- 1Department of Rheumatology, The Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang, China
- 2Department of Rheumatology, Key Laboratory of Myositis, China-Japan Friendship Hospital, Beijing, China
Introduction: Reports of unexpected side effects have accompanied the vaccination of larger proportions of the population against coronavirus disease 2019 (COVID-19), including a few cases of inflammatory myopathy (IM). In a bid to improve understanding of the clinical course of vaccine complications, a systematic review of reported cases of IM following COVID-19 vaccination has been conducted.
Methods: The PRISMA guideline 2020 was followed. Two independent investigators systematically searched PubMed and Embase to identify relevant studies published up to July 2022, using the following keywords: COVID-19 Vaccine, inflammatory myositis. The Joanna Briggs Institute critical appraisal tools were used for the risk of bias.
Results: A total of 24 articles presenting clinical features of 37 patients with IM following COVID-19 vaccine were identified. Female patients composed 59.5% of cases and 82.4% had been vaccinated with BNT162b2 or ChAdOx1. Onset of symptoms occurred within 2 weeks of the first or second vaccine dose in 29 (85.3%) patients and included muscular weakness in 54.1% and skin rash in 71.4% of patients. Myositis specific autoantibodies (MSAs) and myositis associated autoantibodies (MAAs) were reported in 28 patients. Specific clinical subtypes of myositis, reported in 27 patients, included 22 (81.5%) cases of dermatomyositis (DM) and 3 (11.1%) cases of immune-mediated necrotizing myopathy (IMNM). Following treatment, 32 (86.5%) patients showed improvement on follow-up.
Conclusion: COVID-19 vaccine may induce various clinical myositis subtypes and related antibodies. Muscular weakness was the most common presenting symptom. Clinicians should be aware of this unexpected adverse event following COVID-19 vaccination and arrange for appropriate management.
Systematic review registration: INPLASY https://inplasy.com/inplasy-2022-9-0084/ [INPLASY202290084].
Introduction
Inflammatory myopathy (IM) belongs to a rare group of clinically heterogeneous, autoimmune inflammatory disorders characterized by muscular weakness and multi-system involvement, including lung and skin. Dermatomyositis (DM), anti-synthetase syndrome (ASS), immune-mediated necrotizing myopathy (IMNM), polymyositis (PM) and inclusion body myositis (IBM) have long been recognized as distinct subtypes of IM (1–3). The pathogenesis of IM is complicated and poorly understood but an autoimmune response is known to be involved. Vaccines exert a potent stimulatory effect on the immune system and have the potential to induce or exacerbate serologic findings or clinical autoimmune diseases. The pandemic caused by coronavirus disease 2019 (COVID-19) had a devastating and worldwide impact on public health, social life and the economy (4–7). The development of vaccines which successfully prevent severe illness from SARS-CoV-2 infection are considered one of the effective solutions (8).
Safety concerns have surrounded the vaccines since their development, with common adverse effects including local reaction at the site of injection and diverse non-specific flu-like symptoms (9). Most symptoms occur soon after vaccination and resolve within a short period but some serious events such as myopericarditis and cerebral venous thrombosis post COVID-19 vaccination had been reported (10–12). Meanwhile, some rare cases of vaccine-associated IMs have been reported (13–36). The current study systematically reviewed IM cases reported post-COVID-19 vaccination to date. Clinical and laboratory features are described and therapy and prognosis discussed.
Methods
Protocol and registration
We registered our study on INPLASY website (Register number INPLASY202290084) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (37).
Eligibility criteria
The study was undertaken to systematically review IM patients followed with COVID-19 vaccination. A PICOS acronym was used to formulate the questions for this study: (1) participants (patients with IMs), (2) intervention or exposition (COVID-19 vaccination), (3) comparison or control (not applicable), (4) outcomes measures (time, dose and type of COVID-19 vaccination), (5) types of studies included (case reports and series of cases).
We set the exclusion criteria as follows: (1) studies that described non-inflammatory myopathy, (2) reviews of the literature, personal opinions and conference abstracts, (3) full articles not found, (4) duplicate cases from other studies, (5) suspected/ probable cases of SARS-CoV-2 infection.
