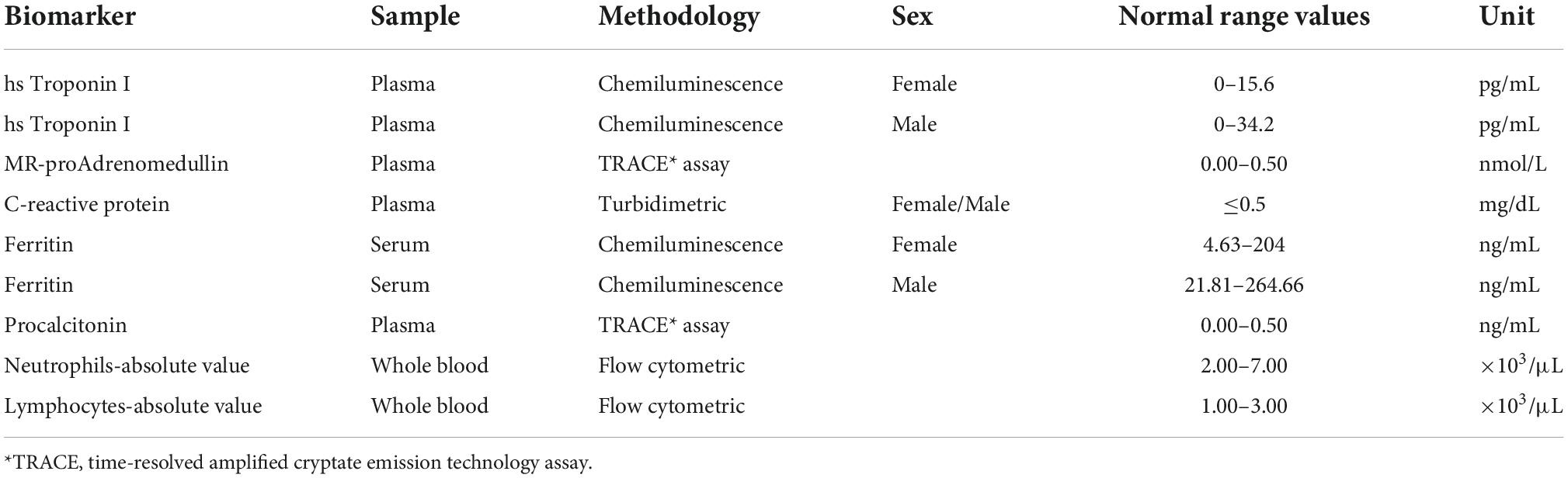
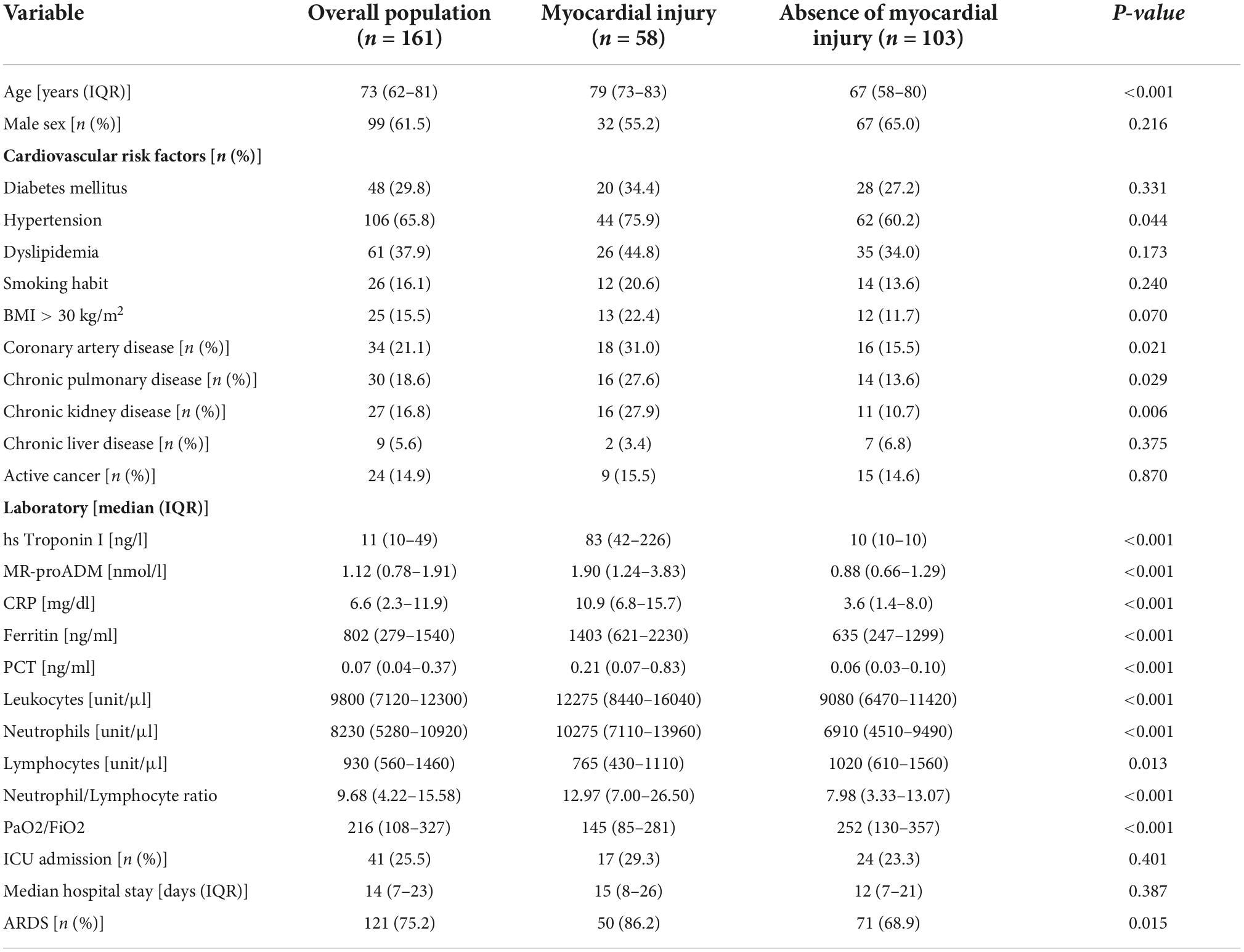
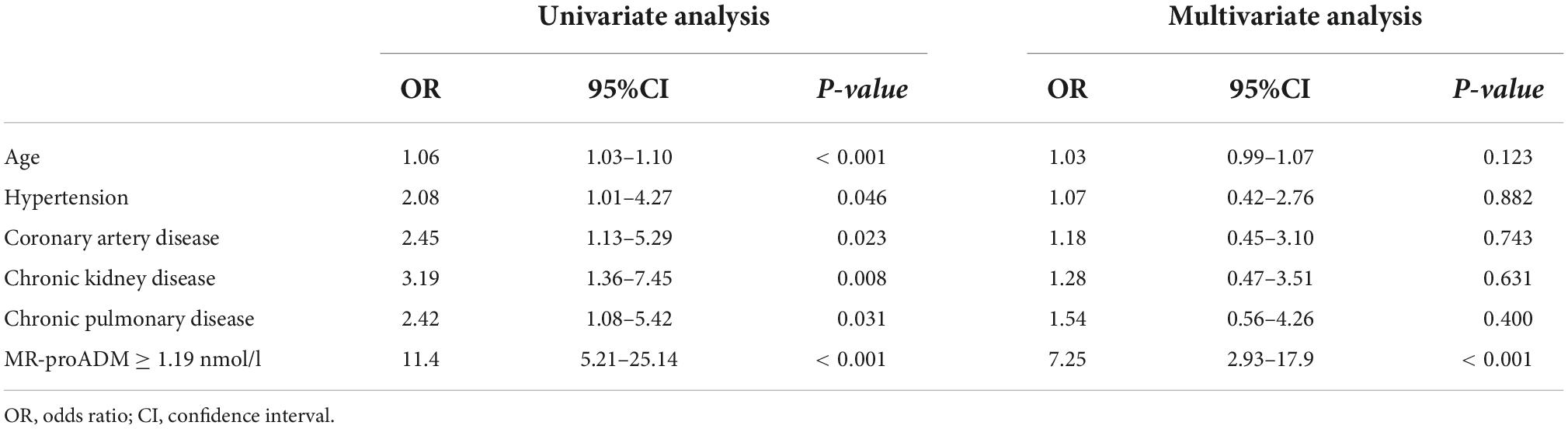
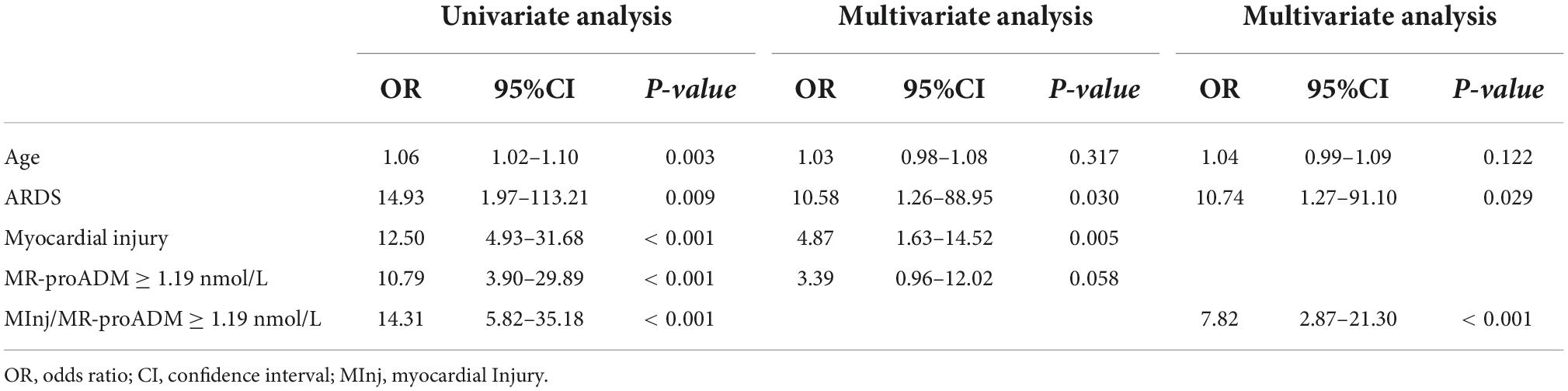
Synergistic effect of myocardial injury and mid-regional proAdrenomedullin elevation in determining clinical outcomes of SARS-CoV-2 patients
- 1Department of Diagnostic and Therapeutic Medicine, University Campus Bio-Medico of Rome, Rome, Italy
- 2Unit of Cardiovascular Science, University Campus Bio-Medico of Rome, Rome, Italy
- 3Division of Infectious Diseases, Department of Internal Medicine, Clinica Universidad de los Andes, Santiago Metropolitan, Chile
- 4Division of Cardiology, Department of Internal Medicine, Pauley Heart Center, Virginia Commonwealth University, Richmond, VA, United States
- 5Division of Infectious Disease and Epidemiology, Department of Internal Medicine, Virginia Commonwealth University, Richmond, VA, United States
- 6Central Virginia, Veterans Administration Hospital, Richmond, VA, United States
- 7Department of Emergency, University Campus Bio-Medico of Rome, Rome, Italy
- 8Unit of Clinical Laboratory Science, University Campus Bio-Medico of Rome, Rome, Italy
- 9Labotarory Research Unit, Fondazione Policlinico Universitario Campus Bio-Medico, Rome, Italy
- 10Unit of Medical Statistics and Molecular Epidemiology, University Campus Bio-Medico of Rome, Rome, Italy
Objective: Coronavirus disease 2019 (COVID-19) is a systemic disease induced by SARS-CoV-2 causing myocardial injury. To date, there are few data on the correlation between mid-regional proAdrenomedullin (MR-proADM) and myocardial injury. The aim of this study was to evaluate whether the association of myocardial injury and elevated mid-regional proAdrenomedullin values could predict mortality of SARS-CoV-2 patients, to offer the best management to COVID-19 patients.
Materials and methods: All patients hospitalized for SARS-CoV-2 infection at the COVID-19 Center of the Campus Bio-Medico of Rome University were included between October 2020 and March 2021 and were retrospectively analyzed. Myocardial injury was defined as rising and/or fall of cardiac hs Troponin I values with at least one value above the 99th percentile of the upper reference limit (≥15.6 ng/L in women and ≥34.2 ng/L in men). The primary outcome was 30-day mortality. Secondary outcomes were the comparison of MR-proADM, CRP, ferritin, and PCT as diagnostic and prognostic biomarkers of myocardial injury. Additionally, we analyzed the development of ARDS, the need for ICU transfer, and length of stay (LOS).
