IL 33 Correlates With COVID-19 Severity, Radiographic and Clinical Finding
- 1Department of Infectious Disease, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia
- 2Faculty of Medical Sciences, Center for Molecular Medicine and Stem Cell Research, University of Kragujevac, Kragujevac, Serbia
- 3Department of Clinical Pharmacy, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia
- 4Department of Virusology and Immunology, Institute for Public Health Kragujevac, Kragujevac, Serbia
- 5Department of Internal Medicine, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia
- 6Department of Abdominal Surgery, Military Medical Academy, Belgrade, Serbia
- 7Department of Radiology, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia
- 8Department of Surgery, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia
- 9University Clinical Center Kragujevac, Kragujevac, Serbia
- 10Department of Otorhinolaringology, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia
- 11Department of Surgery, Faculty of Medicine Foca, University of East Sarajevo, Foca, Bosnia and Herzegovina
- 12Institute for Transfusiology and Haemobiology, Military Medical Academy, Belgrade, Serbia
Objective: The increased level of interleukin (IL)-33 is considered as a predictor of severe coronavirus disease 2019 (COVID-19) infection, but its role at different stages of the disease is still unclear. Our goal was to analyze the correlation of IL-33 and other innate immunity cytokines with disease severity.
Methods: In this study, 220 patients with COVID-19 were included and divided into two groups, mild/moderate and severe/critical. The value of the cytokines, clinical, biochemical, radiographic data was collected and their correlation with disease severity was analyzed.
Results: Most patients in the severe/critical group were male (81.8%) and older (over 64.5 years). We found a statistically significant difference (p < 0.05) in these two groups between clinical features (dyspnea, dry cough, fatigue, and auscultatory findings); laboratory [(neutrophil count, lymphocyte count, monocyte count, hemoglobin, plasma glucose, urea, creatinine, total bilirubin (TBIL), direct bilirubin (DBIL), aspartate aminotransferase (AST), albumin (ALB), lactate dehydrogenase (LDH), creatinine kinase (CK), D-dimer, C-reactive protein (CRP), procalcitonin (PCT), Fe, and Ferritin)], arterial blood gases (oxygen saturation-Sa02, partial pressure of oxygen -p02), and chest X-rays (CXR) lung findings (p = 0.000). We found a significantly higher serum concentration (p < 0.05) of TNF-α, IL-1β, IL-6, IL-12, IL-23, and IL-33 in patients with COVID-19 with severe disease. In the milder stage of COVID-19, a positive correlation was detected between IL-33 and IL-1β, IL-12 and IL-23, while a stronger positive correlation between the serum values of IL-33 and TNF-α, IL-1β, IL-6, and IL-12 and IL-23 was detected in patients with COVID-19 with severe disease. A weak negative correlation (p < 0.05) between pO2 and serum IL-1β, IL-12, and IL-33 and between SaO2 and serum IL-33 was noted. The positive relation (p < 0.05) between the serum values of IL-33 and IL-12, IL-33 and IL-6, and IL-6 and IL-12 is proven.
Conclusion: In a more progressive stage of COVID-19, increased IL-33 facilitates lung inflammation by inducing the production of various innate proinflammatory cytokines (IL-1β, IL-6, TNF-α, IL-12, and IL-23) in several target cells leading to the most severe forms of the disease. IL-33 correlates with clinical parameters of COVID-19 and might represent a promising marker as well as a therapeutic target in COVID-19.