Search strategy
Two independent investigators systematically searched PubMed and Embase to identify relevant studies published up to July 2022. We searched Medical Subject Headings terms and keywords in multiple combinations, including COVID-19 or SARS-CoV-2 vaccine, dermatomyositis, myositis or inflammatory myopathy.
Data collection
Two authors evaluated abstracts and titles of each article and a full-text review was performed as relevant considering all inclusion and exclusion criteria. Another rheumatologist reviewed all articles where there was no consensus in the initial evaluation to determine which articles would be included. After selection and evaluation, data from the included studies were extracted, including study authors and year, publications, study design, and basic information of the patients.
Risk of bias
The risk of bias for each included study was assessed using the Joanna Briggs Institute Critical Appraisal checklist for case reports and case series. Disagreements were solved between the two investigators by consensus or by another independent investigator.
Results
Study selection and general information
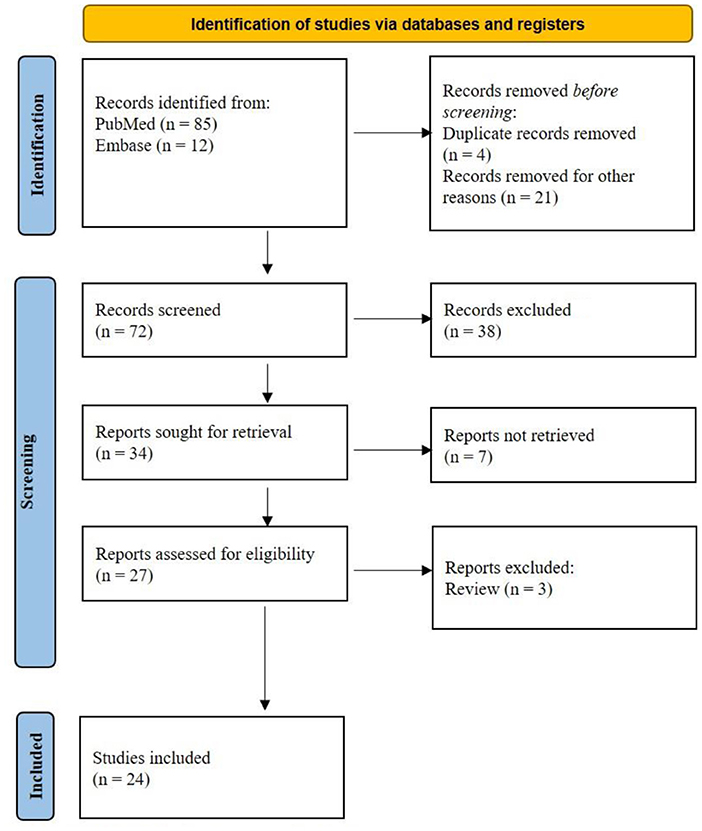
PubMed and Embase provided 97 titles and abstracts for review. Of these, a total of five articles were excluded due to duplication. After screening, 34 full articles were further assessed for eligibility. Eventually, 24 studies (10–33) containing 37 patients were selected for final analysis (Figure 1).
