ and CD8+ T-cells count and suffer from acute respiratory syndrome for 7 to 10 days due to the rapid viral replication (Cheng and Shan, 2020; Zhou et al., 2020a). Clinical features of SARS-CoV-2 infections are similar to SARS-CoV, characterized by fever, dry cough, dyspnoea or shortness of breath, diarrhea, sore throat, muscle ache, and vomiting in some patients (Meo et al., 2020; Wu and McGoogan, 2020).
The SARS-CoV-2 is a member of the family Coronaviridae, a positive-sense, single-stranded RNA virus that enters the mammalian cell through an interaction of viral spike glycoprotein that binds to the angiotensin-converting enzyme 2 (ACE2) receptor (Fehr and Perlman, 2015). Following receptor binding, the virus uses the host cell receptor and endosome to enter the cell and synthesizes viral polyproteins that encode for the replicase-transcriptase complex. The virus then synthesizes RNA using its RNA-dependent RNA polymerase to synthesize structural proteins leading to completion of assembly and release of viral particles (Fehr and Perlman, 2015; Cheng and Shan, 2020). Genomic sequencing of the virus has revealed that SARS-CoV-2 has a high similarity to the bat-derived SARS-CoV, with approximately 79% identity (Wu C. et al., 2020). Studies have shown that SARS-CoV-2 is spread primarily through the respiratory system and droplets, with an incubation period of between 2 and 14 days, and a median period of 4 days (range, 2–7 days) (Livingston et al., 2020). As such, pharmacological agents that target the spike protein or host’s ACE2 proteins used to treat SARS and Middle-East Respiratory Syndrome (MERS) have been suggested as potential agents that could be used to treat patients with COVID-19. Agents proposed to eradicate the coronavirus or at least reduce the effects and hinder the contagion of the SARS-CoV-2 include repurposing currently available drugs such as monoclonal antibodies, antivirals, antimalarial among others (Fehr and Perlman, 2015; Cheng and Shan, 2020).
This intensifying outbreak has led to a surge in registered clinical trials since the infection was first reported (Zhu et al., 2020a). In order to rapidly inform further and better design and conduct of clinical trials, there is an urgent need to provide government agencies on the investigational candidates most suitable for clinical trials. While there are major gaps in knowledge around COVID-19, especially in terms of the effectiveness and safety of various directly acting antiviral agents, a review of the characteristics of published, on-going trials and a synthesis of all available results can help inform current practice and direct future research. This rapid review was performed to provide government bodies on the evidence available in relation to the antiviral drug therapies that have been examined to date.
Methods
Search Strategy
We performed a search of PubMed, EMBASE, Cochrane CENTRAL from inception to March 31st, 2020 to search for articles assessing the use of antivirals in patients with SARS-CoV-2 pneumonia without any language restriction. This was supplemented by a search on ClinicalTrials.gov, WHO International Clinical Trials Registry Platform and Chinese Clinical Trial Registry as well as pre-print articles on medRxiv, arXiv, bioRxiv, and ChinaXiv. Keywords used include: novel coronavirus, COVID-19, 2019-nCoV, antivirals, anti-retroviral and humans. Following peer-review, we updated our search to May 31st, 2020 on the database identified previously. We also expanded our keywords to include the following search terms: SARS-Co-v 2, abidol, tenofovir, EIDD-2801, sofosbuvir/ledipasvir, sofosbuvir/daclatasvir.
Study Selection and Data Abstraction
Articles were screened by two authors (SL, NL, and ST) independently for relevant studies. Studies which described the use of direct acting anti-viral therapies, irrespective of study designs conducted in humans were included. These could include case studies, case reports, cohort studies, observational studies or randomized controlled studies since. In vitro, animal studies and reviews were excluded since studies have suggested that these may not directly translate to clinical effects in human. We excluded drugs which does not act directly on virus such as antibiotics and antimalarial since these drugs have limited role in targeting the functions of the virus and preventing it from replicating in the body. All information was extracted independently by authors with discrepancies resolved thorough consensus. Due to the time constraints, the review was not registered in PROSPERO but the corresponding author can be contacted for the full protocol.
Study Quality and Reporting
The quality of all included studies which were registered and currently underway were assessed subjectively by one author, and classified anecdotally to either low, medium or high. This classification was based upon the study population > study design > sample size of trial and finally the presumed importance of results. Using this approach, a study that reports on patients would be given higher priority over those which had involved healthy subjects. In the event that the study recruited similar populations, a randomized controlled trial would be graded higher priority over a quasi-randomized study > observational study > case series > case report. Finally, a study of similar design that had reported clinical outcomes such as mortality, hospitalization days would be graded higher compared to those which had reported laboratory data only such as presence or absence of SARS-CoV-2 in patients. All data were summarized narratively due to the limited available evidence on the topic.