Introduction
In December 2019, in Wuhan, Hubei province, China, several patients with atypical pneumonia were hospitalized (1). A new virus from the group of coronaviruses was identified and called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease called Coronavirus Disease (COVID-19) (2). SARS-CoV-2 is highly infectious and asymptomatic patients are the main source of infection (3). Because of its easy and quick transmission and interhuman spread, the WHO declared it as a pandemic on March 11, 2020 (1). Recent studies have shown that different biochemical parameters are altered in patients with COVID-19, and some of them are useful as indicators of disease severity (4–6). Patients can have various forms of the disease, from asymptomatic to acute respiratory distress syndrome (ARDS) and respiratory insufficiency (7, 8). The most common manifestations are fever, cough, dyspnea, fatigue, chest pain, loss of sense of smell, headache, nausea or vomiting, and pharyngalgia (7, 9). Chest X-rays (CXR) is not considered a sensitive method for the detection of lung abnormalities in the early stage of the disease, but it is useful for monitoring the lung progression in later stages of the disease (10, 11). Immunopathogenic mechanisms during the disease vary from immunosuppression to uncontrolled immune-inflammatory response (12, 13). While the immunosuppression is conveyed through well-known immunosuppressive cytokines such as transforming growth factor β (TGF-β) and interleukin (IL)-10, the uncontrolled immune response is manifested by the rapid production and excretion of proinflammatory cytokines, such as cytokines of the IL-1 family, tumor necrosis factor α (TNFα), and IL-6. (12, 14) The rapid secretion of cytokines, chemokines, and other proinflammatory molecules, often referred to as cytokine storm, can be clinically manifested as ARDS with consequent respiratory failure (15, 16). Even though ARDS and cytokine storm are well-established entities in modern medicine, the exact mechanisms of respiratory failure and impeding proinflammatory cascade in COVID-19 are still challenging but might be of great significance when it comes to predicting the outcome of the disease and treatment (17).
One of the cytokines that have not been sufficiently studied in the immunopathogenesis of COVID-19 is IL-33. As a member of the IL-1 family, IL-33 induces an inflammatory response within the tissue (18–20). In the lungs, it is secreted mainly by injured epithelial alveolar cells (10, 19). As a previous study showed that the virus can trigger the secretion of IL-33 through activation of SARS CoV-2-derived papain-like protease (PLpro), IL-33 is considered as a predictor of severe forms of COVID-19. However, the potential role of IL-33 in disease genesis and progression has not been studied sufficiently (18, 19).
In this study, we analyzed the correlation of IL-33 and other innate immunity cytokines such as TNF-α, IL-1β, IL-6, IL-12, and IL-23 with disease severity. Also, we analyzed interdependence between disease severity and clinical, biochemical features, and CXR lung findings in 220 patients with COVID-19.
Methods
Patients
In this observational and cross-sectional study, confirmed cases of COVID-19 from May 2020 to December 2020 were involved and examined. COVID-19 was confirmed by real-time reverse transcription–PCR (RT-PCR) analysis. All hospitalized patients with COVID-19 were classified according to disease severity into two groups:
I) Mild/moderate group, patients with fever (37–38°C), fatigue, pharyngalgia, anosmia, nausea and vomiting, headache, dry irritating cough, dyspnea, SaO2 82–100%, pO2 7.1 kPa- 13.3 kPa, with normal or weakened/sharpened respiratory noise, and audible cracks in the lower segments of the lungs, with normal/interstitial thickening and focal consolidation in CXR lung findings (CXR I, II, III);
II) Severe/critical group, patients with fever of 38–40°C, frequent dry irritating cough, dyspnea, fatigue, myalgia, nausea and vomiting, headache, chest pain, anosmia, SaO2 ≤ 70–81%, and pO2 ≤ 5.1–7 kPa which require high-frequency ventilation (HFV), non-invasive mechanical ventilation (Non-Invasive Ventilation, NIV), or invasive mechanical ventilation, with auscultatory attenuated-inaudible respiratory noise with audible whistling or cracks diffusely, with multifocal consolidation, diffuse alveolar changes, ARDS in CXR lung findings (CRX IV, V).
The patients included in this study had no prior treatment with antibiotics, aminosalicylates, corticosteroids, statins, immunosuppressive agents, or any kind of biological therapy for at least 2 months before enrolling in the study.
A total of 220, 110 patients from each of the previously defined groups, were selected at random for the study. The study sample of 110 patients from each group was extracted by simple randomization technique, activating for 110 times random number generator in Excel (RANDBETWEEN).