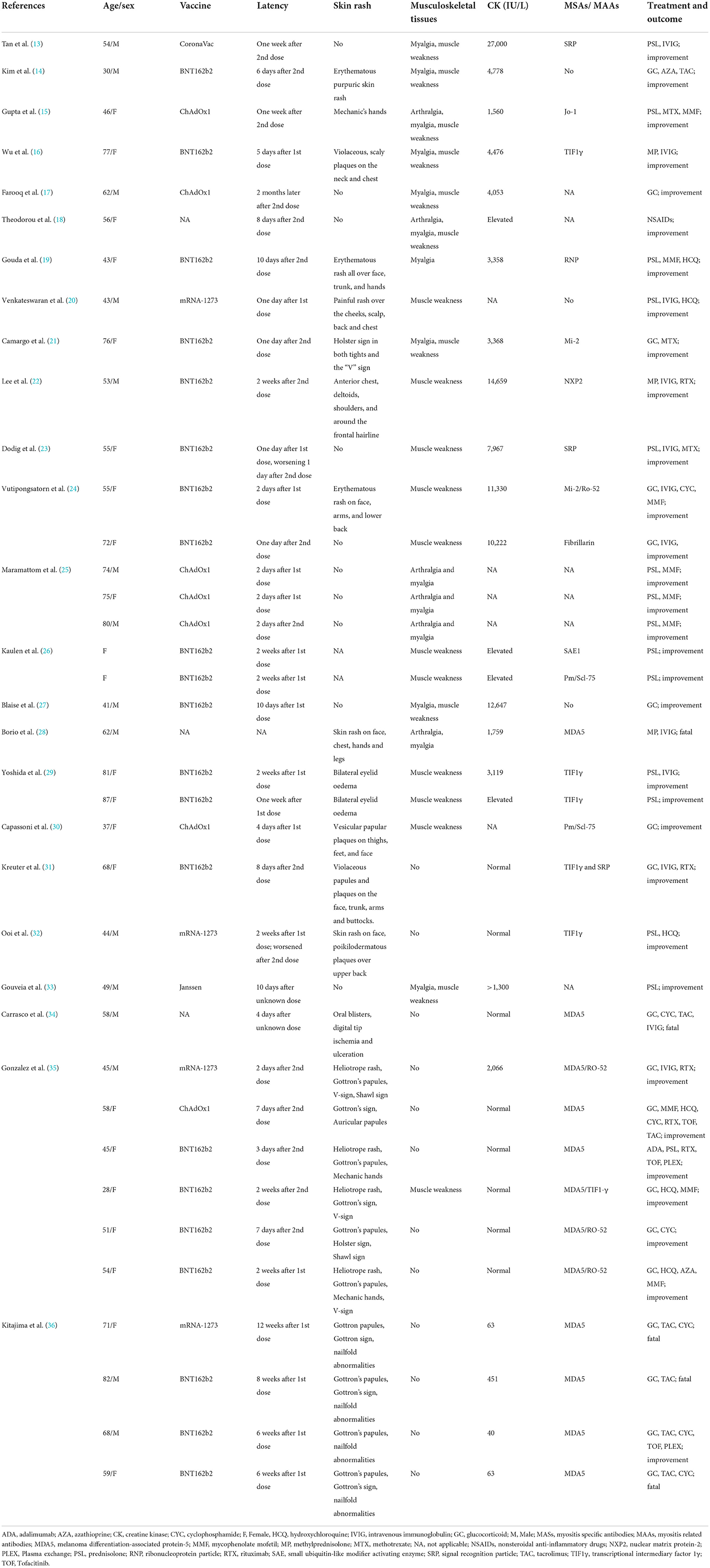
Twenty-two female and 15 male (ratio: 1.5: 1) patients were included with age of onset ranging from 28 to 87 years (median: 56 years). Twenty-four (68.6%) patients were over 50 years old (see Table 1).
COVID-19 vaccine information
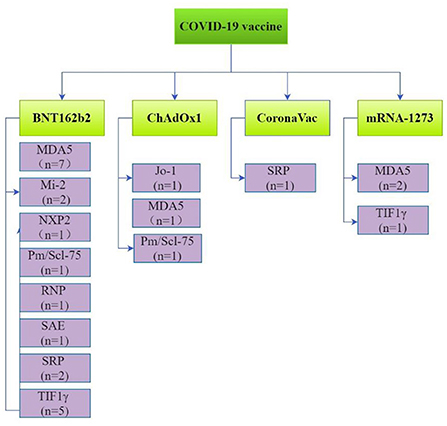
COVID-19 vaccine manufacturers were reported by 21 studies including 34 patients as follows: 21 (61.8%) received Pfizer/BioNTech (BNT162b2); 7 (20.6%) Oxford-AstraZeneca (AZD1222, ChAdOx1); 4 Moderna (mRNA-1273); 1 Janssen and 1 Sinovac Biotech (CoronaVac) vaccines. Thirty-four patients reported the interval between vaccination and symptom onset with 18 (52.9%) patients developing symptoms after the first dose of COVID-19 vaccine and 16 (47.1%) after the second dose. Furthermore, 14/18 (77.8%) patients developed symptoms within 2 weeks of the first dose and 15/16 (93.8%) within 2 weeks of the second dose. Overall, 29/34 (85.3%) patients developed myositis symptoms within 2 weeks of the first or second dose of the COVID-19 vaccine.
