- 1Department of Neurology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
- 2Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China
Background: Vaccination remains the most effective measure to prevent SARS-CoV-2 infection and worse outcomes. However, many myasthenia gravis (MG) patients are hesitant to receive vaccine due to fear of worsening.
Methods: MG patients were consecutively enrolled in two MG centers in North China. The “worsening” after vaccination was self-reported by MG patients, and severity was measured with a single simple question. The general characteristics and disease status immediately prior to the first dose were compared between the worsening and non-worsening groups. Independent factors associated with worsening were explored with multivariate regression analysis.
Results: One hundred and seven patients were included. Eleven patients (10.3%) reported worsening after vaccination, including eight patients with mild or moderate worsening and three patients with severe worsening. Only one of them (0.9%) needed an escalation of immunosuppressive treatments. There were significant differences between the worsening and non-worsening groups in terms of Myasthenia Gravis Foundation of America classes immediately before the first dose and intervals since the last aggravation. Precipitating factors might contribute to the worsening in some patients. Logistic regression revealed that only interval since the last aggravation ≤6 months was associated with worsening after SARS-CoV-2 vaccination (P = 0.01, OR = 8.62, 95% CI: 1.93–38.46).
Conclusion: SARS-CoV-2 vaccines (an overwhelming majority were inactivated vaccines) were found safe in milder Chinese MG patients who finished two doses. Worsening after vaccination was more frequently seen in patients who were presumed as potentially unstable (intervals since last aggravation ≤6 months). However, mild worsening did occur in patients who were presumed to be stable. Precipitating factors should still be sought and treated for better outcome.
Introduction
The coronavirus disease 2019 (COVID-19) is still raging worldwide. Vaccination remains the most effective measure to prevent a SARS-CoV-2 infection and its worse outcomes (1). Several studies have reported the safety of SARS-CoV-2 vaccines in myasthenia gravis (MG) patients (2–5). However, many MG patients are hesitant to receive the vaccine for fear of worsening (6). It is important to know the clinical characteristics of patients who are prone to worsening in order to provide individualized advice. A retrospective analysis was conducted in MG patients who received SARS-CoV-2 vaccines from two MG centers in China to report the safety of SARS-CoV-2 vaccination and to explore the factors which might indicate possible worsening.
Materials and methods
This study was conducted from March 2021 to March 2022 in Qilu Hospital (Qingdao) and Xuanwu Hospital to investigate the possible worsening of MG after SARS-CoV-2 vaccination. The eligible patients should be diagnosed as MG at least 1 month before being included. We designed and sent a structural questionnaire (Supplementary Material 1) to the patients in our registry and confirmed their replies by WeChat (an instant chat tool) or telephone or by a face-to-face visit if needed. We also used this questionnaire in the follow-up of patients who reported worsening.
The “worsening” after vaccination was self-reported by MG patients, which was defined as the occurrence of additional symptoms or the aggravation of existing symptoms in at least one of the six muscle groups (extraocular, facial, bulbar, cervical, limb and respiratory muscles) within 28 days after each vaccination dose. When patients reported worsening, they were required to recall the symptoms and overall severity immediately prior to the first dose as the baseline severity. The overall severity at baseline and maximal worsening after vaccination was determined with a single simple question (what percentage of normal do you feel regarding your severity of MG symptoms, 0–100% of normal), which had been shown to correlate strongly with limb muscle weakness and moderately with bulbar and respiratory symptoms (7). The “normal” refers to a patient’s sense of well-being (100%); the greater is the %, the mild is the worsening. The patients were being kept in close communication to determine whether worsening was related to MG and to find and manage any significant impact on their daily life until they recovered to the status prior to vaccination. If the patients reported a significant impact on their daily life within 14 days after worsening, a face-to-face visit was arranged by the treating neurologists for exclusion of the effects of concurrent diseases and for the determination of escalation in immunosuppressive treatment (IST). The final information of the survey was recorded in our database after confirmation of the self-reports of the patients.