Data Collection
Nasopharyngeal swab samples were taken from all patients with suspected SARS-CoV-2 infection and then sent to designated authoritative laboratories to detect the SARS-CoV-2. For the detection and quantification of viral nucleic acid, we used the RT-PCR for the N gene and Orf gene of SARS-CoV-2. Reverse transcription, duplication, and detection are performed automatically in a JENA Real-time Thermal Cycler. We used specific primers and a fluorescence probe. Samples were considered as positive if an amplification signal was detected on the detection system and internal control, and negative in the absence of any amplification signal on the detection system and a positive result on internal control. The assay was repeated in the absence of signal in the positive control and the presence of a negative control amplification signal.
Blood samples were drawn by venous puncture from all patients included in the study, and stored in four different tubes: one for blood cell counting, another tube for D-dimer, the third one for analyses of the biochemistry in plasma [urea, glycemia, creatinine, C-reactive protein (CRP), procalcitonin (PCT), creatinine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), albumin (ALB), direct bilirubin (DBIL), total bilirubin (TBIL), Iron (Fe), Ferritin, Potassium (K+), and Sodium (Na+)], which are performed in the Central Biochemical Laboratory of UKC Kragujevac by standard methods, using the Beckman Coulter AU 400 Unicel DXC 800 Synchron Clinical System, and fourth for immunoassay.
Several times during the day by ion selective electrode on the automaton, arterial blood gases (SaO2, pO2, partial pressure of carbon dioxide-pCO2), of all patients were measured.
All patients had a CXR using the digital portable anteroposterior (AP) technique. Radiographic features were described according to Yoon et al. (21) following a 5-point scoring scale: 1–normal; 2–patchy atelectasis and/or hyperinflation and/or bronchial wall thickening; 3–focal consolidation; 4–multifocal consolidation; and 5–diffuse alveolar changes.
Measurement of Cytokine Levels in Sera
As described in our previous study, at 8:00 am, the blood was collected from the patients with COVID-19 (22). The sera were separated and stored at −80°C before use. The commercially available ELISA tests were used following the instructions of the manufacturer (R&D Systems, Minneapolis, Minn, USA) to measure the concentrations of TNF-α, IL-1β, IL-6, IL-12, IL-23, and IL-33 in serum samples.
Statistical Analysis
Statistical Analysis Software, IBM SPSS (version 23.0) (IBM, Armonk, New York, USA) was used for performing all data analyses. The significance tests used for suitable purposes were the Chi-square test, Student's t-test, or Mann–Whitney U test. The data were shown as mean ± SE of the mean. Pearson's or Spearman's correlation assessed the possible relationship between variables. The strength of correlation was defined as negative or positive weak (−0.3 to −0.1 or.1 to.3), moderate (−0.5 to −0.3 or.3 to.5), or strong (−1.0 to −0.5 or 1.0 to.5). Also, the basic regression model was used to identify possible predictors for disease severity. Multiple linear regression analysis was used to predict Il-33 levels based on COVID-19 severity and other cytokine levels. Receiver operating characteristic (ROC) curve analysis was used to identify optimal cut-off values of IL33 levels in order to identify the detection of COVID-19 severity. The statistical significance was set at p < 0.05.