Clinical characteristics
The vast majority of patients (26/37; 70.3%) had muscle involvement with 13 (35.1%%) cases showing myalgia and 20 (54.1%) muscle weakness. Pharyngeal muscles were involved in four patients, causing dysarthria and dysphagia. From available data, 25 patients (71.4%) with skin symptoms, including the Heliotrope sign, Gottron's rash, “V” sign, mechanic hand and other atypical rashes. Available clinical data indicated that 12/30 (40%) patients had fever, 8 (26.7%) had arthralgia and 14 (46.7%) dyspnea. Interstitial lung disease (ILD) was reported in 13 (43.3%) patients.
T2-weighted magnetic resonance imaging (MRI) was performed for 15 patients and demonstrated increased uptake in muscle groups. Inflammatory myopathy was confirmed by muscle biopsy or skin biopsy in 18 patients. Serum CK increased significantly in 21 patients, ranging from 451 to 27,000 IU/L. Specific clinical subtypes of myositis were reported for 27 patients, 22 (81.5%) of whom had DM, 3 (11.1%) had IMNM, 1 (3.7%) had ASS, 1 (3.7%) had PM and the other 10 patients received no specific classification (see Figure 2).
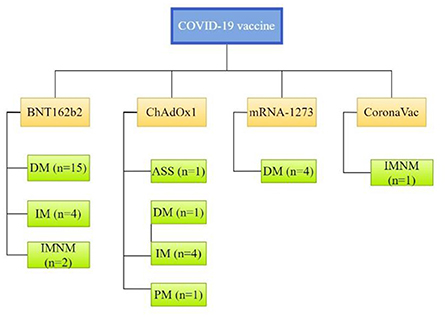
Figure 2. Clinical subtypes of myositis associated with individual CoV-19 vaccines. ASS, anti-synthetase syndrome; DM, dermatomyositis; IM, inflammatory myopathy; IMNM, immune-mediated necrotizing myopathy; PM, polymyositis.
Myositis specific antibodies (MASs) and myositis related antibodies (MAAs) were reported in 28 patients. These included antibodies against melanoma differentiation-associated protein-5 (MDA5; n = 11), transcriptional intermediary factor 1γ (TIF1γ; n = 6), signal recognition particle (SRP; n = 3), Mi-2 (n = 2), nuclear matrix protein-2 (NXP2; n = 1), small ubiquitin-like modifier activating enzyme (SAE; n = 1), Jo-1 (n = 1), PM/Scl-75 (n = 2), fibrillarin (n = 1) and ribonucleoprotein particle (RNP; n = 1; Figure 3).
Treatment and prognosis
A range of treatments was reported by the literature under review. One patient received no immunotherapy except for non-steroidal anti-inflammatory drugs (NSAIDs). Thirty-six patients were treated with glucocorticoids, with nine receiving oral and/or intravenous glucocorticoid monotherapy and 27 patients receiving glucocorticoids combined with intravenous immunoglobulin (IVIG) or other immune agents, such as methotrexate (MTX), cyclophosphamide (CYC) or Rituximab (RTX). Thirty-two (86.5%) patients had experienced partial or complete remission by follow-up and 5 patients of MDA5-DM had poor prognosis due to rapid progression of ILD and lung infection.
Risk of bias in included studies
High risk of bias was identified in this analysis due to the lack of description of the demographic characteristics for some patients in the case reports and case series. In addition, this review described rarity of such events; therefore, the included studies were highly heterogeneous. Although the representative sample of each study was not a cause for concern, this caused the lack of statistical data for additional analysis.