Data on general characteristics such as gender, onset age, thymoma, acetylcholine receptor (AChR) antibody or muscle-specific kinase (MuSK) antibody, muscle involvement (ocular/generalized) at onset, and maximal Myasthenia Gravis Foundation of America (MGFA) classes during total MG duration until the first dose were extracted from our MG database. The status immediately before the first dose was acquired from the MG database and by active communication with the patients, including the age when they received the first dose, MGFA classes immediately before the first dose (IBFD MGFA class), post-intervention status immediately before the first dose (IBFD PIS) (8), Myasthenia Gravis Status and Treatment Intensity (MGSTI) levels (9), and the interval since last aggravation to the first dose. The IBFD PIS was defined by only impacts on daily life of the patients without considering the persistence of this status. MGSTI contained six levels: level 0 refers to complete stable remission (CSR), level 1–3 refers to pharmacologic remission (PR) or minimal manifestation (MM) with mono or dual oral therapies of glucocorticoids and immunosuppressants, and level 4 refers to symptomatic but not requiring IV immunoglobulin, plasma exchange, or hospitalization. The PIS used in MGSTI levels was consistent with the definition of PIS by MGFA (8), which required at least 1 year for being classified as CSR, PR, and MM. We used the cutoff of the MGSTI score in reference to the study of rituximab in anti-MuSK MG patients (9). Last aggravation referred to the nearest worsening which needs escalation of ISTs in patients with a total duration >6 months or the onset of MG in patients with a total duration ≤6 months. By this definition, the status of the patients during the intervals is improved or unchanged or even worsened slightly but need no escalation of ISTs. Precipitating factors (infection, fatigue, emotional stress, suspected drug use, etc.), IST escalation, and intervals between the worsening after vaccination and recovery to the status prior to vaccination were recorded. This study was approved by the Ethics committee of both hospitals, and informed consents were obtained from the patients or their authorized family members.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range) and compared using t-test or the Mann–Whitney U-test. Categorical variables were expressed as frequencies (percentages) and compared using the chi-square test or Fisher’s exact test. Logistic regression was conducted to determine the independent factors associated with worsening. Statistical analyses were performed using IBM SPSS, version 20.0 (SPSS Inc., Chicago, IL, USA). A two-tailed p <0.05 was considered significant.