Results
Clinical Feature in Patients With COVID
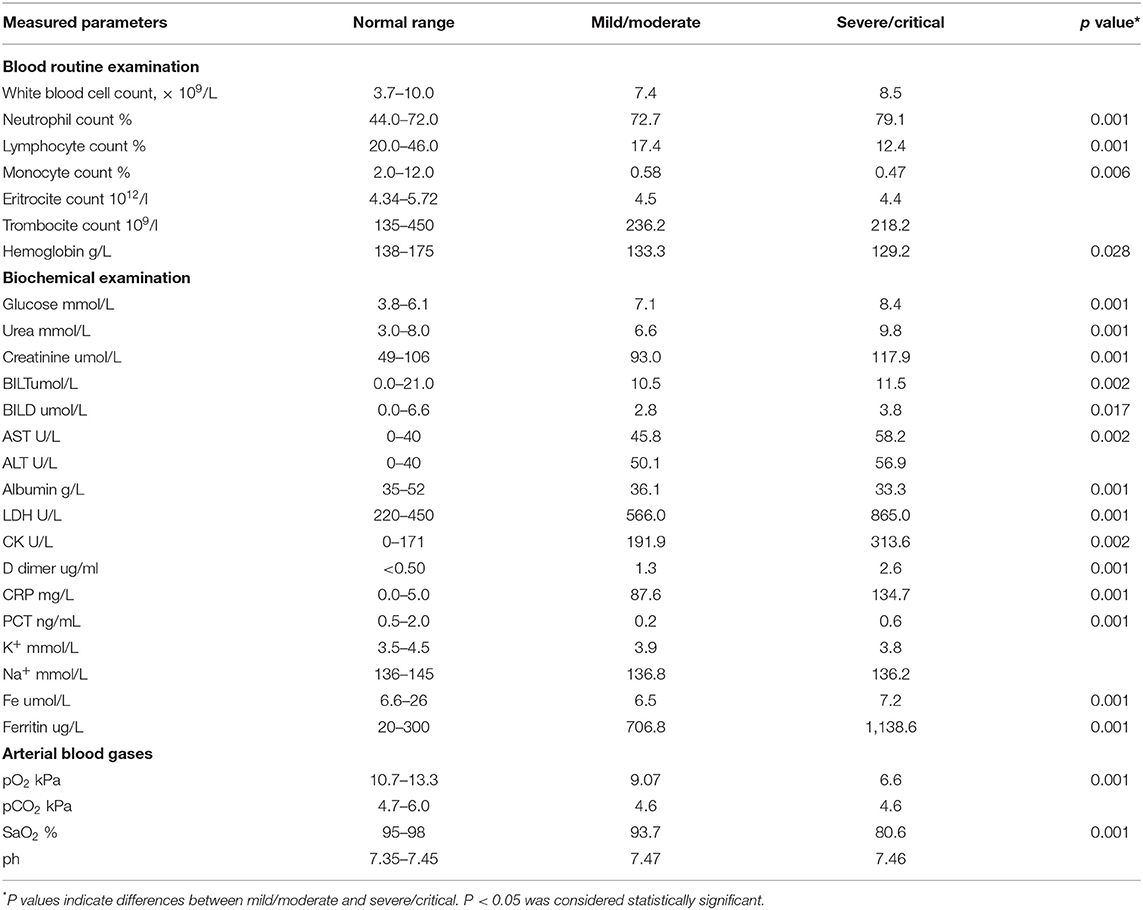
All recruited patients met the criteria for COVID-19 (23). Among them, 140 (63.3%) were male. The mean age was 55.5 years. The COVID-19 patients were divided into two groups, based on disease severity. The patients in group II were significantly older (p = 0.001; Table 1). The median age in group I (mild/moderate) was 57.2 ± 1.5 and for group II (severe/critical) 64.5 ± 1.2 years. The analysis of the data did not show any significant difference regarding the distribution of gender. The analysis revealed a lower percentage of female patients and a higher percentage of male patients in group II, but this difference did not reach statistical significance (p = 0.071; Table 1). The most common symptoms in these two groups were fever 185 (84.0%), dry cough 176 (80%), fatigue 171 (77.7%), dyspnea 108 (49.0%), nausea and vomiting 88 (40%), and myalgia 57 (25.9%). Less common were anosmia 31 (14%), headache 29 (13.1%), chest pain 25 (11.3%), and pharyngalgia 6 (2.7%) (Table 1). The most frequent auscultatory finding in group I was attenuated breathing sound in 66.3% of patients, which CXR findings described as focal consolidation in 51.8 % and interstitial thickening in 38.1%, while in group II, 71.8% of patients had auscultatory attenuated breathing sound and 70.9% audible cracks diffusely with multifocal consolidation in 53.6% and ARDS in 46.3% of hospitalized patients. The percentage of patients with dyspnea, fatigue, auscultatory attenuated breathing sound, and audible cracks diffusely was significantly higher in the severe/critical group than in the mild/moderate group (p < 0.05; Table 1), which is in line with the CXR findings of the patients. The patients in the mild/moderate group had normal CRX findings, interstitial thickening, or focal consolidation zones (CRX I, II, III). Only one patient had multifocal consolidation (Table 1). All the patients in the severe/critical group had multifocal consolidation zones or diffuse alveolar changes (CRX IV, V) (p = 0.000). We found some significant differences in laboratory features between the mild/moderate group and the severe/critical group. In patients with severe/critical outcomes an increase in neutrophil count (p = 0.001), levels of plasma glucose (p = 0.001), urea (p = 0.001), creatinine (p = 0.001), AST (p = 0.002), TBIL (p = 0.002), DBIL (p = 0.017), LDH (p = 0.001), CK (p = 0.002), D-dimer (p = 0.001), CRP (p = 0.001), PCT (p = 0.001), Fe (p = 0.001), and Ferritin (p = 0.001) was noted with reduced lymphocyte count (p = 0.001), monocyte count (p = 0.006), decreased levels of hemoglobin (p = 0.028), and albumin (p = 0.001) and lower values of arterial blood gases SaO2 (p = 0.001) and pO2 (p = 0.001) compared with the mild/moderate group (Table 2). There were no statistically significant differences in white blood cell count, erythrocytes, and platelets; ALT level, K+, Na+, pH of blood, and pCO2 (Table 2).