Discussion
The current literature review indicates that COVID-19 vaccination-associated IM patients were predominantly women with a median age of 56 years. Most patients were vaccinated with Pfizer/BioNTech's (BNT162b2) and more than half reported symptoms following the first dose. Most patients had muscle weakness and a range of myositis subtypes had occurred. Most patients responded well to glucocorticoids and/or immunosuppressants.
As with other autoimmune conditions, the precise etiology of IM is unknown, although genetic and environmental factors determine susceptibility (38). The category of environmental factors may be considered to include a number of vaccines (39). Vaccine-induced autoimmunity may be due to molecular mimicry between host cell and antigen or to a direct response to vaccine adjuvants. A clear link between IMs and vaccination has yet to be found but there remain many case reports of vaccine associated myositis (40, 41). Vaccines against hepatitis B, influenza, tetanus toxoid, H1N1 and BCG are all thought to have a temporal association with DM (42).
Whereas, the latency between COVID-19 vaccination and development of myositis symptoms varied, over half the cases had symptoms within 2 weeks of the first dose. Indeed, symptom onset occurred within 1 week for most of the patients. This represents a similar latency period to that observed with other vaccine induced myositis (42).
The majority of cases followed BNT162b2 or ChAdOx1 vaccinations, in agreement with previous reports. A study conducted in Germany evaluated neurological autoimmune diseases following COVID-19 vaccinations in which 20 of 21 patients were vaccinated with BNT162b2 (n = 12) or ChAdOx1 (n = 8) (26).
A range of clinical IM subtypes, such as DM, ASS, IMNM and PM, and induction of different myositis antibodies, such as TIF1γ, SRP, NXP2, Mi-2 and Pm/Scl-75, were found to follow COVID-19 vaccination. Furthermore, subtypes and antibody types were dependent on vaccine manufacturer. These observations indicate the heterogeneity of post COVID-19 vaccine myositis.
Dodig et al. reported the case of a 55-year-old female patient who developed myalgia, chills, fatigue and vague generalized weakness the day after her first dose of BNT162b2 vaccine. By day 21, after she had received a second dose of BNT162b2 vaccine, the symptoms had worsened to progressive weakness and on day 49 she presented at an emergency department unable to walk with a serum CK level of 7,967 IU/L (23). This reminds us that if the adverse reactions to the first vaccine dose are serious, the second dose may cause further aggravation. Specialist guidance is required for those who have a serious reaction to the first vaccine dose as to whether a second dose should be recommended.
Although the COVID-19 vaccine induces myositis symptoms, such as muscle weakness and rash, fortunately, most patients improve and symptoms may be completely relieved after receiving glucocorticoids or other immunotherapy. About half of DM patient with MDA5 antibody progressed to the complication of severe ILD which led to respiratory failure and poor prognosis despite active treatment. The anti-MDA5 antibody positive form of DM is prone to RPILD with a high mortality rate (43). Whether the vaccine accelerates disease progress is unclear and requires further research.
Many COVID-19 vaccines work by inducing a T-cell-mediated immune response to a protein translated from vaccine-delivered mRNA. mRNA vaccines show a significant level of immunogenicity and strong induction of type I interferon is a distinguishing feature (44). It is noteworthy that IM patients, especially those with DM, have an increase in type I interferon-inducible genes in muscle fibers, endothelial cells, skin and peripheral blood (45).
Limitations
Some limitations should be considered. This review acknowledges that most of the studies were case reports, and involved small sample sizes. The protocol was submitted retrospectively. This should be noted as a major limitation. Protocols should be written prospectively i.e., before the project is conducted. Due to the heterogeneity of these studies, we cannot perform further pooled analysis of their data. However, the risk of bias shows that the articles do not follow all guidelines to appropriately report the cases of IM patients followed COVID-19 vaccination. To more clearly show the relationship between myositis and COVID-19 vaccination, more studies and multi-center data should be conducted in the future.