Results
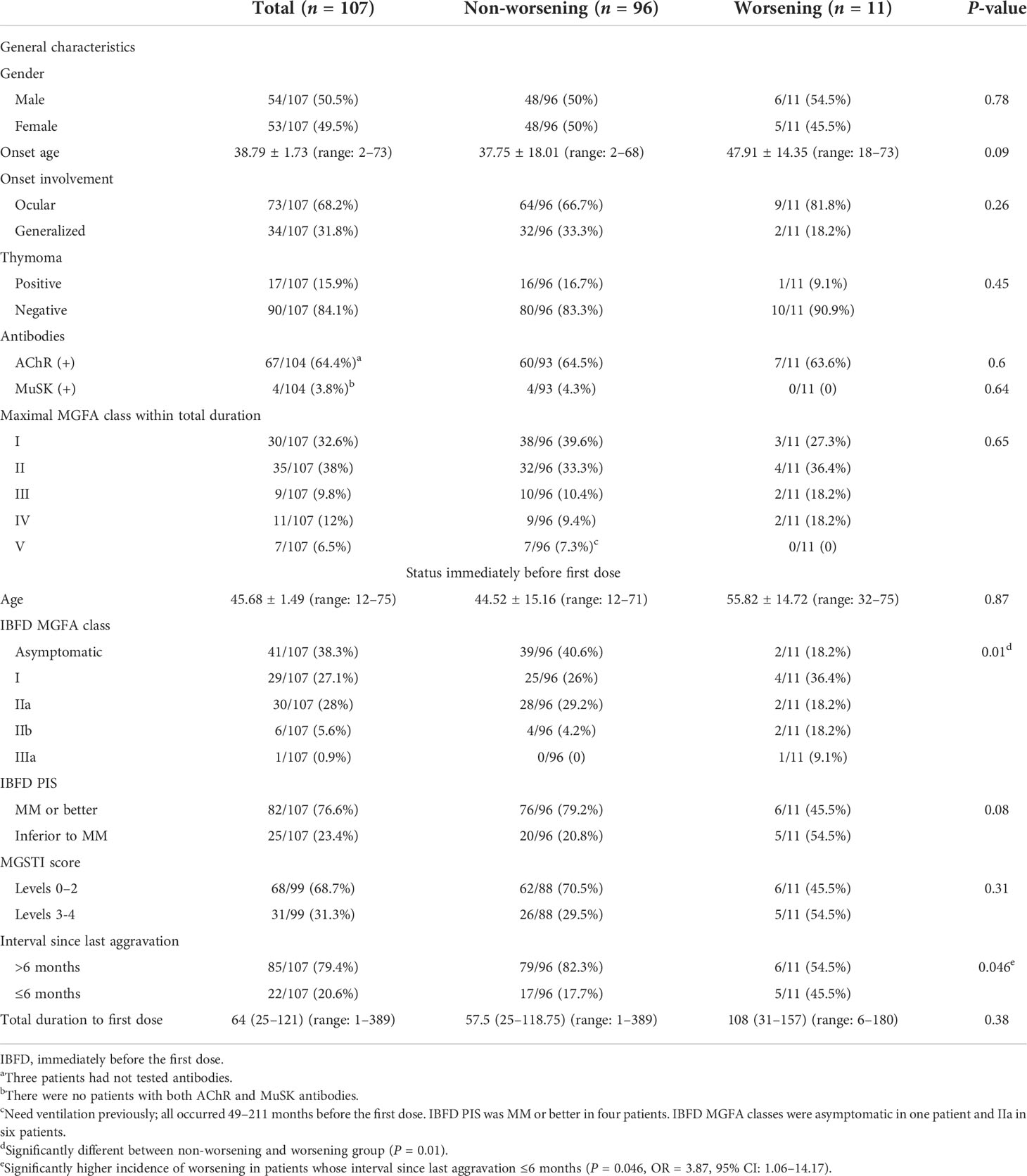
A total of 107 MG patients received at least one dose of SARS-CoV-2 vaccine, 105 patients received homologous inactivated vaccines, and two patients received homologous recombinant subunit vaccines. Ninety-seven patients received the second dose at 26 (22–32) days after the first dose, and 35 patients received the third dose at 187 (184–200) days after the second dose. The first dose was given to 22 patients with interval since last aggravation ≤6 months (20.6%), including 10 patients whose total MG duration was ≤6 months. Their IBFD PIS at first dose was MM or better in 10 patients and inferior to MM in 12 patients. There were no SARS-CoV-2 infections in all patients during the follow-up. The detailed baseline information and the status immediately before the first dose are shown in Table 1.
Eleven patients (10.3%) reported 12 episodes of worsening after COVID-19 vaccination, including six (5.6%) after the first dose and six (6.2%) after the second dose. No patient reported worsening after the third dose. Since the first and the second doses were in close time interval and most of the patients finished two doses, the baseline data of patients who received the first dose were used in the subsequent analysis. Six patients were examined face to face by the treating neurologists when their worsening lasted more than 14 days or when they visit for routine follow-up, and the other five patients who recovered before 14 days were evaluated based on the online survey. There was a significant difference in IBFD MGFA classes between the worsening and non-worsening groups (P = 0.01). A significantly higher incidence of worsening was noted in patients with interval since last aggravation ≤6 months (P = 0.046, OR = 3.87, 95% CI: 1.06–14.17). Moreover, there were marginal differences in onset age and IBFD PIS (MM or better) between the two groups. Logistic regression was conducted by taking worsening as dependent variable and patient characteristics (gender, onset age, onset muscle involvement, thymoma, and AChR or MuSK antibodies), age at first dose, interval since last aggravation, IBFD MGFA classes, IBFD PIS, and MGSTI score as independent variables, and it was revealed that interval since last aggravation ≤6 months was the only independent factor associated with worsening after vaccination (P = 0.01, OR = 8.62, 95% CI: 1.93–38.46).