Results: A total of 161 patients were included in this study. Of these, 58 (36.0%) presented myocardial injury at admission. An MR-proADM value ≥ 1.19 nmol/L was defined as the optimal cut-off to identify patients with myocardial injury (sensitivity 81.0% and specificity 73.5%). A total of 121 patients (75.2%) developed ARDS, which was significantly more frequent among patients with myocardial injury (86.2 vs. 68.9%, p = 0.015). The overall 30-day mortality was 21%. Patients with myocardial injury presented significantly higher mortality compared to those without the same (46.6 vs. 6.8%, p < 0.001). When dividing the entire study population into four groups, based on the presence of myocardial injury and MR-proADM values, those patients with both myocardial injury and MR-proADM ≥ 1.19 nmol/L presented the highest mortality (53.2%, p < 0.001). The combination of myocardial injury and MR-proADM values ≥ 1.19 nmol/L was an independent predictor of death (OR = 7.82, 95% CI = 2.87–21.30; p < 0.001).
Conclusion: The study is focused on the correlation between myocardial injury and MR-proADM. Myocardial injury induced by SARS-CoV-2 is strongly associated with high MR-proADM values and mortality.
Introduction
Coronavirus disease 2019 (COVID-19) is a systemic disease induced by Severe Acute Respiratory Distress Syndrome Coronavirus 2 (SARS-CoV-2) causing widespread endothelial damage primarily involving the pulmonary and cardiovascular systems (1–4).
Acute cardiac injury in COVID-19 patients is present in upto 15–50% of critically ill patients and is represented by myocardial injury, endothelitis, heart failure, Takotsubo cardiomyopathy, acute coronary syndromes, pulmonary thromboembolism, and arrhythmias (2, 5–8).
Myocardial injury is defined as an increase in myocardial enzyme levels (Troponin) with at least one value above the 99th percentile upper reference limit in absence of myocardial ischemia and can be caused by several mechanisms (9). Myocardial injury occurs due to indirect or direct myocardial damage with a mortality of 60% (8).