Conclusion
We acknowledge that temporal association does not equate with causality and extensive epidemiological studies would be required to prove causality. A thorough clinical and pathological examination of further patients is required to elucidate the association between COVID-19 vaccination and IM. However, if vaccinated individuals experience severe and continuous muscle pain and weakness, clinicians should consider vaccine-induced inflammatory myositis which may be confirmed by measuring muscle enzyme levels and myositis autoantibodies and performing muscle biopsy for a definitive diagnosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YD: data curation and formal analysis. YG: data curation, methodology, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National High Level Hospital Clinical Research Funding (2022-NHLHCRF-YS-02).
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1007637/full#supplementary-material
References
1. Pipitone N, Salvarani C. Up-to-date treatment and management of myositis. Curr Opin Rheumatol. (2020) 32:523–7. doi: 10.1097/BOR.0000000000000745
2. Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. (2018) 5:109–29. doi: 10.3233/JND-180308
3. Lundberg IE, Fujimoto M, Vencovsky J, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Primers. (2021) 7:86. doi: 10.1038/s41572-021-00321-x
4. To KK, Sridhar S, Chiu KH, Hung DL Li X, Hung IF, et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg Microbes Infect. (2021) 10:507–35. doi: 10.1080/22221751.2021.1898291
5. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. (2021) 19:141–54. doi: 10.1038/s41579-020-00459-7
6. Saud A, Naveen R, Aggarwal R, Gupta L. COVID-19 and myositis: what we know so far. Curr Rheumatol Rep. (2021) 23:63. doi: 10.1007/s11926-021-01023-9
7. Dalakas MC. Guillain-Barré syndrome: The first documented COVID-19-triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e781. doi: 10.1212/NXI.0000000000000781
8. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
9. Hernández AF, Calina D, Poulas K, Docea AO, Tsatsakis AM. Safety of COVID-19 vaccines administered in the EU: should we be concerned? Toxicol Rep. (2021) 8:871–9. doi: 10.1016/j.toxrep.2021.04.003
10. Ling RR, Ramanathan K, Tan FL, Tai BC, Somani J, Fisher D, et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med. (2022) 10:679–88. doi: 10.1016/S2213-2600(22)00059-5
11. Fan BE, Shen JY, Lim XR, Tu TM, Chang CCR, Khin HSW, et al. Cerebral venous thrombosis post BNT162b2 mRNA SARS-CoV-2 vaccination: a black swan event. Am J Hematol. (2021) 96:E357–61. doi: 10.1002/ajh.26272
12. Tu TM Yi SJ, Koh JS, Saffari SE, Hoe RHM, Chen GJ, et al. incidence of cerebral venous thrombosis following SARS-CoV-2 infection vs mRNA SARS-CoV-2 vaccination in Singapore. JAMA Netw Open. (2022) 5:e222940. doi: 10.1001/jamanetworkopen.2022.2940
13. Tan CY, Toh TH, Toh YF, Toh YF, Wong KT, Shahrizaila N, et al. A temporal association between COVID-19 vaccination and immune-mediated necrotizing myopathy. Muscle Nerve. (2022) 65:E24–E26. doi: 10.1002/mus.27531
14. Kim JH, Kim JH, Woo CG. Clinicopathological characteristics of inflammatory myositis induced by COVID-19 vaccine (Pfizer-BioNTech BNT162b2): a case report. J Korean Med Sci. (2022) 37:e91. doi: 10.3346/jkms.2022.37.e91
15. Gupta K, Sharma GS, Kumar A. COVID-19 vaccination-associated anti-Jo-1 syndrome. Reumatologia. (2021) 59:420–2. doi: 10.5114/reum.2021.111836
16. Wu M, Karim M, Ashinoff R. COVID-19 vaccine-associated dermatomyositis. JAAD Case Rep. (2022) 23:58–60. doi: 10.1016/j.jdcr.2022.02.023