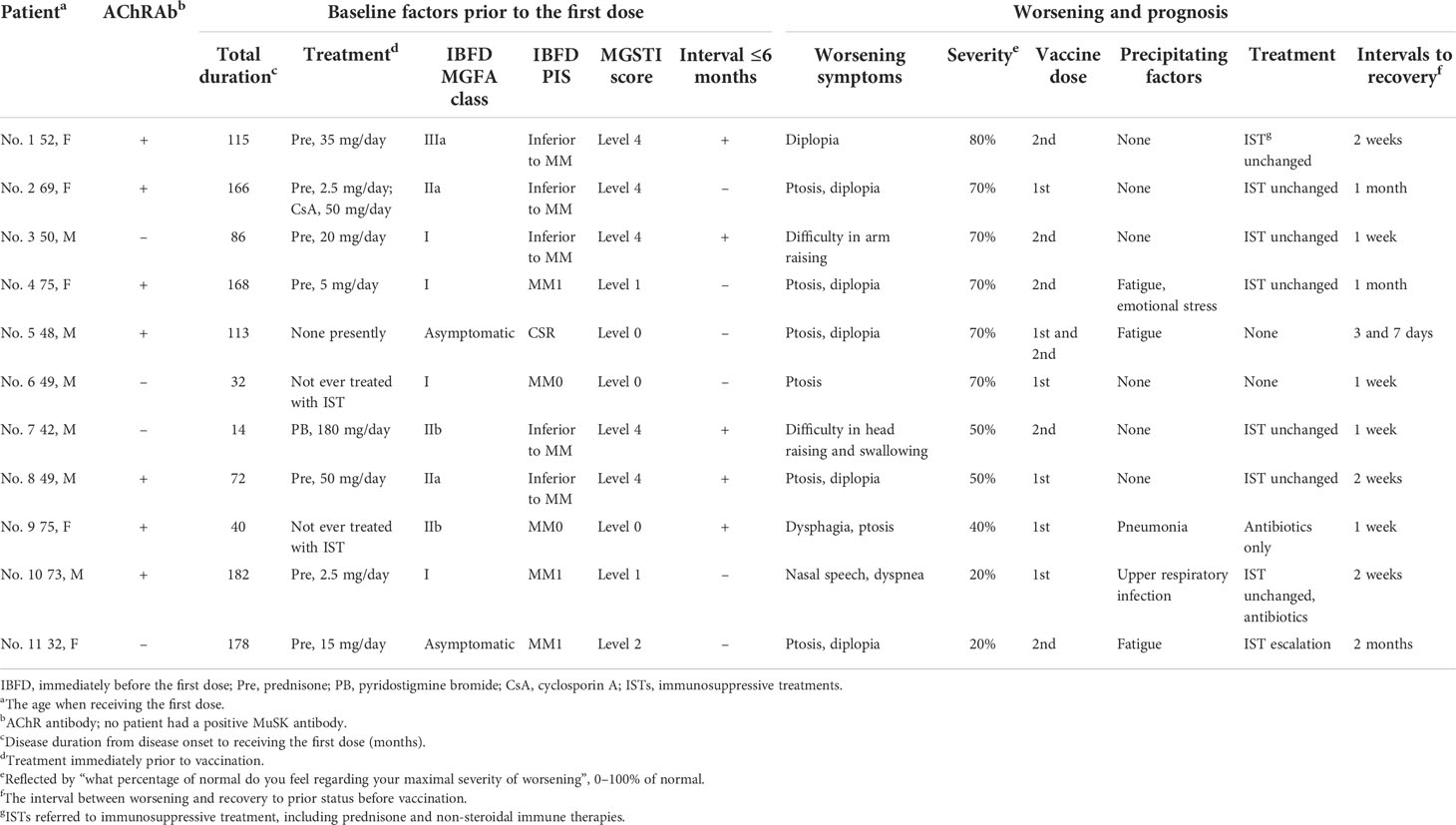
Details on the 11 patients who reported worsening are presented in Table 2. Eight patients (including one who suffered two worsening episodes after each dose) suffered from mild worsening (50–80% of normal), two of whom reported precipitating factors. Four patients recovered to the status prior to vaccination without IST escalation within 14 days. Two patients reported moderate worsening severity at 40 or 20% of normal, and they had pneumonia at 3 days after the first dose or an upper respiratory infection at 5 days before the first dose. Worsening occurred 18 days or 1 day after vaccination. The interval since last aggravation was 31 months in one patient and 49 months in the other, and MGSTI was level 0 in one patient and level 1 in the other. Treated with antibiotics, they recovered in 7 and 14 days, respectively. Only one patient needed an escalation of prednisone dosage and an addition of tacrolimus 10 days after worsening, which was determined by the treating neurologist. She had suffered from long-term exhaustion before the first dose and reported 20% of normal. The interval since last aggravation was 41 months, and the MGSTI was level 2. She recovered 2 months later (Table 2).
Discussions
The safety profile of MG patients after SARS-CoV-2 vaccination could be partly learned from the experiences of SARS-CoV-2 vaccination in autoimmune diseases, such as rheumatic disease, as well as experiences of influenza vaccines in MG patients. In 2021, an international registry including 5,121 inflammatory/autoimmune rheumatic and musculoskeletal disease (I-RMDs) patients who received SARS-CoV-2 vaccination reported that an overwhelming majority of the patients tolerated the vaccination well, with rare reports