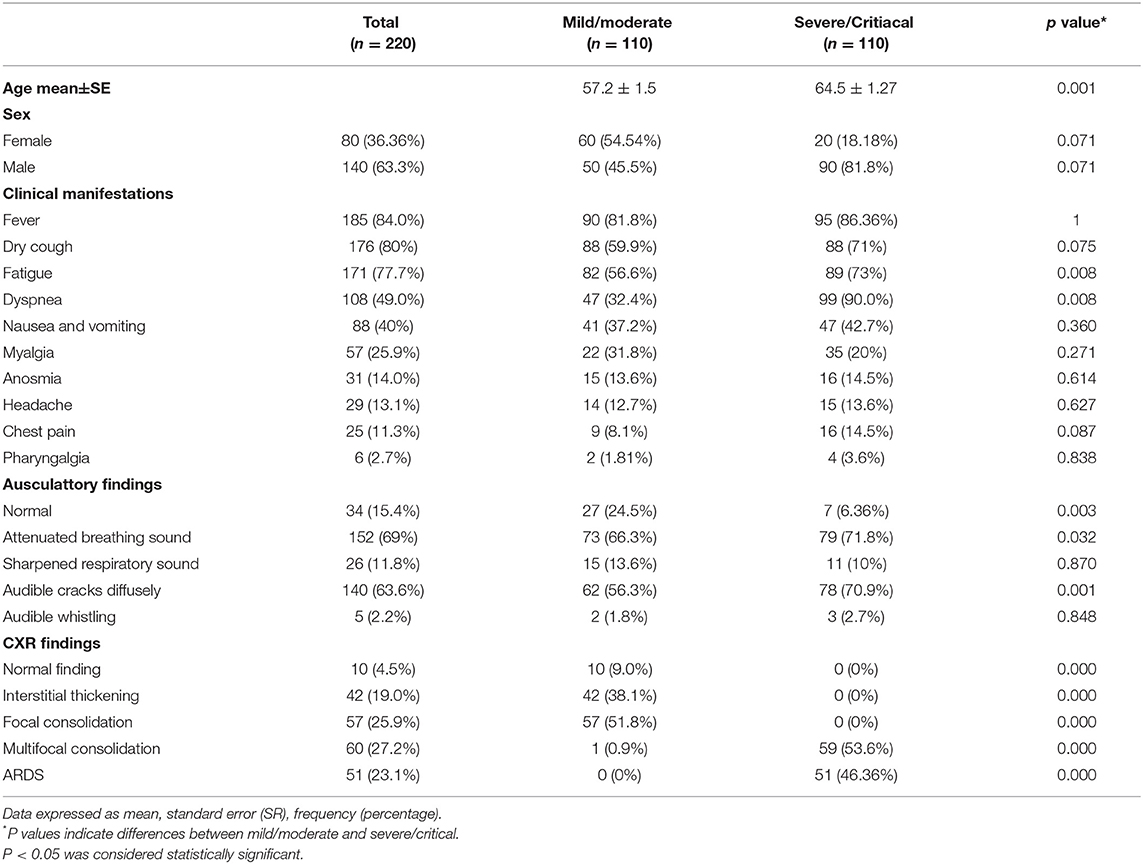
Table 1. Demographics and clinical characteristics of patients with coronavirus disease 2019 (COVID-19).