17. Farooq M, Mohammed Y, Zafar M, Dharmasena D, Rana UI, Kankam O. COVID-19 vaccine-induced pneumonitis, myositis and myopericarditis. Cureus. (2022) 14:e20979. doi: 10.7759/cureus.20979
18. Theodorou DJ, Theodorou SJ, Axiotis A, Gianniki M, Tsifetaki N. COVID-19 vaccine-related myositis. QJM. (2021) 114:424–5. doi: 10.1093/qjmed/hcab043
19. Gouda W, Albasri A, Alsaqabi F, Al Sabah HY, Alkandari M, Abdelnaby H. Dermatomyositis following BNT162b2 mRNA COVID-19 vaccination. J Korean Med Sci. (2022) 37:e32. doi: 10.3346/jkms.2022.37.e32
20. Venkateswaran K, Aw DC, Huang J, Angkodjojo S. Dermatomyositis following COVID-19 vaccination. Dermatol Ther. (2022) 35:e15479. doi: 10.1111/dth.15479
21. Camargo Coronel A, Jiménez Balderas FJ, Quiñones Moya H, Hernández Zavala MR, Mandinabeitia Rodríguez P, Hernández Vázquez JR, et al. Dermatomyositis post vaccine against SARS-COV2. BMC Rheumatol. (2022) 6:20. doi: 10.1186/s41927-022-00250-6
22. Lee AYS, Lee C, Brown DA, Suan D. Development of anti-NXP2 dermatomyositis following Comirnaty COVID-19 vaccination. Postgrad Med J. (2022) postgradmedj-2022-141510. doi: 10.1136/postgradmedj-2022-141510
23. Dodig D, Fritzler MJ, Naraghi A, Tarnopolsky MA, Lu JQ. Immune-mediated necrotizing myopathy after BNT162b2 vaccination in a patient with antibodies against receptor-binding domain of SARS-CoV-2 and signal recognition particle. Muscle Nerve. (2022) 65:E11–3. doi: 10.1002/mus.27483
24. Vutipongsatorn K, Isaacs A, Farah Z. Inflammatory myopathy occurring shortly after severe acute respiratory syndrome coronavirus 2 vaccination: two case reports. J Med Case Rep. (2022) 16:57. doi: 10.1186/s13256-022-03266-1
25. Maramattom BV, Philips G, Thomas J, Santhamma SGN. Inflammatory myositis after ChAdOx1 vaccination. Lancet Rheumatol. (2021) 3:e747–9. doi: 10.1016/S2665-9913(21)00312-X
26. Kaulen LD, Doubrovinskaia S, Mooshage C, Jordan B, Purrucker J, Haubner C, et al. Neurological autoimmune diseases following vaccinations against SARS-CoV-2: a case series. Eur J Neurol. (2022) 29:555–63. doi: 10.1111/ene.15147
27. Blaise M, Rocher F, Spittler H, Sanchez A, Lanteri E, Coco L, et al. Severe necrotizing myopathy after COVID-19 vaccine with BNT162b2 and regimen with ipilimumab plus nivolumab in a patient with advanced melanoma. J Eur Acad Dermatol Venereol. (2022) 36:e100–2. doi: 10.1111/jdv.17760
28. Borio G, Terracciano C, Buttafava F, Vercelli A, Pagani L, Zanzani C, et al. Skin rash and interstitial pneumonia can be a fatal combination: a rare case of anti-melanoma differentiation-associated gene 5 (MDA5)-associated interstitial lung disease. Eur J Case Rep Intern Med. (2021) 8:002860. doi: 10.12890/2021_002860
29. Yoshida A, Ikegami T, Igawa K. Two cases of anti-TIF1-γ antibody positive dermatomyositis with manifested symptoms after SARS-CoV-19 vaccination. J Eur Acad Dermatol Venereol. (2022) 36:e517–20. doi: 10.1111/jdv.18060
30. Capassoni M, Ketabchi S, Cassisa A, Caramelli R, Molinu AA, Galluccio F, et al. AstraZeneca (AZD1222) COVID-19 vaccine-associated adverse drug event: a case report. J Med Virol. (2021) 93:5718–20. doi: 10.1002/jmv.27175
31. Kreuter A, Lausch S, Burmann SN, Paschos A, Michalowitz AL. Onset of amyopathic dermatomyositis following mRNA-based SARS-CoV-2 vaccination. J Eur Acad Dermatol Venereol. (2022) 36:e669–e672. doi: 10.1111/jdv.18211
32. Ooi XT, Choi EC, Lee JS. Manifestation of a cancer-associated TIF-1 gamma dermatomyositis after COVID-19 vaccine. Int J Dermatol. (2022) 14. doi: 10.1111/ijd.16358
33. Gouveia J, Barros C, Caldeira M, Ferreira C, Freitas R. Inflammatory myopathy secondary to SARS-CoV-2 vaccination. Isr Med Assoc J. (2022) 24:444.