Indirect myocardial injury evidenced by the increase of Troponin is present in up to 36% in the early course of SARS-CoV-2 infection and it is associated with an increased risk of requiring mechanical ventilation, fatal ventricular arrhythmias, and a 59.6% of risk mortality (10–15).
A direct myocardial injury affects hs Troponin I in case of acute coronary syndrome and could affect adrenomedullin expression that is expressed by cardiomyocytes, pericytes, cardiofibroblasts, endothelial cells, epicardial adipose cells, vascular endothelial cells, smooth muscle cells, and migratory angiogenic cells (16, 17).
Currently, the understanding of the underlying physiopathological mechanisms of the onset of myocardial injury is still limited and there are only little data on the correlation between myocardial injury and MR-proADM. This biomarker helps clinicians in identifying those patients with severe disease and at higher risk of death (4, 18–22).
The aim of this study was to evaluate whether the association of myocardial injury and elevated mid-regional proAdrenomedullin values could predict mortality of SARS-CoV-2 patients, to offer the best management to COVID-19 patients.
Materials and methods
Patient selection and characteristics
All patients hospitalized with SARS-CoV-2 infection at the COVID-19 Center of the Campus Bio-Medico of Rome University were included between October 2020 and March 2021 and were retrospectively analyzed. The COVID-19 Center includes the Medicine Department with a sub-intensive care unit.
We included all patients with a positive reverse transcription polymerase chain reaction test (RT-PCR) for SARS-CoV-2, with hs Troponin I and MR-proADM assessment. We excluded patients < 18 years old and pregnant women.
The study was approved by the Ethical Committee of the University Campus Bio-Medico of Rome.
All methods were performed in accordance with the relevant guidelines and regulations available at that moment.
The control group consisted of patients with SARS-CoV-2 infection without increased hs Troponin I or acute coronary syndrome (ACS), pericarditis, or myocarditis admitted to the COVID-19 center in the same period.
Clinical outcomes and definitions
Primary outcome of the study was 30-day mortality. Secondary outcomes were the comparison of MR-proADM, CRP, ferritin, and PCT as diagnostic and prognostic biomarkers of myocardial injury. Additionally, we analyzed the development of ARDS, the need for ICU transfer, and length of stay (LOS).
Myocardial injury was defined by the rise and/or fall of cardiac hs Troponin I values with at least one value above the 99th percentile of the upper reference limit; ARDS was defined according to the Berlin definition (9, 10, 23).
The following data were collected at inclusion: demographic characteristics (age and gender), onset symptoms, relevant comorbidities, immune status (active malignancy or other causes of immunosuppression), concomitant antimicrobial, use of antiretroviral medication, immunosuppressive treatments, and clinical presentation.
All patients received a complete physical examination including body temperature, blood pressure, heart and respiratory rate, cardiac, pulmonary, abdominal, and neurological evaluation, electrocardiogram, and chest tomography, while an echocardiogram was performed only if clinically needed.
Laboratory tests performed at inclusion were complete blood count (CBC), hs Troponin I, MR-proADM, CRP, ferritin, PCT, D-Dimer, INR, TTPA, liver function test, creatinine, arterial blood gases, and serum lactate.
All patients received standard of care based on disease severity and need for oxygen support. When needed, patients received low-molecular weight heparin, remdesivir, and steroid therapy.
All included patients were followed until death or 30-day follow-up, whichever came first.
Laboratory markers
Diagnosis of COVID-19 was performed by molecular testing through RT-PCR on a nasopharyngeal swab and/or endotracheal aspirate, detecting spike three SARS-CoV-2 genes (S, N, and E or S, RdRP, and N genes) (4).
Myocardial injury was considered when hs Troponin I was ≥15.6 ng/L in women and ≥34.2 ng/L in men.
Ferritin, hs Troponin I, and CRP were measured by Alinity c (Abbott, diagnostics) following the manufacturer’s instruction. Normal ranges are shown in Table 1. CBC was performed on a whole blood sample by Sysmex XE-9000 (Dasit, Italy) following the manufacturer’s instruction. NLR was calculated by the ratio between absolute values of neutrophils and lymphocytes. MR-proADM and PCT plasma concentrations were measured on an automated Kryptor analyzer, using a time-resolved amplified cryptate emission (TRACE) technology assay (Kryptor PCT; Brahms AG; Hennigsdorf, Germany), with commercially available immunoluminometric assays (Brahms) (24–27).
Statistical analysis
As appropriate, continuous variables were reported as mean (standard deviation) or as median (interquartile range). Categorical variables were reported as frequencies and percentages. Comparisons between continuous variables were performed using Student’s t-test or the Mann-Whitney U-test. Comparison between categorical variables was evaluated using the Fisher exact test or the Pearson chi-square test, as appropriate. The normal distribution of continuous variables was tested with the Shapiro-Wilk test. Correlation between continuous variables was assessed using the Spearman rank test. A receiver operating characteristic (ROC) curve analysis was used to test the ability of laboratory values to discriminate between patients with and without myocardial injury and patients who died and did not during the hospital stay. The optimal cutoff point was calculated by determining the value that provided the greatest sum of sensitivity and specificity. All baseline clinical features were evaluated in univariate analysis for the association with myocardial injury and death using logistic regression. Only variables with a p-value < 0.05 were considered for the final multivariable logistic regression models, providing odds ratios (ORs) and 95% confidence intervals (CI). Statistical analysis was performed using Stata/IC version 14.0 (STATA Corp., College Station, TX, USA), and p-values < 0.05 (2-tailed) were considered significant.
Results
Study population
A total of 161 patients were included in this study. Of these, 58/161 (36%) presented myocardial injury at admission. The characteristics of the patients are shown in Table 2. A total of 99/161 (61.5%) patients were males. Among them, 32/99 (32%) developed myocardial injury (p = 0.21).
Patients with myocardial injury were significantly older (79 [IQR = 73–83] vs. 67 [IQR = 58–80] years old, p < 0.001) than those without myocardial injury. These patients had a more frequent history of Hypertension (75.9 vs. 60.2%, p = 0.044), coronary artery disease (31 vs. 15.5%, p = 0.021), chronic pulmonary disease (27.6 vs. 13.6%, p = 0.029), and chronic kidney disease (27.9 vs. 10.7%, p = 0.006). Among the patients with myocardial injury, 3/58 (5.17%) had acute coronary syndrome.
In the overall population, 41/161 patients (25.5%) were admitted to the intensive care unit during the hospitalization, and the median hospital stay was 14 (7–23 IQR) days. A total of 121/161 patients (75.2%) developed ARDS, which was significantly more frequent among patients with myocardial injury (86.2 vs. 68.9%, p = 0.015). In-hospital mortality was 21% (34/161 of the overall population) and 46.6% (27/58 of patients with myocardial injury).
Myocardial injury
A significant correlation was found between hs Troponin I and MR-proADM levels (Spearman r = 0.569, p < 0.001). An MR-proADM value ≥ 1.19 nmol/L was defined as the optimal cut-off to identify patients with myocardial injury. This cut-off had 81.0% of sensitivity and 73.5% of specificity.
As reported in Table 2, patients with myocardial injury at admission showed significantly higher values of MR-proADM, CRP, ferritin, PCT, and neutrophil/lymphocyte ratio. At ROC curve analysis, all laboratory markers were able to discriminate between patients with and without myocardial injury (Figure 1 and Table 3). However, MR-proADM showed the greatest area under the curve ([AUC] 0.818, 95% CI = 0.750–0.875; p < 0.001). Pairwise comparison showed that the AUC of MR-proADM was significantly greater than the AUC of ferritin (p = 0.010) and neutrophil/lymphocyte ratio (0.021), but similar to that of CRP and PCT.