Results
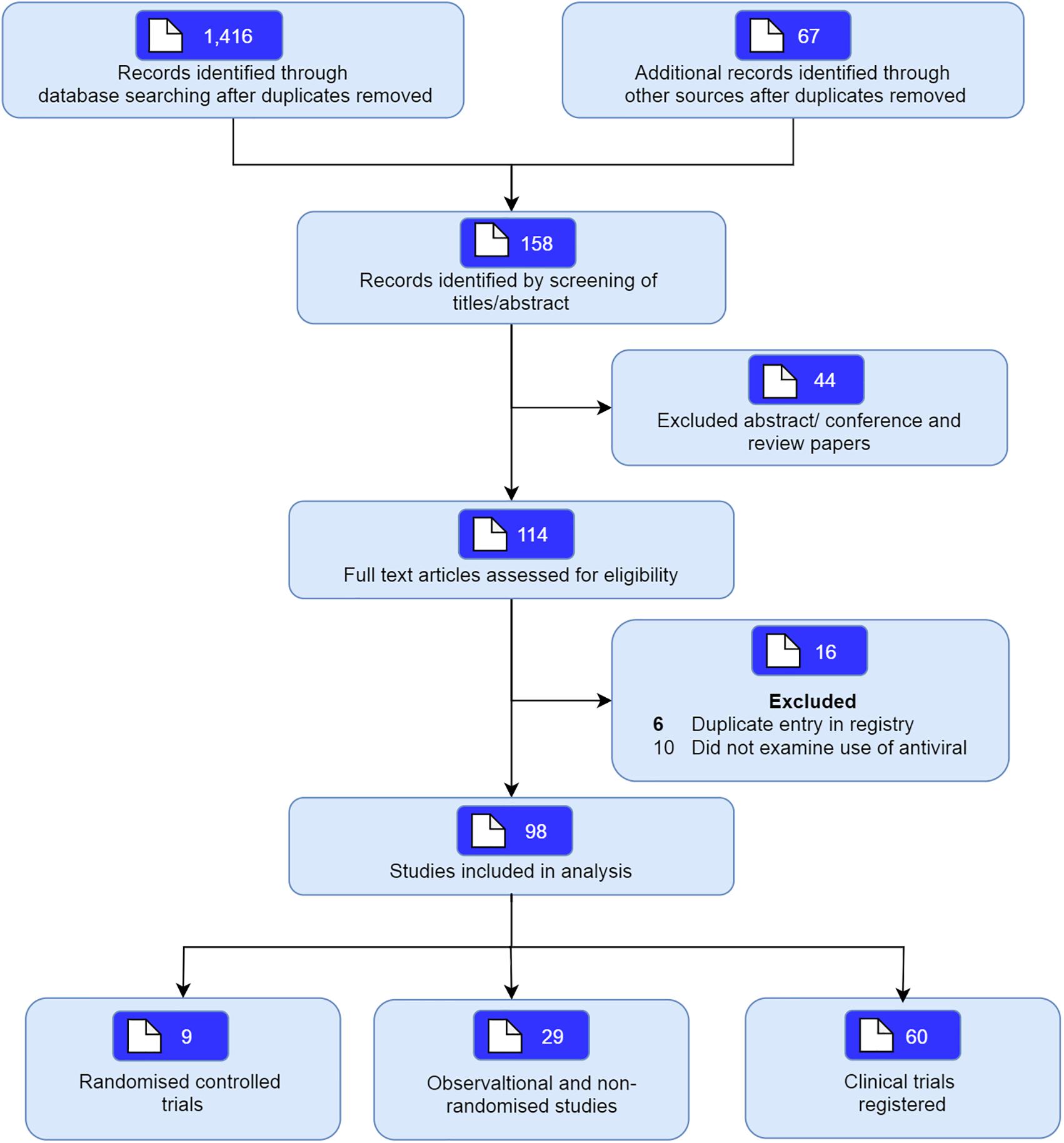
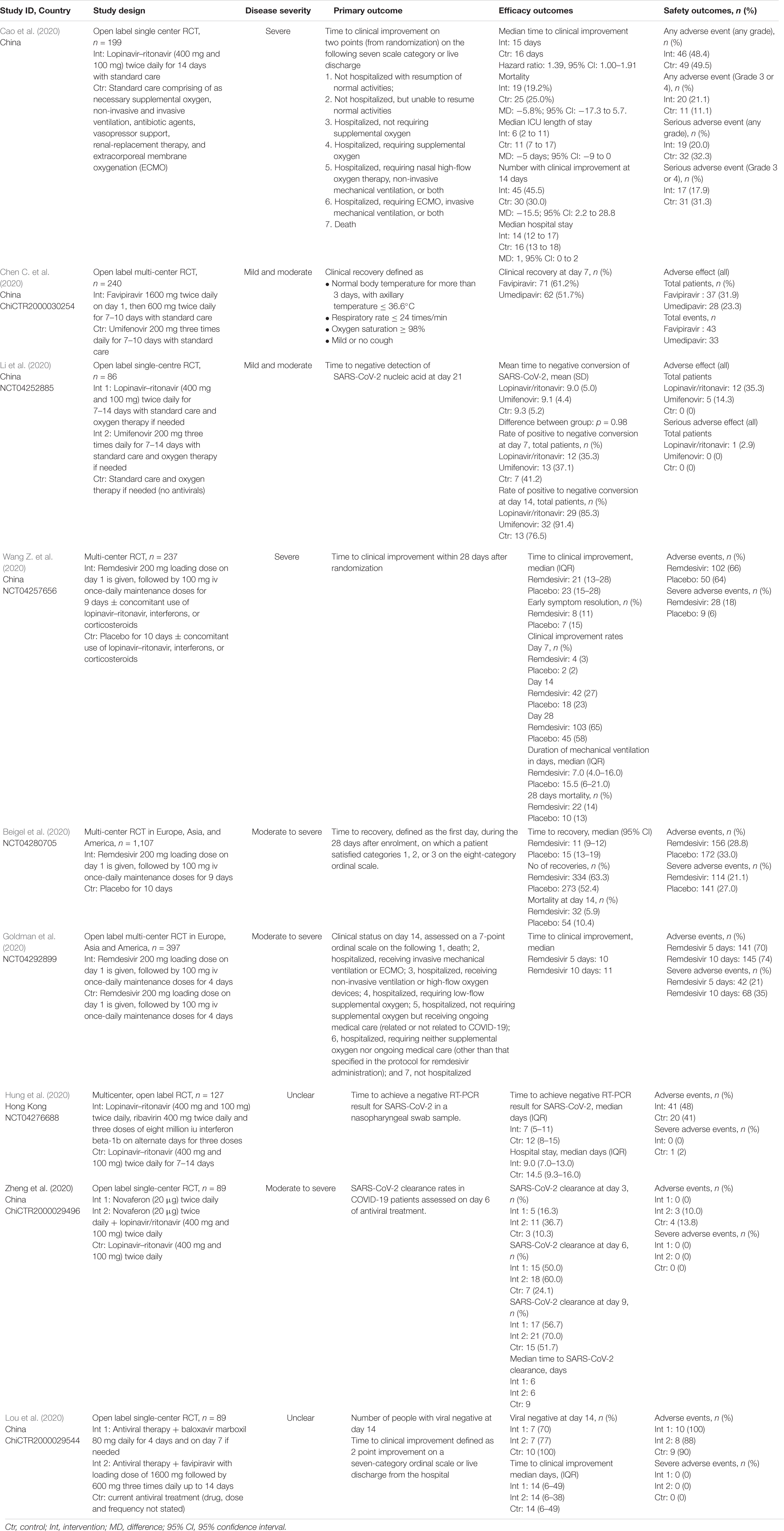
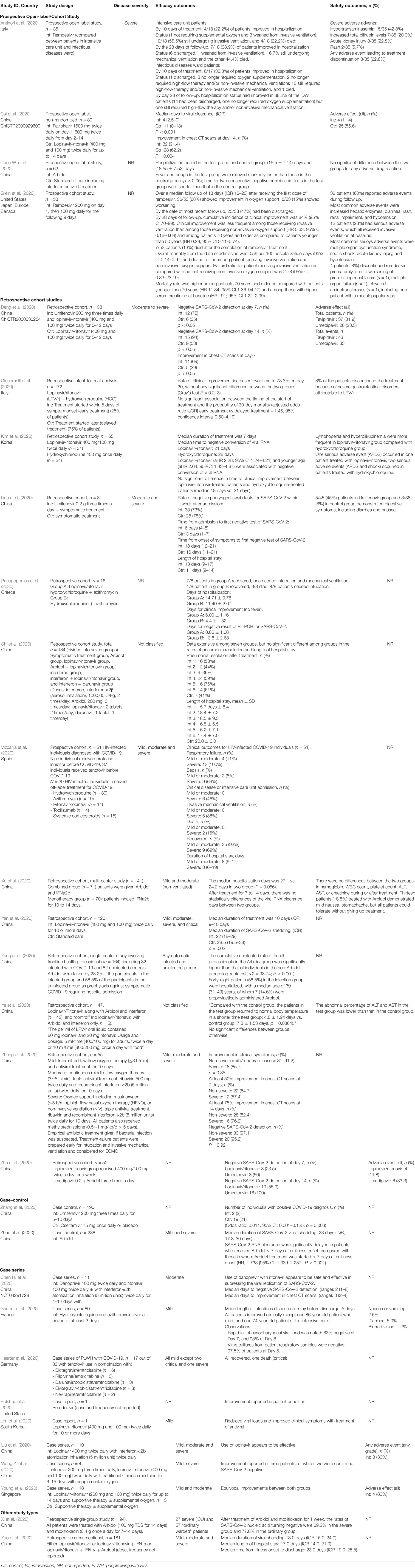
The database search identified a total of 1,416 articles of which 158 potentially relevant studies were screened. Forty-four studies were excluded based upon screening of abstract, and a further 16 were excluded since they did not include individuals with SARS-CoV-2, or were an in vitro studies. A total of 98 studies including nine randomized studies (RCTs) (Beigel et al., 2020; Cao et al., 2020; Chen C. et al., 2020; Goldman et al., 2020; Hung et al., 2020; Li et al., 2020; Lou et al., 2020; Wang Y. et al., 2020; Zheng et al., 2020b) and 29 non-randomized studies (Antinori et al., 2020; Cai et al., 2020; Chen W. et al., 2020; Chen H. et al., 2020; Deng et al., 2020; Gautret et al., 2020; Giacomelli et al., 2020; Grein et al., 2020; Haerter et al., 2020; Holshue et al., 2020; Kim et al., 2020; Lian et al., 2020; Lim et al., 2020; Liu et al., 2020; Panagopoulos et al., 2020; Shi et al., 2020; Vizcarra et al., 2020; Wang Z. et al., 2020; Xi et al., 2020; Xu et al., 2020; Yan et al., 2020; Yang et al., 2020; Ye et al., 2020; Young et al., 2020; Zhang et al., 2020; Zheng et al., 2020a; Zhou et al., 2020b; Zhu et al., 2020b; Zuo et al., 2020) examining the use of antivirals in COVID-19 were included (Figure 1). We also included another 60 registered clinical trials which were at clinical phases 2, 3, or 4 (Supplementary Appendix Table 1, Supplementary Appendix Figure 1, and