34. Carrasco L, Arthur A, Gaitan Rueda J, Case C. A rapidly progressive and rare illness: autoantibodies against melanoma differentiation-associated protein 5 (anti-MDA5): amyopathic dermatomyositis with progressive interstitial lung disease that developed after covid-19 vaccine. Chest J. (2021) 160:A680–A681. doi: 10.1016/j.chest.2021.07.646
35. Gonzalez D, Gupta L, Murthy V, Gonzalez EB, Williamson KA, Makol A, et al. Anti-MDA5 dermatomyositis after COVID-19 vaccination: a case-based review. Rheumatol Int. (2022) 4:1–13. doi: 10.1007/s00296-022-05149-6
36. Kitajima T, Funauchi A, Nakajima T, Marumo S, Imura Y, Fukui M. Anti-melanoma differentiation-associated gene 5 antibody-positive interstitial lung disease after vaccination with COVID-19 mRNA vaccines. J Rheumatol. (2022) 15:jrheum.220259. doi: 10.3899/jrheum.220259
37. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
38. Reed AM, Ytterberg SR. Genetic and environmental risk factors for idiopathic inflammatory myopathies. Rheum Dis Clin North Am. (2002) 28:891–916. doi: 10.1016/S0889-857X(02)00029-7
39. Orbach H, Tanay A. Vaccines as a trigger for myopathies. Lupus. (2009) 18:1213–6. doi: 10.1177/0961203309345734
40. Mamarabadi M, Baisre A, Leitch M, Hsu V, Kanduri JS, Chen S. Case of anti-single recognition particle-mediated necrotizing myopathy after influenza vaccination. J Clin Neuromuscul Dis. (2018) 19:211–6. doi: 10.1097/CND.0000000000000208
41. Ferri C, Colaci M, Manzini CU, Sebastiani M, Giuggioli D, Brugioni L. Polymyositis following pandemic influenza A (H1N1) and 2009-10 seasonal trivalent vaccines. Case Rep Rheumatol. (2012) 2012:836930. doi: 10.1155/2012/836930
42. Stübgen JP. A review on the association between inflammatory myopathies and vaccination. Autoimmun Rev. (2014) 13:31–9. doi: 10.1016/j.autrev.2013.08.005
43. González-Moreno J, Raya-Cruz M, Losada-Lopez I, Cacheda AP, Oliver C, Colom B. Rapidly progressive interstitial lung disease due to anti-MDA5 antibodies without skin involvement: a case report and literature review. Rheumatol Int. (2018) 38:1293–6. doi: 10.1007/s00296-018-3991-7
44. Wadman M. Public needs to prep for vaccine side effects. Science. (2020) 370:1022. doi: 10.1126/science.370.6520.1022
Keywords: inflammatory myopathy, dermatomyositis, coronavirus disease 2019 vaccine, SARS-CoV-2 vaccine, myositis specific autoantibodies
Citation: Ding Y and Ge Y (2022) Inflammatory myopathy following coronavirus disease 2019 vaccination: A systematic review. Front. Public Health 10:1007637. doi: 10.3389/fpubh.2022.1007637
Received: 01 August 2022; Accepted: 06 October 2022;
Published: 21 October 2022.
Edited by:
Haider Abdul-Lateef Mousa, University of Basrah, IraqReviewed by:
Zisis Kozlakidis, International Agency for Research on Cancer (IARC), FranceRyan Ruiyang Ling, National University of Singapore, Singapore
Copyright © 2022 Ding and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongpeng Ge, gyp2016@163.com
 Yukang Ding1
Yukang Ding1 Yongpeng Ge
Yongpeng Ge