of worsening (4.4%, including 0.6% severe and 1.5% requiring medication changes) (10). In MG patients, no exacerbation of MG was found after influenza vaccination in most studies (11–14). However, aggravation which needed IST escalation did occur as a rare event (2/133, 1.5%) (15).
There have been several reports on the worsening of MG following SARS-CoV-2 vaccines. One case report described a myasthenic crisis after SARS-CoV-2 vaccination (16). In 294 MG patients who received mRNA vaccines in Japan (3), three patients (1%) were hospitalized for IVIg and/or methylprednisolone pulse; however, the number of patients with mild worsening was not reported. In 55 MG patients who received mRNA vaccines in Israel (4), eight patients (14.5%) reported worsening, and only three patients (5.5%) required additional medication to treat their symptoms. In 104 MG patients who received mRNA vaccines in Italy (5), eight patients (7.7%) reported worsening, including two patients (1.9%) who needed IST escalation. Chang et al. (2) reported 22 Chinese MG patients who received SARS-CoV-2 vaccines (21 inactivated vaccines and one recombinant subunit vaccines)—18 patients had complete resolution of symptoms for at least 2 months, three were in MGFA I class, and one was in MGFA II class before vaccination; only two patients (9.1%) reported mild worsening which resolved quickly within a few days without IST escalation. In our study, no patient received mRNA SARS-CoV-2 vaccines. In our study, 11 patients (10.3%) reported worsening, and only one patient (0.9%) was confirmed as aggravation which needed IST escalation. Our results were consistent with the abovementioned results in the overall safety profiles of vaccination in MG, although different vaccines were given.