Supplementary Appendix Figure 2). Most of the trials will be mainly conducted in China but also from other countries including France, Canada, Hong Kong, Iran, Brazil, Egypt, Pakistan, Thailand, United States, Spain, and Korea. The pharmaceutical interventions found for COVID-19 treatment include remdesivir, oseltamivir, favipiravir, danoprevir, ritonavir, darunavir, baloxavir marboxil, azvudine, triazavirin, umifenovir, lopinavir either alone or in combination with other products such as human immunoglobulin, interferons, carrimycin, bevacizumab, cobicistat, and traditional Chinese medicines (see Tables 1, 2 for characteristics of studies identified).
Most of the registered trials were very small in size with sample size of fewer than 100 patients, with a median sample size of 145 (IQR: 60–343). In most trials, participants had to be aged 18 years and above. Most of these trials were in the recruiting stages (n = 39) or the preparation stages (n = 19). There was only limited data available on the efficacy of antivirals on COVID-19 and their clinical impact. Most of the trials will examine a myriad of primary outcomes, including time to clinical improvements, number of individuals requiring mechanical ventilation, number of individuals hospitalized into ICU, length of hospitalization, mortality as well as absence of virological indicators. Three studies also used physical functioning scores based upon an ordinal 7-point scale from the WHO master protocol and the National Early Warning Score 2 (NEWS2).