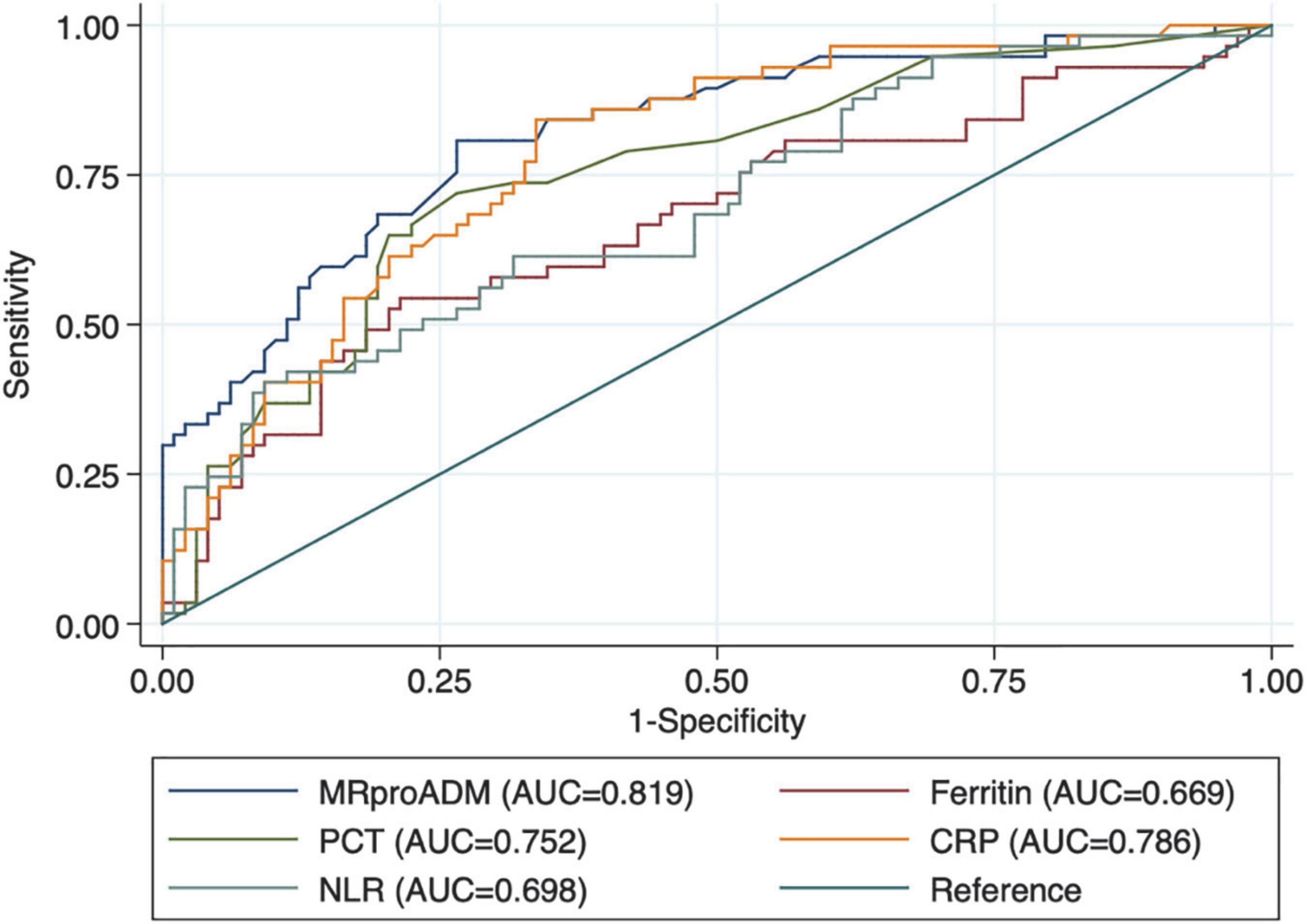
Figure 1. Receiver operating characteristic curves (ROC) of biomarkers in patients with myocardial injury.
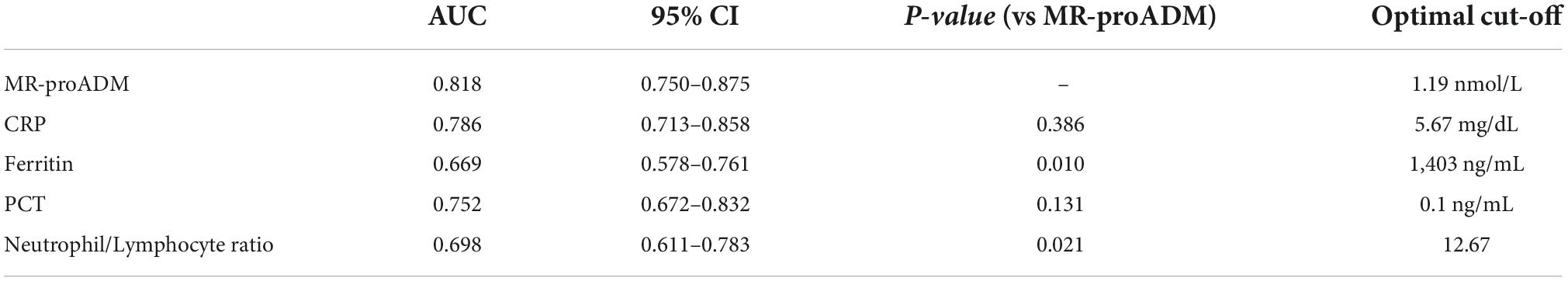
Table 3. Receiver operating characteristic (ROC) curves of laboratory markers for myocardial damage and pairwise comparison between mid-regional proAdrenomedullin (MR-proADM), C-reactive protein, ferritin, procalcitonin, and neutrophil/lymphocyte ratio.
Predictors of myocardial injury
At univariate analysis (Table 4), age, hypertension, a history of coronary artery disease, chronic pulmonary disease, chronic kidney disease, and MR-proADM ≥ 1.19 nmol/L were significantly associated with an increased risk of myocardial injury. In the multivariate analysis (Table 4), an MR-proADM value of ≥1.19 nmol/L was an independent predictor of increased risk of myocardial injury (OR = 7.25, 95% CI = 2.93–17.9, p < 0.001).
Predictors of death
Overall, 30-day death occurred in 34 (21.1%) patients and was significantly more frequent among those with myocardial injury (46.6 vs. 6.8%, p < 0.001). In the ROC curve analysis, MR-proADM was able to discriminate between patients who died and those who did not (AUC = 0.822, 95% CI = 0.751–0.877; p < 0.001; optimal cut-off ≥ 1.19 nmol/L). Among patients with MR-proADM values ≥ 1.19 nmol/L (n = 72), the incidence of death was significantly higher compared with those patients with low MR-proADM values (40.3 vs. 5.9%, p < 0.001). Also, when only considering patients with myocardial injury, MR-proADM was able to discriminate between patients who died and those who did not (AUC = 0.690, 95% CI = 0.551–0.820; p = 0.007; optimal cut-off ≥ 4.01 nmol/L). This cut-off had 40.7% of sensitivity and 89.7% of specificity.