In this study, with the structured questionnaire, MG worsening was identified by additional symptoms or changes of severity in previous symptoms, which were ascertained by close communication or face-to-face visit. The single simple question provided a simple, quick, and valid evaluation of the worsening severity. Therefore, our survey was feasible on the background of SARS-CoV-2 pandemic. Our database provided detailed information to analyze the factors that might be associated with worsening after vaccination. Status immediately prior to vaccination is most important. The interval since last aggravation (aggravation or onset) ≤6 months prior to vaccination is presumed as possible instability. During this time, the patients might hesitate to receive vaccination. The IBFD MGFA class reflects the severity on vaccination, and the IBFD PIS reflects the impacts of MG on daily life prior to vaccination. The MGSTI score reflects the stability of MG and the ongoing treatment potency. Our result showed that the severity and impact of MG on daily life immediately prior to vaccination and the potential instability status were associated with worsening after vaccination. Logistic regression analysis revealed that the only independent factor for worsening following vaccination was a potentially unstable status. There was no association between worsening and other factors, such as gender, onset age, pathogenic antibodies, thymoma, onset muscle involvement, and maximal MGFA classes. Even seven patients who previously needed invasive ventilation 49–211 months before the first dose reported no worsening after vaccination. Moreover, the comprehensive status of stability and treatment potency (MGSTI levels) were not different between the worsening and non-worsening groups. This indicated that the potential short-term instability of MG instead of ongoing stability of MG was associated with worsening after vaccination.
There were some limitations in this study. First, this was a retrospectively real-world study, which rendered inevitable selection bias by including most mild patients due to the reluctance of the treating physician to give vaccination in more severe patients. Second, the number of patients who had received three doses was small. Lumping 10 patients who only received one dose and 97 patients who received two doses into one cohort might underestimate the incidence of worsening. However, the present result could indicate the incidence of worsening after two doses and the factors associated with worsening in light of their effect sizes. Third, we lack the data of precipitating factors in 96 non-worsening patients due to difficulty in acquiring these data through this online survey. The precipitating factors were acquired after asking about every detail of the 11 worsening patients.
Conclusion
SARS-CoV-2 vaccines (an overwhelming majority were inactivated vaccines) were found safe in milder Chinese MG patients who finished two doses. Worsening was more frequently seen in patients who were presumed as potential unstable (duration from last aggravation or onset ≤6 months). However, even patients who were presumed as stable could have mild worsening after vaccination. Precipitating factors should still be sought and treated if needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University and Ethics Committee of Xuanwu Hospital, Capital Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s) and minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
Y-XY and H-FL conceptualized and designed the study and revised the manuscript. H-YL and L-YS interpreted the data and wrote the manuscript. H-YL and S-MH performed statistical analysis. L-YS and MS maintained the research database. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (nos. 81771362 and 82171397 to H-FL) and Research Grant from Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University (QDKY2021RX06 to Y-XY).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.923017/full#supplementary-material
References
1. Patone M, Handunnetthi L, Saatci D, Pan J, Katikireddi SV, Razvi S, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med (2021) 27(12):2144–53. doi: 10.1038/s41591-021-01556-7