Direct Antivirals Used in Covid-19
Protease Inhibitors
Successful entry of the SARS-CoV-2 into the cell will depend on the activation of envelope glycoprotein by host cell protease. As such, protease enzyme inhibitors are considered an excellent drug target for patients with COVID-19 (Table 3). Examples of such drugs include lopinavir, ritonavir, darunavir, danoprevir and the experimental drug ASC-09. Among these agents, the most commonly examined protease inhibitor was lopinavir/ritonavir combination, using a dosing regimen of 400 mg/100 mg lopinavir/ritonavir twice daily for up to 14 days which was reported in 18 published studies (Cai et al., 2020; Cao et al., 2020; Deng et al., 2020; Giacomelli et al., 2020; Kim et al., 2020; Li et al., 2020; Lim et al., 2020; Liu et al., 2020; Shi et al., 2020; Vizcarra et al., 2020; Wang Y. et al., 2020; Yan et al., 2020; Ye et al., 2020; Young et al., 2020; Zhu et al., 2020b; Zuo et al., 2020). These studies were conducted in China (n = 13), South Korea (n = 2), Italy, Singapore, and Greece (n = 1 each). Results from the published randomized controlled studies suggest that there is limited clinical efficacy of the combination (Cao et al., 2020; Hung et al., 2020; Li et al., 2020; Zheng et al., 2020b). In a recently completed RCT in China, the lopinavir/ritonavir combination was reported to have limited efficacy, with no difference in time to clinical improvement (median, 16 days), duration of intensive care unit stay, days of mechanical ventilation, or days of oxygen support (Cao et al., 2020). Authors reported that there appears to be some benefit when patients were given the drug therapy earlier (within 12 days of symptom onset) as they experienced a shorter time to clinical improvement (HR 1.25; 1.77–2.05 versus 1.30; 0.84–1.99). Nevertheless, given the significant drug-drug interaction and potential risk of adverse events including gastrointestinal distress such as nausea and diarrhea and hepatotoxicity, caution should be exercised while using this combination given that nearly 20% to 30% of patients have elevated transaminases at presentation (Wu and McGoogan, 2020; Wu C. et al., 2020).
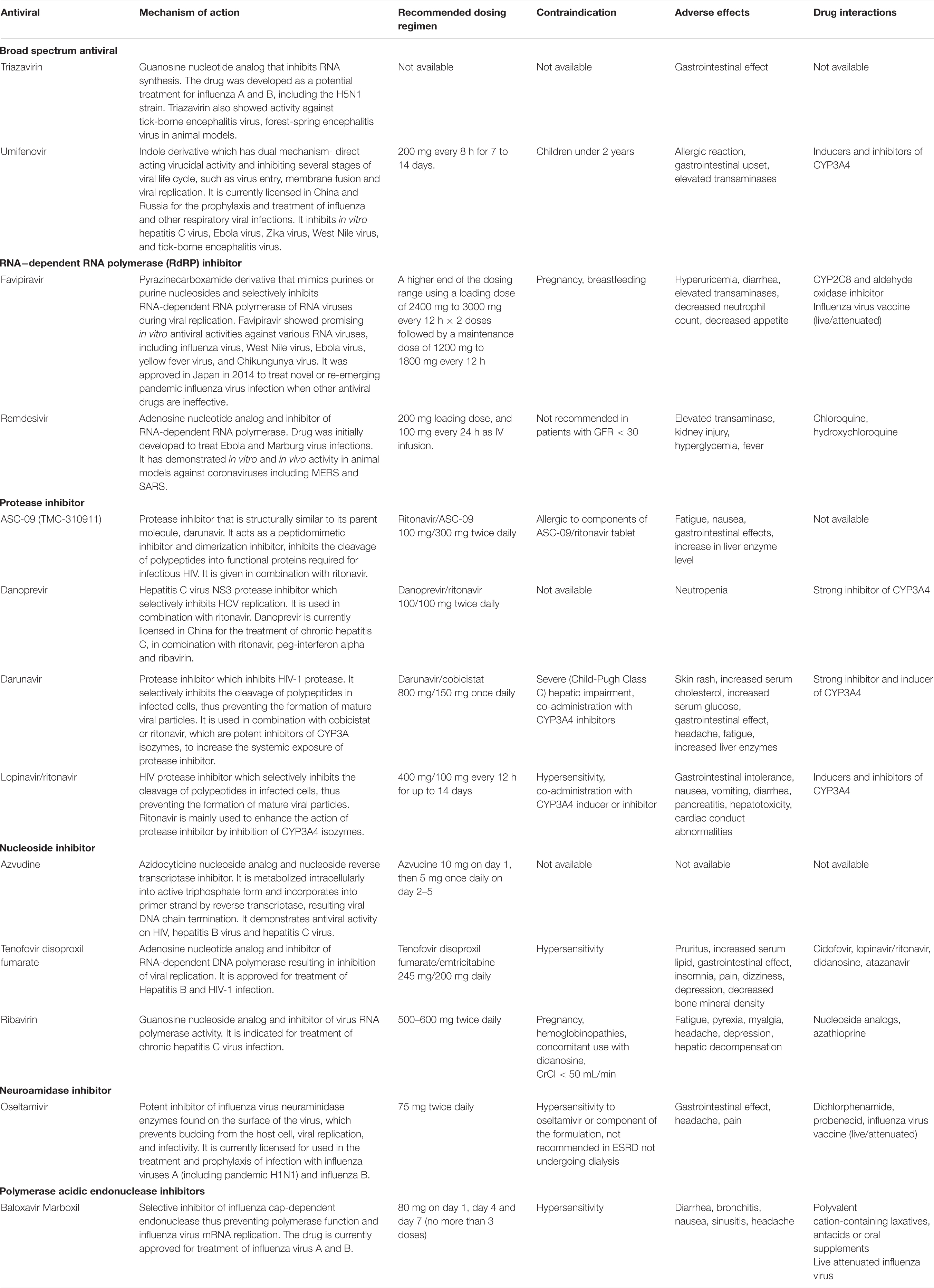
Table 3. Overview of mechanism of action of antivirals and recommended doses for use in COVID-19 patients.