When dividing the entire study population in four groups based on the presence of myocardial injury and MR-proADM values, 75 patients (46.6%) had no myocardial injury and MR-proADM < 1.19 nmol/L, 11 patients (6.8%) had a myocardial injury and MR-proADM < 1.19 nmol/L, 28 patients (17.4%) had no myocardial injury and MR-proADM ≥ 1.19 nmol/L, and 47 patients (29.2%) had a myocardial injury and MR-proADM ≥ 1.19 nmol/L. Death occurred in 3/75 (4.0%), 2/11 (18.2%), 4/28 (14.2%), 25/47 (53.2%), respectively (p < 0.001; Figure 2).
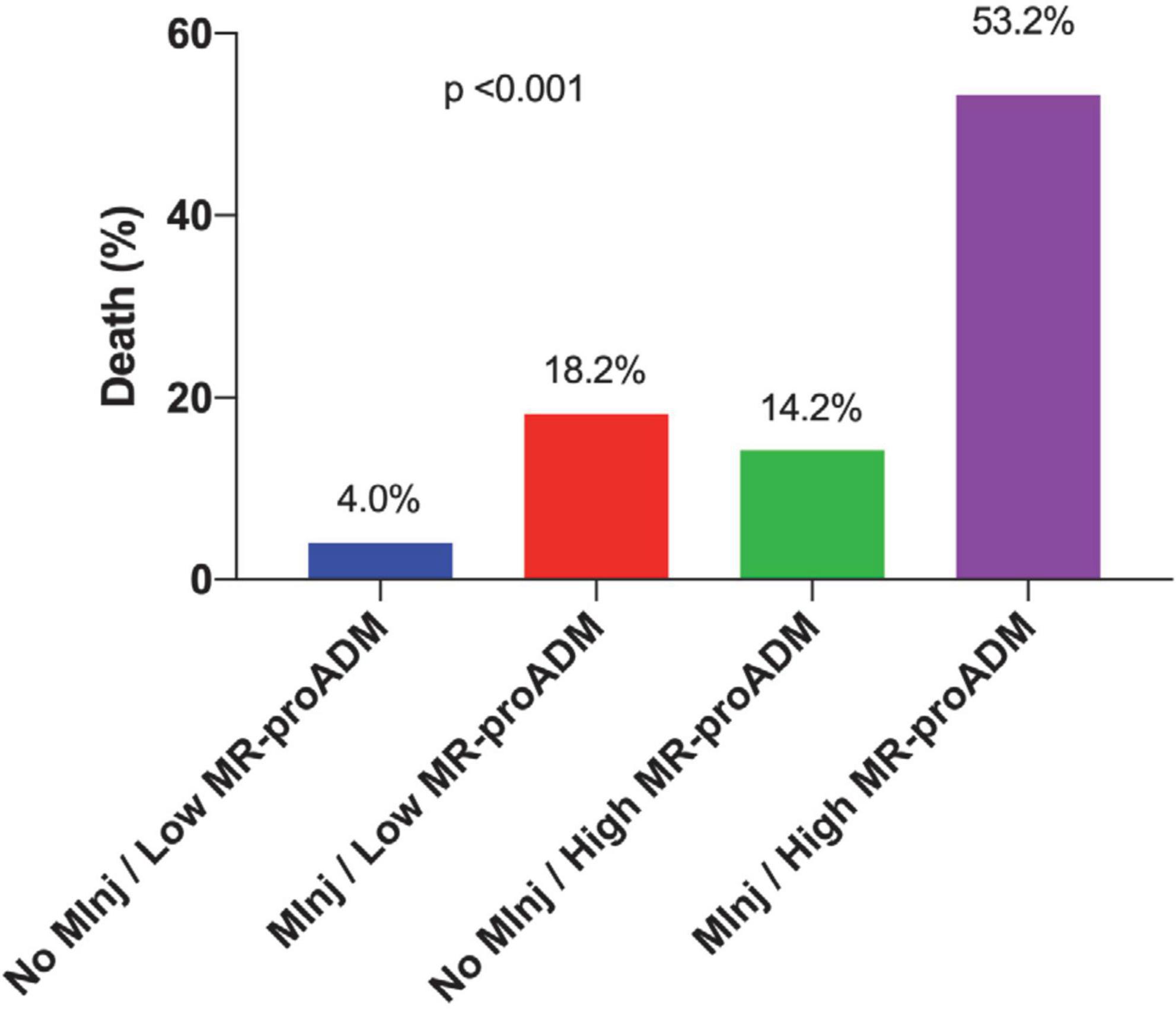
Figure 2. Incidence of in-hospital death according to the presence of myocardial injury and mid-regional proAdrenomedullin (MR-proADM) values of ≥1.19 nmol/L.
In the univariate analysis (Table 5), age, ARDS, myocardial injury, MR-proADM values of ≥1.19 nmol/L, and the combination of myocardial injury and MR-proADM values of ≥1.19 nmol/L were significantly associated with an increased risk of death.
When myocardial injury and MR-proADM values of ≥1.19 nmol/L were entered separately in the same multivariate model, myocardial injury (OR = 4.87, 95% CI = 1.63–14.52, p = 0.005) and ARDS (OR = 10.58, 95% CI = 1.26–88.95, p = 0.030) were independent predictors of increased risk of death (Table 5). In a separate model, the combination of myocardial injury and MR-proADM values ≥ 1.19 nmol/L was an independent predictor of death with an OR of 7.82 (95% CI = 2.87–21.30, p < 0.001; Table 5).
Discussion
The epidemiological data on myocardial injury in the literature is discordant. In the same way, the pathophysiological mechanism of myocardial injury onset is still unclear.
Consistent with the published data, 36% of the study population developed myocardial injury (11, 12). Among this group of patients, 27/58 (46.6%) died, in comparison with the 60% described in the literature (11, 12).
The mortality of patients with myocardial injury with both elevated values of hs Troponin I and MR-proADM ≥ 1.19 nmol/L reached 53.2% vs. mortality of 14.8% in the case of the elevated value of hs Troponin I only. Furthermore, the elevation of both biomarkers allowed the identification of patients with myocardial injury at higher mortality risk. In fact, if they were both negative the mortality was only 4%, but if both of them were positive, the mortality reached 53.2%.
These results agree with previous reports, where MR-proADM ≥ 2 nmol/L identified those patients affected by moderate/severe COVID-19 with high mortality risk related to multiple organ dysfunction syndrome, while values ≥3 nmol/L were predictive for ARDS development (4).
While an MR-proADM value of ≥1.19 nmol/L allows identifying patients with myocardial injury with high sensitivity and specificity, an MR-proADM value of ≥4.01 nmol/L allows identifying patients with myocardial injury at high risk of death with high specificity.
Therefore, the dosage of MR-proADM allows stratifying patients with myocardial injury at high risk of death by identifying patients who may also benefit from therapy with adrecizumab.
The median value of hs Troponin I in case of myocardial injury resulted in 83 vs. 11 ng/L of the overall population. Some studies had reported an optimal cut-off of 17 ng/L for Troponin T to predict mortality and of 0.03 μg/L for Troponin I in COVID-19 patients with cardiovascular disease (15, 28). These data suggest a role of hs Troponin I, not only as a marker of ischemia but also as a relevant biomarker of global stress for myocardial injury. In this way, hs Troponin I could be used to guide the prognosis and clinical management of the patients.