2. Ruan Z, Tang Y, Li C, Sun C, Zhu Y, Li Z, et al. COVID-19 vaccination in patients with myasthenia gravis: A single-center case series. Vaccines (Basel) (2021) 9(10):1112. doi: 10.3390/vaccines9101112
3. Ishizuchi K, Takizawa T, Sekiguchi K, Motegi H, Oyama M, Nakahara J, et al. Flare of myasthenia gravis induced by COVID-19 vaccines. J Neurol Sci (2022) 436:120225. doi: 10.1016/j.jns.2022.120225
4. Lotan I, Hellmann MA, Friedman Y, Stiebel-Kalish H, Steiner I, Wilf-Yarkoni A. Early safety and tolerability profile of the BNT162b2 COVID-19 vaccine in myasthenia gravis. Neuromuscul Disord (2022) 32(3):230–5. doi: 10.1016/j.nmd.2022.01.013
5. Farina A, Falso S, Cornacchini S, Spagni G, Monte G, Mariottini A, et al. Safety and tolerability of SARS-Cov2 vaccination in patients with myasthenia gravis: a multicenter experience. Eur J Neurol (2022) 29(8):2505–10. doi: 10.1111/ene.15348
6. Zhou Q, Zhou R, Yang H, Yang H. To be or not to be vaccinated: That is a question in myasthenia gravis. Front Immunol (2021) 12:733418. doi: 10.3389/fimmu.2021.733418
7. Abraham A, Breiner A, Barnett C, Katzberg HD, Bril V. The utility of a single simple question in the evaluation of patients with myasthenia gravis. Muscle Nerve (2018) 57(2):240–4. doi: 10.1002/mus.25720
8. Jaretzki A 3rd, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, et al. Myasthenia gravis: recommendations for clinical research standards. task force of the medical scientific advisory board of the myasthenia gravis foundation of America. Neurology (2000) 55(1):16–23. doi: 10.1212/wnl.55.1.16
9. Hehir MK, Hobson-Webb LD, Benatar M, Barnett C, Silvestri NJ, Howard JF Jr, et al. Rituximab as treatment for anti-MuSK myasthenia gravis: Multicenter blinded prospective review. Neurology (2017) 89(10):1069–77. doi: 10.1212/WNL.0000000000004341
10. Machado PM, Lawson-Tovey S, Strangfeld A, Mateus EF, Hyrich KL, Gossec L, et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR coronavirus vaccine (COVAX) physician-reported registry. Ann Rheum Dis (2022) 81(5):695–709. doi: 10.1136/annrheumdis-2021-221490
11. Auriel E, Regev K, Dori A, Karni A. Safety of influenza and H1N1 vaccinations in patients with myasthenia gravis, and patient compliance. Muscle Nerve (2011) 43(6):893–4. doi: 10.1002/mus.22077
12. Zinman L, Thoma J, Kwong JC, Kopp A, Stukel TA, Juurlink DN. Safety of influenza vaccination in patients with myasthenia gravis: a population-based study. Muscle Nerve (2009) 40(6):947–51. doi: 10.1002/mus.21440
13. Tackenberg B, Schneider M, Blaes F, Eienbröker C, Schade-Brittinger C, Wellek A, et al. Acetylcholine receptor antibody titers and clinical course after influenza vaccination in patients with myasthenia gravis: A double-blind randomized controlled trial (ProPATIent-trial). EBioMedicine (2018) 28:143–50. doi: 10.1016/j.ebiom.2018.01.007
14. Strijbos E, Tannemaat MR, Alleman I, de Meel R, Bakker JA, van Beek R, et al. A prospective, double-blind, randomized, placebo-controlled study on the efficacy and safety of influenza vaccination in myasthenia gravis. Vaccine (2019) 37(7):919–25. doi: 10.1016/j.vaccine.2019.01.007
15. Seok HY, Shin HY, Kim JK, Kim BJ, Oh J, Suh BC, et al. The impacts of influenza infection and vaccination on exacerbation of myasthenia gravis. J Clin Neurol (2017) 13(4):325–30. doi: 10.3988/jcn.2017.13.4.325
Keywords: safety, myasthenia gravis, SARS-CoV-2, vaccination, survey
Citation: Li H-Y, Shao L-Y, Song M, Hu S-M, Yue Y-X and Li H-F (2022) Safety of inactivated SARS-CoV-2 vaccines in myasthenia gravis: A survey-based study. Front. Immunol. 13:923017. doi: 10.3389/fimmu.2022.923017
Received: 18 April 2022; Accepted: 27 June 2022;
Published: 05 August 2022.
Edited by:
Maria Giovanna Danieli, Università Politecnica delle Marche, ItalyReviewed by:
Valentina Damato, University of Florence, ItalyHonghao Wang, Southern Medical University, China
Arad Dotan, Sheba Medical Center, Israel
Copyright © 2022 Li, Shao, Song, Hu, Yue and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao-Xian Yue, yyx12550@163.com; Hai-Feng Li, drlhf@163.com
 Hong-Yan Li
Hong-Yan Li Li-Yuan Shao2
Li-Yuan Shao2 Shi-Min Hu
Shi-Min Hu Yao-Xian Yue
Yao-Xian Yue Hai-Feng Li
Hai-Feng Li