While there are no RCTs on the other protease inhibitors darunavir and danoprevir, real world evidence have been reported from Germany and China (Chen H. et al., 2020; Haerter et al., 2020; Shi et al., 2020). Haerter et al. (2020) in Germany reported the outcomes of a case series of patients living with HIV treated with antiretroviral treatment including darunavir. Of the four patients treated, one died while the other three patients recovered. Shi et al. (2020) similarly reported in their case series on the limited efficacy of darunavir in terms of reducing duration from illness onset to admission and clinical symptoms. Only one small study reported the safety of danoprevir in patients with COVID-19. However, taken together these data are difficult to interpret given the concomitant use of drug therapies, lack of comparator treatment and heterogeneity of disease severity.
Broad Spectrum Antiviral
Another drug commonly examined is umifenovir, a broad spectrum antiviral licensed in China and Russia for influenza. Umifenovir prevents viral host cell entry by inhibiting the membrane fusion of the viral envelope and host cell cytoplasmic membrane (Blaising et al., 2014; Fink et al., 2018; Haviernik et al., 2018). The drug was suggested to have some effects in reducing the risk of COVID-19 transmission and has been examined for post-exposure prophylaxis using a dose of 200 mg orally every 8 h. In an early pilot study from China, treatment with umifenovir was found to reduce SARS-CoV-2 viral loads, with 94% of patients treated with umifenovir reported negative SARS-CoV-2 viral load compared to 53% in the control (Deng et al., 2020). Nevertheless, the results from two RCTs suggested limited efficacy in treating COVID-19 (Chen C. et al., 2020; Li et al., 2020), as the recovery rates were comparable with control.
RNA−Dependent RNA Polymerase (RdRP) Inhibitor
Favipiravir is another oral antiviral that has been examined recently. Favipiravir is a pyrazinecarboxamide derivative and guanine analog which selectively inhibits the RNA-dependent RNA polymerase (RdRP) of RNA viruses (Furuta et al., 2009). RdRP is required during the replication process of RNA viruses as it determines the replication rates and mutation of the virus to adapt to the new host environment, which ultimately influences its fidelity. As such, targeting of RdRP has become another mainstay in the treatment of SARS-CoV-2. In a pilot pre-post study in China, 80 patients with COVID-19 were treated with favipiravir with a loading dose of 1600 mg followed by a maintenance dose of 600 mg three times daily for up to 14 days. After 14 days of treatment, the authors found that patients treated with favipiravir had better treatment outcomes in terms of disease progression and viral clearance compared to those treated with lopinavir/ritonavir (Cai et al., 2020). Two recently completed RCTs in China had reported promising clinical results due to the higher 7-day recovery rates, and symptom improvements such as fever and cough (Chen C. et al., 2020; Lou et al., 2020). With no significant adverse events were reported, favipiravir is currently being examined in several clinical trials as a potential target drug for SARS-CoV-2.
Remdesivir is another nucleotide analog inhibitor of RdRP that have been extensively examined as a potential anti SARS-CoV-2 medication. The earliest report on the use of remdesivir was reported by Holshue et al. (2020), which reported improvement in the patient’s condition after treatment. Since then, two RCTs on remdesivir has been conducted using a dose of remdesivir 200 mg on day 1, followed by 100 mg daily for up to 10 days. In the first RCT of 237 patients with COVID-19 by Wang Y. et al. (2020) in China, the authors found that more patients on remdesivir had clinical improvements after 28 days, and they reported faster time to symptoms improvements compared to control. Beigel et al. (2020) meanwhile reported a large multi-center RCT in Europe, Asia, and America on 1,107 patients treated with either remdesivir or placebo for 10 days. In their study, they found that the median time to recovery was much faster with remdesivir treatment, with a significantly higher number of patient who recovered. Nevertheless, there are uncertainties about the adverse effects of the drugs, and more clinical trials are underway to examine the potential of this drug in SARS-CoV-2.
Nucleosidase and Neuroamidase Inhibitors
Another class of drugs that has been used in SARS-CoV-2 is the neuroamidase inhibitors such as oseltamivir. Given that the COVID-19 outbreak in China occurred during the peak influenza season, a large proportion of patients had received oseltamivir therapy prior to the discovery of SARS-CoV-2 as these agents have been used for various influenza subtype and other RNA viruses to inhibit the spread of the influenza virus (Wang et al., 2014; Malosh et al., 2018). Several clinical trials are currently evaluating the effectiveness of oseltamivir either alone or as a combination such as with chloroquine and favipiravir, but given its pharmacological action, there is limited role of these drugs in the management of COVID-19 once influenza has been excluded.
Similarly, the neuroamidase inhibitors ribavirin and azvudine have been recommended in the initial stages for management of COVID-19, given that the symptoms were thought to be due to pneumonia. There is currently no evidence to suggest that ribavirin when used alone offers any benefit in the management of COVID-19. The combination therapy of ribavirin, lopinavir/ritonavir and interferon beta-1b was recently shown to have some positive results and would need to be explored further (Hung et al., 2020). However, as ribavirin causes a dose-dependent hematological toxicity, and is a known teratogen, there is limited value of this drug in the treatment of COVID-19.
Polymerase Acidic Endonuclease Inhibitors
The only drug in its class examined identified in the current review was baloxavir marboxil. This drug targets the viral polymerase acidic protein to block the endonuclease function, resulting in the inhibition of virus mRNA transcription and infection (Koszalka et al., 2019; Locke et al., 2019). Only one small clinical study in China has been identified in the current review, but due to the small sample the implications will be limited (ChiCTR2000029548).
Discussion
With no therapeutic agent is currently known to be effective for COVID-19, multiple different antivirals have been examined based upon the early in vitro evidence against SARS-CoV. While several case series and reports showed improvements with use of lopinavir-ritonavir, the recently published study by Cao et al. (2020) have showed limited benefits highlighting the difficulty in finding an appropriate agents for rapid implementation in such outbreaks. It remains unfortunate that this therapy is ineffective, given that this would have represented an immediate and safe oral therapy for COVID-19. For most of the current trials reported, these are underpowered and unlikely to provide the healthcare community with the necessary high quality evidences needed to combat this pandemic if taken individually. In addition, most of the trials registered will only include patients aged 18 and above, and thus will unlike to provide the necessary information on children, adolescents, pregnant women or even those with respiratory diseases (Lee, 2020).