Our study shows that MR-proADM ≥ 1.19 nmol/L expresses myocardial injury with high diagnostic accuracy (sensitivity 81% and specificity 73.5%) when compared to ferritin and NLR ratio.
Of all bio-markers, MR-proADM was found to be the most specific of myocardial injury and SARS-CoV-2-related mortality.
MR-proADM ≥ 1.19 nmol/L has been shown to be an independent predictor of increased risk of myocardial injury and it has been significantly associated with risk factors of myocardial injury such as age, hypertension, history of coronary artery disease, and chronic pulmonary or kidney disease.
Age ≥ 65 years, male sex, and multicomorbidities increase the possibilities for developing severe SARS-CoV-2 infection, while pre-existing cardiovascular diseases, such as hypertension, diabetes mellitus, coronary artery disease, and heart failure, are associated with a worse prognosis (10, 14, 29–31).
Myocardial injury and MR-proADM ≥ 1.19 nmol/L were independent predictors of death (p < 0.001).
According to the literature, myocardial injury was also a predictor of in-hospital mortality.
Also, considering that acute cardiac injury in patients who died of COVID-19 has been reported in 35%, with detection of SARS-CoV-2 within the myocardium in 47% of post-mortem studied hearts (2, 5–7). Furthermore, one-third of severely ill COVID-19 patients develop acute kidney failure. Many of them require hemodyalitic procedures. This complication could weaken the diagnostic accuracy of Troponin value in the assessment of cardiac injury (10). It would be desirable to evaluate in further studies the combined dosage of hs Troponin I and MR-proADM, which could allow us to estimate with greater accuracy the real incidence of myocardial injury also in the absence of chest pain, troponin assessment, or evaluation of myocardial contractility.
The study has the limitation of being a single-center study and therefore the data obtained should be further confirmed by multicentric studies.
To our knowledge, this is one of the few studies that focused on the correlation between myocardial injury and MR-proADM. Values of MR-proADM ≥ 1.19 nmol/L correlate with myocardial injury and widespread endothelitis severity.
A myocardial injury might occur during SARS-CoV-2 infection as a consequence of myocardial, pulmonary, and endothelial damage. The mechanisms involved are represented by hypoxia that induces a decreased oxygen supply to the heart, causing modest or massive elevation of Troponin concentration, which is not necessarily correlated with deterioration of systolic left ventricular function but could be associated with right ventricular dysfunction due to acute right ventricular overload secondary to parenchymal or vascular lung disease resulting in subendocardial damage of the right ventricular myocardium in 19% of cases and by cytokine-induced injury (10, 15, 28, 32–35).
Adrenomedullin (ADM) is a protein that is released by endothelial and vascular smooth muscle cells following volume overload with the aim to preserve the endothelial barrier function. It binds to receptors prevalently found in cardiovascular and pulmonary systems (36–38). ADM induces vasodilatation, with consequent blood flow increase by reducing vasoconstriction acting as an inhibitor of the renin–angiotensin–aldosterone system (RAS). Furthermore, ADM contributes to endothelial integrity decreasing vascular permeability and acts as a bronchodilator.
Hypoxia, inflammatory cytokines, bacterial or viral products, shear stress, and vascular leakage represent stimuli for ADM up-regulation as it happens during SARS-CoV-2 infection, contributing to the failure of the ADM regulation (4, 39–41).
Disruption of the ADM system leads to (1) decrease of vascular resistance and capacitance vessels determining blood flow increase with hypoxic cardiac ischemia. (2) RAS activity reduces vasoconstriction, which leads to vascular leakage, increasing inflammation, and activation of the coagulation cascade. Additionally, RAS activation increases edema, oxidation, proliferation, and fibrosis, resulting in hypoxic cardiac ischemia and diffuse endothelitis that can lead to multiorgan failure (4, 42–50).
The mid-regional proAdrenomedullin (MR-proADM) is a peptide derived from ADM in a 1:1 ratio that can be used as a biomarker of organ failure, disease severity, and mortality in patients with COVID-19 (4, 51).
The alterations in endothelial cell lining are adaptive or maladaptive depending on disease extension, the time elapsed from disease onset, long-lasting viral shedding, and the host’s genetic heritage that expresses more or less ADM receptors, determining the extent of the immune-metabolic-inflammatory response. Instead, SARS-CoV-2 loads or variants have not so far indicated to influence the extent of organ damage (1, 52, 53).
Therefore, the role of ADM in COVID-19-related organ damage might suggest the use of new therapeutic agents, such as monoclonal antibodies. Adrecizumab, a humanized, monoclonal, non-neutralizing ADM-binding antibody could be used to improve vascular integrity, tissue congestion, and thereby clinical outcomes (18, 19).
Furthermore, the high incidence of myocardial injury caused by SARS-CoV-2 corresponds to that observed in other viral infections, such as Influenza, in which myocardial damage was detected as asymptomatic cardiac involvement in 0–53% of cases, with the presence of electrocardiogram alterations on roughly 50% of patients or highlighted post-mortem by the presence of myocarditis, pericarditis or acute coronary syndrome (14, 15, 54–58). Viral infections, indeed, can determine endothelial dysfunction up to apoptosis rousing coronary vasoconstriction and procoagulant state causing activation of plaque to hemodynamic instability (59).
Vaccination represents the best preventive method for both adults and children with effectiveness rates of 65–95 vs. 50–60% for Influenza, respectively, mostly in high-risk patients (>65 years, young children, presence of comorbidities, and immunocompromised patients), and it could be useful to prevent cardiovascular damage reducing mortality (59–65).
Conclusion
Myocardial injury induced by SARS-CoV-2 is relevant.
The elevation of hs Troponin I and MR-proADM allows the identification of patients with myocardial injury at higher mortality risk.
An MR-proADM value of ≥1.19 nmol/L identifies patients with myocardial injury, and a MR-proADM value of ≥4.01 nmol/L identifies patients with myocardial injury at high risk of death.
Therefore, the dosage of MR-proADM allows stratifying patients with myocardial injury at high risk of death to offer the best management to critically ill COVID-19 patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee of the University Campus Bio-Medico of Rome. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
SS led the study design, data collection, data analysis, data interpretation, and manuscript writing. FM, GD’A, and DL assisted with data collection and analysis of the validation dataset. FM performed the statistical analysis. FS assisted the patients. MF, LL, DL, and GB assisted with computer queries, data analysis, and manuscript preparation. CB, AA, JM, and RM assisted with data collection and analysis of the development dataset as well as study design, data interpretation, and manuscript writing. CB, JM, AA, SC, and SA assisted with chart review, data analysis, and supervised all aspects of the investigation, as well as assisting with study design, data interpretation, and manuscript writing. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
We thank Stefano Spoto for English language revision.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADM, adrenomedullin; ARDS, acute respiratory distress syndrome; AUC, areas under the curve; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; MR-proADM, mid-regional-proAdrenomedullin; PCT, procalcitonin; ROC, receiver operating characteristic; SARS-CoV-2, severe acute respiratory distress syndrome coronavirus 2; SOFA, sequential organ failure assessment.
References
1. Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol. (2020) 92:455–9. doi: 10.1002/jmv.25688
2. Potere N, Valeriani E, Candeloro M, Tana M, Porreca E, Abbate A, et al. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit Care. (2020) 24:389. doi: 10.1186/s13054-020-03022-1
3. World Health Organization [WHO]. Clinical Management of Severe Acute Respiratory Infection (SARI) when COVID-19 Disease is Suspected: Interim Guidance. Geneva: World Health Organization (2020).