These trials also included a wide range of primary outcomes including time to clinical improvements, number of individuals requiring mechanical ventilation, number of individuals hospitalized into ICU, length of hospitalization, mortality as well as absence of virological indicators. As most of the outcomes that will be reported varied, and will include subjective outcomes, this may lead to measurement bias. Importantly, few of the current trials have reported on mortality in their study either as a primary or secondary outcome. While the case fatality rates differs between countries, ranging from as low as 0.3% to as high as 11.0%, these reports have not been forthcoming in all the included studies and should be given attention (Rajgor et al., 2020). In addition, most of the current studies are not coordinated, leading to inconsistencies among trials in their definitions of conditions and inclusion criteria, the design and delivery of intervention and comparison, as well as measurement of the outcomes. Cognisant of this, the World Health Organization (WHO) is initiating a clinical trials experts group which will aim to develop a master protocol for a RCT to evaluate efficacy of therapeutics against nCov (World Health Organization, 2020). Other impending initiatives include the strengthening of management and coordination of the promising drugs such as remdesivir and favipiravir, which should be prioritized for clinical studies. This is based upon the potential activity of both agents against RNA polymerase, established use in novel influence and also oral bioavailability. This ideally should involve the pharmacist who can help in the development of treatment protocols, monitoring of drug adverse events as well as assist in the expanded access of these new investigational drugs (Lee et al., 2019; Stevens et al., 2020).
Investigators should also consider using other clinical trial designs including step-wedge design which may reduce the need for large sample sizes (Baio et al., 2015). In addition, a database should also be setup to share all available existing data between sites and countries, which effectively create a real-world evidence study network, which can increase the speed of information dissemination especially in pandemics such as COVID-19. Indeed, there is a need for researchers to report as much details as possible to ensure reproducibility of results especially as these studies currently use very weak outcomes which can limit the efficacy assessment. Nevertheless, the development of clinical trials during an outbreak is an adaptive process, with new evidence being generated at an impressive rate. As such, we believe that these results generated will inform the adaptation of existing and new trials that are being developed. Indeed, with progressive release of trial results, there is a need for a living systematic review to progressively update the pooled results with each additional trial included. This is crucial in view of the small sample size of individual studies.
Nevertheless, we acknowledge that there are some limitations to this review, given that we had only one reviewer who had conducted the search. In addition, this review also included several pre-print articles which have not been peer-reviewed and thus may not provide the academic rigor normally required for published studies. However, in view of the evolving situation of COVID-19 and the need for rapid understanding of this disease, the decision to include these studies were needed in order for us to provide the readers with the most updated information available. Most of the published treatment data to date are derived from observational studies which have relatively small sample size, which may introduce risk regarding the magnitude of effect sizes.
In summary, this updated review of antivirals in COVID-19 showed that there is limited information available to guide clinical practice as well as the need for a more coordinated research network to seek the best therapeutic options especially in pandemics. While several agents reviewed have suggested some potential benefits of therapy, the evidence remains inconclusive.
Author Contributions
SL conceived the study, conducted the analysis, and wrote the draft. ST, YL, and NL collected the data, conducted the analysis, and edited the draft. All authors approved the final draft.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01857/full#supplementary-material
References
Antinori, S., Cossu, M. V., Ridolfo, A. L., Rech, R., Bonazzetti, C., Pagani, G., et al. (2020). Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: clinical outcome and differences in post-treatment hospitalisation status. Pharmacol. Res. 158:104899. doi: 10.1016/j.phrs.2020.104899
Baio, G., Copas, A., Ambler, G., Hargreaves, J., Beard, E., and Omar, R. Z. (2015). Sample size calculation for a stepped wedge trial. Trials 16:354.
Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., et al. (2020). Remdesivir for the treatment of Covid-19 — Preliminary report. N. Engl. J. Med. doi: 10.1056/NEJMc2022236 [Epub ahead of print].
Blaising, J., Polyak, S. J., and Pécheur, E. I. (2014). Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 107, 84–94. doi: 10.1016/j.antiviral.2014.04.006
Cai, Q., Yang, M., Liu, D., Chen, J., Shu, D., Xia, J., et al. (2020). Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering doi: 10.1016/j.eng.2020.03.007 [Epub ahead of print].
Cao, B., Wang, Y., Wen, D., Liu, W., Wang, J., Fan, G., et al. (2020). A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 382, 1787–1799.
Chen, C., Huang, J., Cheng, Z., Wu, J., Chen, S., Zhang, Y., et al. (2020). Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv [Preprint]. doi: 10.1101/2020.03.17.20037432.t
Chen, W., Yao, M., Fang, Z., Lv, X., Deng, M., and Wu, Z. (2020). A study on clinical effect of arbidol combined with adjuvant therapy on COVID-19. J. Med. Virol. doi: 10.1002/jmv.26142 [Epub ahead of print].
Chen, H., Zhang, Z., Wang, L., Huang, Z., Gong, F., Li, X., et al. (2020). First clinical study using HCV protease inhibitor danoprevir to treat naïve and experienced COVID-19 patients. medRxiv [Preprint]. doi: 10.1101/2020.03.22.20034041
Cheng, Z. J., and Shan, J. (2020). 2019 Novel coronavirus: where we are and what we know. Infection 48, 155–163. doi: 10.1007/s15010-020-01401-y
Deng, L., Li, C., Zeng, Q., Liu, X., Li, X., Zhang, H., et al. (2020). Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J. Infect. 81, e1–e5. doi: 10.1016/j.jinf.2020.03.002
Fehr, A. R., and Perlman, S. (2015). Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 1282, 1–23. doi: 10.1007/978-1-4939-2438-7_1
Fink, S. L., Vojtech, L., Wagoner, J., Slivinski, N. S. J., Jackson, K. J., Wang, R., et al. (2018). The antiviral drug arbidol inhibits Zika Virus. Sci. Rep. 8:8989.
Furuta, Y., Takahashi, K., Shiraki, K., Sakamoto, K., Smee, D. F., Barnard, D. L., et al. (2009). T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 82, 95–102. doi: 10.1016/j.antiviral.2009.02.198
Gautret, P., Lagier, J.-C., Parola, P., Hoang, V. T., Meddeb, L., Sevestre, J., et al. (2020). Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med. Infect. Dis. 34:101663. doi: 10.1016/j.tmaid.2020.101663
Giacomelli, A., Pagani, G., Ridolfo, A. L., Oreni, A., Conti, F., Pezzati, L., et al. (2020). Early administration of lopinavir/ritonavir plus hydroxychloroquine does not alter the clinical course of SARS-CoV-2 infection: a retrospective cohort study. medRxiv [Preprint]. doi: 10.1101/2020.06.05.20123299
Goldman, J. D., Lye, D. C. B., Hui, D. S., Marks, K. M., Bruno, R., Montejano, R., et al. (2020). Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. doi: 10.1056/NEJMoa2015301 [Epub ahead of print].
Grein, J., Ohmagari, N., Shin, D., Diaz, G., Asperges, E., Castagna, A., et al. (2020). Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 382, 2327–2336.
Haerter, G., Spinner, C. D., Roider, J., Bickel, M., Krznaric, I., Grunwald, S., et al. (2020). COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection 1–6. doi: 10.1101/2020.07.11.20151688
Haviernik, J., Štefánik, M., Fojtíková, M., Kali, S., Tordo, N., Rudolf, I., et al. (2018). Arbidol (Umifenovir): a broad-spectrum antiviral drug that inhibits medically important arthropod-borne flaviviruses. Viruses 10:184. doi: 10.3390/v10040184
Holshue, M. L., Debolt, C., Lindquist, S., Lofy, K. H., Wiesman, J., Bruce, H., et al. (2020). First Case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 382, 929–936.
Hung, I. F.-N., Lung, K.-C., Tso, E. Y.-K., Liu, R., Chung, T. W.-H., Chu, M.-Y., et al. (2020). Triple combination of interferon beta-1b, lopinavir & ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395, 1695–1704.
Kim, J.-W., Kim, E. J., Kwon, H. H., Jung, C. Y., Kim, K. C., Choe, J.-Y., et al. (2020). Lopinavir-ritonavir versus hydroxychloroquine for viral clearance and clinical improvement in patients with mild to moderate coronavirus disease 2019. Korean J. Intern. Med. doi: 10.3904/kjim.2020.224 [Epub ahead of print].
Koszalka, P., Tilmanis, D., Roe, M., Vijaykrishna, D., and Hurt, A. C. (2019). Baloxavir marboxil susceptibility of influenza viruses from the Asia-Pacific, 2012–2018. Antiviral Res. 164, 91–96. doi: 10.1016/j.antiviral.2019.02.007
Lee, S., Mak, V., and Tang, Y. (2019). Pharmacist services in nursing home: a systematic review and meta-analysis. Br. J. Clin. Pharmacol. 85, 2668–2688.
Lee, S. W. H. (2020). Coronavirus (COVID-19): what the tuberculosis (TB) community can learn. Prog. Drug Discov. Biomed. Sci. 3:a0000090. doi: 10.3687/pmmb.a0000090
Li, Y., Xie, Z., Lin, W., Cai, W., Wen, C., Guan, Y., et al. (2020). An exploratory randomized, controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI). medRxiv [Preprint]. doi: 10.1101/2020.03.19.20038984
Lian, N., Xie, H., Lin, S., Huang, J., Zhao, J., and Lin, Q. (2020). Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin. Microbiol. Infect. 26, 917–921. doi: 10.1016/j.cmi.2020.04.026
Lim, J., Jeon, S., Shin, H.-Y., Kim, M. J., Seong, Y. M., Lee, W. J., et al. (2020). Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J. Korean Med. Sci. 35:e79.
Liu, F., Xu, A., Zhang, Y., Xuan, W., Yan, T., Pan, K., et al. (2020). Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. 95, 183–191. doi: 10.1016/j.ijid.2020.03.013
Livingston, E., Bucher, K., and Rekito, A. (2020). Coronavirus Disease 2019 and Influenza 2019-2020. JAMA 323, 1122–1122.
Locke, S. C., Splawn, L. M., and Cho, J. C. (2019). Baloxavir marboxil: a novel cap-dependent endonuclease (CEN) inhibitor for the treatment of acute uncomplicated influenza. Drugs Today 55, 359–366.
Lou, Y., Liu, L., and Qiu, Y. (2020). Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. medRxiv [Preprint].
Malosh, R. E., Martin, E. T., Heikkinen, T., Brooks, W. A., Whitley, R. J., and Monto, A. S. (2018). Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin. Infect. Dis. 66, 1492–1500. doi: 10.1093/cid/cix1040
Meo, S., Alhowikan, A., Al-Khlaiwi, T., Meo, I., Halepoto, D., Iqbal, M., et al. (2020). Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharmacol. Sci. 24, 2012–2019.
Panagopoulos, P., Petrakis, V., Panopoulou, M., Trypsianis, G., Penlioglou, T., Pnevmatikos, I., et al. (2020). Lopinavir/ritonavir as a third agent in the antiviral regimen for SARS-CoV-2 infection. J. Chemother. 1–5. doi: 10.1080/1120009x.2020.1775424 [Epub ahead of print].
Rajgor, D. D., Lee, M. H., Archuleta, S., Bagdasarian, N., and Quek, S. C. (2020). The many estimates of the COVID-19 case fatality rate. Lancet Infect. Dis. 20, 776–777. doi: 10.1016/s1473-3099(20)30244-9
Shi, X., Lu, Y., Li, R., Tang, Y., Shi, N., Song, F., et al. (2020). Evaluation of antiviral therapies for coronavirus disease 2019 pneumonia in Shanghai, China. J. Med. Virol. 16:10.1002/jmv.25893.