4. Spoto S, Agrò FE, Sambuco F, Travaglino F, Valeriani E, Fogolari M, et al. High value of mid-regional proadrenomedullin in COVID-19: a marker of widespread endothelial damage, disease severity, and mortality. J Med Virol. (2021) 93:2820–7. doi: 10.1002/jmv.26676
5. Cheng MP, Cau A, Lee TC, Brodie D, Slutsky A, Marshall J, et al. Acute cardiac injury in coronavirus disease 2019 and other viral infections—a systematic review and meta-analysis. Crit Care Med. (2021) 49:1558–66. doi: 10.1097/CCM.0000000000005026
6. Pellegrini D, Kawakami R, Guagliumi G, Sakamoto A, Kawai K, Gianatti A, et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. (2021) 143:1031–42. doi: 10.1161/CIRCULATIONAHA.120.051828
7. Roshdy A, Zaher S, Fayed H, Coghlan JG. COVID-19 and the heart: a systematic review of cardiac autopsies. Front Cardiovasc Med. (2021) 7:626975. doi: 10.3389/fcvm.2020.626975
8. Lee CCE, Ali K, Connell D, Mordi IR, George J, Lang EM, et al. COVID-19-associated cardiovascular complications. Diseases. (2021) 9:47. doi: 10.3390/diseases9030047
9. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138:e618–51. doi: 10.1161/CIR.0000000000000617
10. Eberli FR, Kurz D. Cardiovascular aspects of COVID-19. Swiss Med Wkly. (2020) 150:w20417. doi: 10.4414/smw.2020.20417
11. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. (2020) 76:533–46. doi: 10.1016/j.jacc.2020.06.007
12. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. (2020) 142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549
13. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802. doi: 10.1001/jamacardio.2020.0950
14. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5:811. doi: 10.1001/jamacardio.2020.1017
15. Demir OM, Ryan M, Cirillo C, Desai N, Pericao A, Sinclair H, et al. Impact and determinants of high-sensitivity cardiac troponin-T concentration in patients with COVID-19 admitted to critical care. Am J Cardiol. (2021) 147:129–36. doi: 10.1016/j.amjcard.2021.01.037
16. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong J-C, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. (2020) 126:1456–74. doi: 10.1161/CIRCRESAHA.120.317015
17. Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med. (2020) 27:taaa041. doi: 10.1093/jtm/taaa041
18. Karakas M, Jarczak D, Becker M, Roedl K, Addo MM, Hein F, et al. Targeting endothelial dysfunction in eight extreme-critically Ill patients with COVID-19 using the anti-adrenomedullin antibody adrecizumab (HAM8101). Biomolecules. (2020) 10:E1171. doi: 10.3390/biom10081171
19. Kita T, Kitamura K. Translational studies of adrenomedullin and related peptides regarding cardiovascular diseases. Hypertens Res. (2022) 45:389–400. doi: 10.1038/s41440-021-00806-y
20. Domizi R, Damiani E, Scorcella C, Carsetti A, Giaccaglia P, Casarotta E, et al. Mid-regional proadrenomedullin (MR-proADM) and microcirculation in monitoring organ dysfunction of critical care patients with infection: a prospective observational pilot study. Front. Med. (2021) 8:680244. doi: 10.3389/fmed.2021.680244
21. Montrucchio G, Balzani E, Lombardo D, Giaccone A, Vaninetti A, D’Antonio G, et al. Proadrenomedullin in the management of COVID-19 critically Ill patients in intensive care unit: a systematic review and meta-analysis of evidence and uncertainties in existing literature. J Clin Med. (2022) 11:4543. doi: 10.3390/jcm11154543
22. Sozio E, Tascini C, Fabris M, D’Aurizio F, De Carlo C, Graziano E, et al. MR-proADM as prognostic factor of outcome in COVID-19 patients. Sci Rep. (2021) 11:5121. doi: 10.1038/s41598-021-84478-1
23. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
24. Angeletti S, Cella E, Prosperi M, Spoto S, Fogolari M, De Florio L, et al. Multi-drug resistant Pseudomonas aeruginosa nosocomial strains: molecular epidemiology and evolution. Microbial Pathog. (2018) 123:233–41. doi: 10.1016/j.micpath.2018.07.020
25. De Florio L, Riva E, Giona A, Dedej E, Fogolari M, Cella E, et al. MALDI-TOF MS identification and clustering applied to Enterobacter species in nosocomial setting. Front Microbiol. (2018) 9:1885. doi: 10.3389/fmicb.2018.01885
26. Spoto S, Fogolari M, De Florio L, Minieri M, Vicino G, Legramante J, et al. Procalcitonin and MR-proAdrenomedullin combination in the etiological diagnosis and prognosis of sepsis and septic shock. Microbial Pathog. (2019) 137:103763. doi: 10.1016/j.micpath.2019.103763
27. Spoto S, Lupoi DM, Valeriani E, Fogolari M, Locorriere L, Beretta Anguissola G, et al. Diagnostic accuracy and prognostic value of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in septic patients outside the intensive care unit. Medicina. (2021) 57:811. doi: 10.3390/medicina57080811
28. He F, Quan Y, Lei M, Liu R, Qin S, Zeng J, et al. Clinical features and risk factors for ICU admission in COVID-19 patients with cardiovascular diseases. Aging Dis. (2020) 11:763. doi: 10.14336/AD.2020.0622
29. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
30. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. (2020) 323:1239. doi: 10.1001/jama.2020.2648
31. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. (2020) 180:934. doi: 10.1001/jamainternmed.2020.0994
32. Cremer S, Jakob C, Berkowitsch A, Borgmann S, Pilgram L, Tometten L, et al. Elevated markers of thrombo-inflammatory activation predict outcome in patients with cardiovascular comorbidities and COVID-19 disease: insights from the LEOSS registry. Clin Res Cardiol. (2021) 110:1029–40. doi: 10.1007/s00392-020-01769-9
33. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically ill patients in the seattle region — case series. N Engl J Med. (2020) 382:2012–22. doi: 10.1056/NEJMoa2004500
34. Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. (2020) 142:342–53. doi: 10.1161/CIRCULATIONAHA.120.047971
35. Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry M-C, et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. (2020) 41:3827–35. doi: 10.1093/eurheartj/ehaa664
36. Dschietzig T, Azad HA, Asswad L, Böhme C, Bartsch C, Baumann G, et al. The adrenomedullin receptor acts as clearance receptor in pulmonary circulation. Biochem Biophys Res Commun. (2002) 294:315–8. doi: 10.1016/S0006-291X(02)00474-6
37. Voors AA, Kremer D, Geven C, ter Maaten JM, Struck J, Bergmann A, et al. Adrenomedullin in heart failure: pathophysiology and therapeutic application. Eur J Heart Fail. (2019) 21:163–71. doi: 10.1002/ejhf.1366
38. Citgez E, Zuur-Telgen M, van der Palen J, van der Valk P, Stolz D, Brusse-Keizer M. Stable-state midrange proadrenomedullin is associated with severe exacerbations in COPD. Chest. (2018) 154:51–7. doi: 10.1016/j.chest.2018.02.006