Stevens, M. P., Patel, P. K., and Nori, P. (2020). Involving antimicrobial stewardship programs in COVID-19 response efforts: all hands on deck. Infect. Control Hosp. Epidemiol. 41, 744–745. doi: 10.1017/ice.2020.69
Vizcarra, P., Pérez-Elías, M. J., Quereda, C., Moreno, A., Vivancos, M. J., Dronda, F., et al. (2020). Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV S2352-3018, 30164–30168.
Wang, R. R., Yang, Q. H., Luo, R. H., Peng, Y. M., Dai, S. X., Zhang, X. J., et al. (2014). Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS One 9:e105617. doi: 10.1371/journal.pone.0105617
Wang, Y., Zhang, D., Du, G., Du, R., Zhao, J., Jin, Y., et al. (2020). Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395, 1569–1578.
Wang, Z., Chen, X., Lu, Y., Chen, F., and Zhang, W. (2020). Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Bio Sci. Trends 14, 64–68. doi: 10.5582/bst.2020.01030
World Health Organization (2020). WHO R&D Blueprint - Novel Coronavirus COVID-19 Therapeutic Trial Synopsis. Geneva: World Health Organization.
Wu, C., Chen, X., Cai, Y., Zhou, X., Xu, S., Huang, H., et al. (2020). Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int. Med. 180, 1–11.
Wu, F., Zhao, S., Yu, B., Chen, Y.-M., Wang, W., Song, Z.-G., et al. (2020). A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269.
Wu, Z., and McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323, 1239–1242.
Xi, W.-N., Jin, D., Sun, K., Yu, R., Yao, X. B., Zou, B.-S., et al. (2020). Treatment with arbidol and moxifloxacin in ordinary and severe adult patients infected with COVID-19. MedRxiv [Preprint]. doi: 10.1101/2020.05.30.20117598
Xu, P., Huang, J., Fan, Z., Huang, W., Qi, M., Lin, X., et al. (2020). Arbidol/IFN-α2b therapy for patients with corona virus disease 2019: a retrospective multicenter cohort study. Microbes Infect. 22, 200–205. doi: 10.1016/j.micinf.2020.05.012
Yan, D., Liu, X.-Y., Zhu, Y.-N., Huang, L., Dan, B.-T., Zhang, G.-J., et al. (2020). Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection. Eur. Respirat. J. 56:2000799. doi: 10.1183/13993003.00799-2020
Yang, C., Ke, C., Yue, D., Li, W., Hu, Z., Liu, W., et al. (2020). Effectiveness of arbidol for COVID-19 prevention in health professionals. Front. Public Health 8:249. doi: 10.3389/fpubh.2020.00249
Ye, X., Luo, Y., Xia, S., Sun, Q., Ding, J., Zhou, Y., et al. (2020). Clinical efficacy of lopinavir/ritonavir in the treatment of coronavirus disease 2019. Eur. Rev. Med. Pharmacol. Sci. 24, 3390–3396.
Young, B. E., Ong, S. W. X., Kalimuddin, S., Low, J. G., Tan, S. Y., Loh, J., et al. (2020). Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 323, 1488–1494.
Zhang, J.-N., Wang, W.-J., Peng, B., Peng, W., Zhang, Y.-S., Wang, Y.-L., et al. (2020). Potential of arbidol for post-exposure prophylaxis of COVID-19 transmission-a preliminary report of a retrospective cohort study. Curr. Med. Sci. 30, 1–6.
Zheng, C., Wang, J., Guo, H., Lu, Z., Ma, Y., Zhu, Y., et al. (2020). Risk-adapted treatment strategy for COVID-19 patients. Int. J. Infect. Dis. 94, 74–77. doi: 10.1016/j.ijid.2020.03.047
Zheng, F., Zhou, Y., Zhou, Z., Ye, F., Huang, B., Huang, Y., et al. (2020). A novel protein drug, novaferon as the potential antiviral drug for COVID-19. medRxiv [Preprint]. doi: 10.1101/2020.04.24.20077735
Zhou, F., Yu, T., Du, R., Fan, G., Liu, Y., Liu, Z., et al. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062. doi: 10.1016/s0140-6736(20)30566-3
Zhou, Y., He, X., Zhang, J., Xue, Y. E., Liang, M., Yang, B., et al. (2020). Prolonged SARS-CoV-2 viral shedding in patients with COVID-19 was associated with delayed initiation of arbidol treatment: a retrospective cohort study. medRxiv [Preprint]. doi: 10.1101/2020.06.09.20076646
Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., et al. (2020). A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733.
Zhu, Z., Lu, Z., Xu, T., Chen, C., Yang, G., Zha, T., et al. (2020). Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 81, e21–e23. doi: 10.1016/j.jinf.2020.03.060
Zuo, Y., Liu, Y., Zhong, Q., Zhang, K., Xu, Y., and Wang, Z. (2020). Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID-19: a retrospective study in two designated hospitals in Anhui, China. J. Med. Virol. doi: 10.1002/jmv.26127 [Epub ahead of print].
Keywords: rapid review, systematic review, COVID-19, antivirals, pandemic
Citation: Teoh SL, Lim YH, Lai NM and Lee SWH (2020) Directly Acting Antivirals for COVID-19: Where Do We Stand? Front. Microbiol. 11:1857. doi: 10.3389/fmicb.2020.01857
Received: 15 April 2020; Accepted: 15 July 2020;
Published: 05 August 2020.
Edited by:
Slobodan Paessler, The University of Texas Medical Branch at Galveston, United StatesReviewed by:
Oliver Planz, University of Tübingen, GermanySiew Pheng Lim, Denka Life Innovation Research (DLIR), Singapore
Copyright © 2020 Teoh, Lim, Lai and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaun W. H. Lee, shaun.lee@monash.edu
 Siew L. Teoh1
Siew L. Teoh1 Shaun W. H. Lee
Shaun W. H. Lee