39. Cheung MY, Tang F. Adrenomedullin: exciting new horizons. EMI. (2012) 6:4–17. doi: 10.2174/187221412799015263
40. Anderson FA, Spencer FA. Risk factors for venous thromboembolism. Circulation. (2003) 107(23 Suppl 1):I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6
41. Porfidia A, Valeriani E, Pola R, Porreca E, Rutjes AWS, Di Nisio M. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb Res. (2020) 196:67–74. doi: 10.1016/j.thromres.2020.08.020
42. Wilson DC, Schefold JC, Baldirà J, Spinetti T, Saeed K, Elke G. Adrenomedullin in COVID-19 induced endotheliitis. Crit Care. (2020) 24:411. doi: 10.1186/s13054-020-03151-7
43. Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascón GA, et al. The endothelium in sepsis. Shock. (2016) 45:259–70. doi: 10.1097/SHK.0000000000000473
44. Montrucchio G, Sales G, Rumbolo F, Palmesino F, Fanelli V, Urbino R, et al. Effectiveness of mid-regional pro-adrenomedullin (MR-proADM) as prognostic marker in COVID-19 critically ill patients: an observational prospective study. PLoS One. (2021) 16:e0246771. doi: 10.1371/journal.pone.0246771
45. Spoto S, Valeriani E, Caputo D, Cella E, Fogolari M, Pesce E, et al. The role of procalcitonin in the diagnosis of bacterial infection after major abdominal surgery: advantage from daily measurement. Medicine. (2018) 97:e9496. doi: 10.1097/MD.0000000000009496
46. Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. (2021) 21:319–29. doi: 10.1038/s41577-021-00536-9
47. Trincot CE, Xu W, Zhang H, Kulikauskas MR, Caranasos TG, Jensen BC, et al. Adrenomedullin induces cardiac lymphangiogenesis after myocardial infarction and regulates cardiac edema via connexin 43. Circ Res. (2019) 124:101–13. doi: 10.1161/CIRCRESAHA.118.313835
48. Jougasaki M, Burnett JC. Adrenomedullin: potential in physiology and pathophysiology. Life Sci. (2000) 66:855–72. doi: 10.1016/S0024-3205(99)00358-6
49. Meens MJ, Kwak BR, Duffy HS. Role of connexins and pannexins in cardiovascular physiology. Cell Mol Life Sci. (2015) 72:2779–92. doi: 10.1007/s00018-015-1959-2
50. Romiti GF, Cangemi R, Toriello F, Ruscio E, Sciomer S, Moscucci F, et al. Sex-specific cut-offs for high-sensitivity cardiac troponin: is less more? Cardiovasc Therap. (2019) 2019:1–12. doi: 10.1155/2019/9546931
51. Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH, et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. (2020) 92:1875–83. doi: 10.1002/jmv.26050
52. García de Guadiana-Romualdo L, Martínez Martínez M, Rodríguez Mulero MD, Esteban-Torrella P, Hernández Olivo M, Alcaraz García MJ, et al. Circulating MR-proADM levels, as an indicator of endothelial dysfunction, for early risk stratification of mid-term mortality in COVID-19 patients. Int J Infect Dis. (2021) 111:211–8. doi: 10.1016/j.ijid.2021.08.058
53. Zella D, Giovanetti M, Benedetti F, Unali F, Spoto S, Guarino M, et al. The variants question: what is the problem? J Med Virol. (2021) 93:6479–85. doi: 10.1002/jmv.27196
54. Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health. (2013) 103:e43–51. doi: 10.2105/AJPH.2012.301137
55. Ison MG, Campbell V, Rembold C, Dent J, Hayden FG. Cardiac findings during uncomplicated acute influenza in ambulatory adults. Clin Infect Dis. (2005) 40:415–22. doi: 10.1086/427282
56. Mamas MA, Fraser D, Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol. (2008) 130:304–9. doi: 10.1016/j.ijcard.2008.04.044
57. Watanabe M, Panetta GL, Piccirillo F, Spoto S, Myers J, Serino FM, et al. Acute Epstein-Barr related myocarditis: an unusual but life-threatening disease in an immunocompetent patient. J Cardiol Cases. (2020) 21:137–40. doi: 10.1016/j.jccase.2019.12.001
58. Kallen AJ, Brunkard J, Moore Z, Budge P, Arnold KE, Fosheim G, et al. Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season. Ann Emerg Med. (2009) 53:358–65. doi: 10.1016/j.annemergmed.2008.04.027
59. Naghavi M, Barlas Z, Siadaty S, Naguib S, Madjid M, Casscells W. Association of influenza vaccination and reduced risk of recurrent myocardial infarction. Circulation. (2000) 102:3039–45. doi: 10.1161/01.CIR.102.25.3039
60. Spoto S, Valeriani E, Locorriere L, Anguissola GB, Pantano AL, Terracciani F, et al. Influenza B virus infection complicated by life-threatening pericarditis: a unique case-report and literature review. BMC Infect Dis. (2019) 19:40. doi: 10.1186/s12879-018-3606-7
61. Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. (2012) 12:36–44. doi: 10.1016/S1473-3099(11)70295-X
62. Spoto S, Valeriani E, Riva E, De Cesaris M, Tonini G, Vincenzi B, et al. A Staphylococcus aureus coinfection on a COVID-19 pneumonia in a breast cancer patient. IJGM. (2020) 13:729–33. doi: 10.2147/IJGM.S261760
63. Zahid MN, Moosa MS, Perna S, Buti EB. A review on COVID-19 vaccines: stages of clinical trials, mode of actions and efficacy. Arab J Basic Appl Sci. (2021) 28:225–33. doi: 10.1080/25765299.2021.1903144
64. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharmaceut Anal. (2020) 10:102–8. doi: 10.1016/j.jpha.2020.03.001
Keywords: myocardial injury, mid-regional proAdrenomedullin, COVID-19, Troponin I (tni), SARS-CoV-2
Citation: Spoto S, Mangiacapra F, D’Avanzo G, Lemme D, Bustos Guillén C, Abbate A, Markley JD, Sambuco F, Markley R, Fogolari M, Locorriere L, Lupoi DM, Battifoglia G, Costantino S, Ciccozzi M and Angeletti S (2022) Synergistic effect of myocardial injury and mid-regional proAdrenomedullin elevation in determining clinical outcomes of SARS-CoV-2 patients. Front. Med. 9:929408. doi: 10.3389/fmed.2022.929408
Received: 26 April 2022; Accepted: 16 September 2022;
Published: 26 October 2022.
Edited by:
Luis Garcia De Guadiana-Romualdo, Santa Lucía University General Hospital, SpainReviewed by:
Rita Indirli, University of Milan, ItalyMuhammad Suleman, University of Veterinary and Animal Sciences, Pakistan
Cristina Andres, Rio Carrion Hospital, Spain
Copyright © 2022 Spoto, Mangiacapra, D’Avanzo, Lemme, Bustos Guillén, Abbate, Markley, Sambuco, Markley, Fogolari, Locorriere, Lupoi, Battifoglia, Costantino, Ciccozzi and Angeletti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Spoto, s.spoto@policlinicocampus.it
†These authors have contributed equally to this work
 Silvia Spoto
Silvia Spoto Fabio Mangiacapra2†
Fabio Mangiacapra2†  Giorgio D’Avanzo
Giorgio D’Avanzo César Bustos Guillén
César Bustos Guillén Antonio Abbate
Antonio Abbate John Daniel Markley
John Daniel Markley Marta Fogolari
Marta Fogolari Luciana Locorriere
Luciana Locorriere Domenica Marika Lupoi
Domenica Marika Lupoi Giulia Battifoglia
Giulia Battifoglia Massimo Ciccozzi
Massimo Ciccozzi Silvia Angeletti
Silvia Angeletti