Patients With Severe COVID-19 Had Increased IL-33 and Proinflammatory Cytokines in Sera
Values of cytokines of interest were analyzed in the serum of COVID-19 patients. Significantly higher concentration of TNF-α (p = 0.001), IL-1β (p = 0.001), IL-6 (p = 0.001), IL-12 (p = 0.001), IL-23 (p = 0.043), and IL-33 (p = 0.001) have been found in the sera of COVID-19 patients with severe disease (Figure 1).
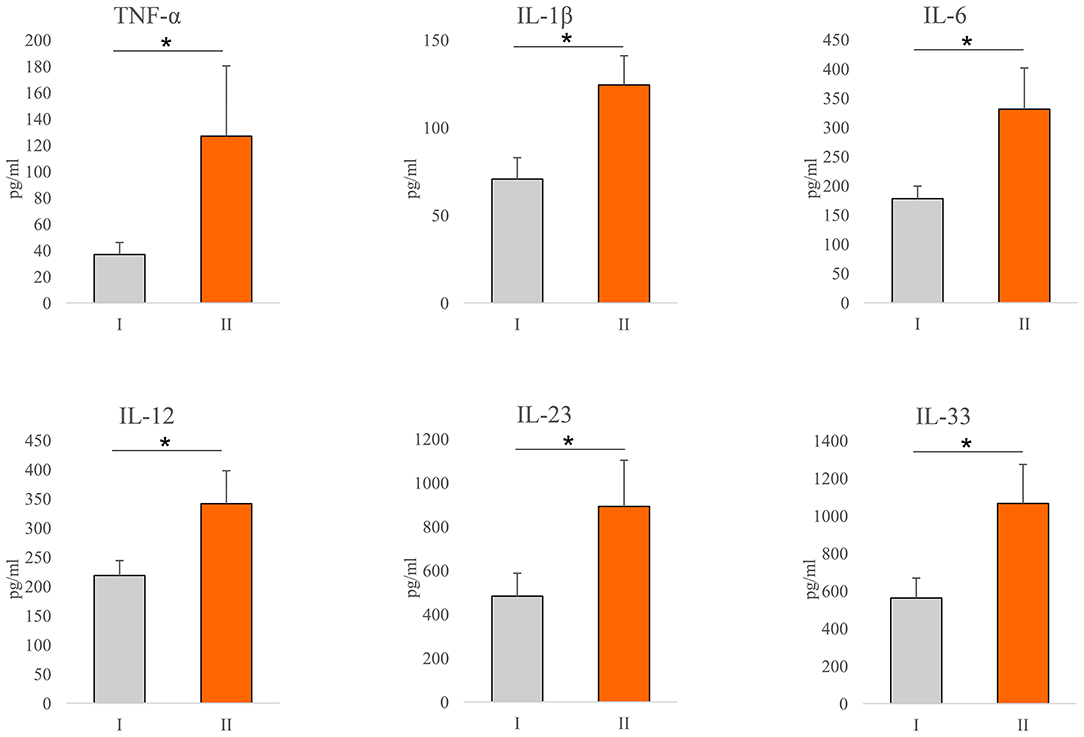
Figure 1. Systemic profile of innate immunity cytokines. According to the disease severity, all Coronavirus disease 2019 (COVID-19) patients were divided into two groups (I and II). The serum concentration of tumor necrosis factor α (TNFα), interleukin (IL)-1β, IL-6, IL-12, IL-23, and IL-33 were determined by ELISA. For statistical significance determination, Mann–Whitney Rank Sum test was used. *P < 0.05.
Stronger Inter-correlation Between IL-33 and Proinflammatory Cytokines in the Severe Stage of COVID-19
In order to determine the relationship between IL-33 and proinflammatory mediators in all stages of COVID-19, we analyzed the ratio of the systemic values of IL-33 with the values of TNF-α, IL-1β, IL-6, IL-12, and IL-23. We did not find a difference in the ratios between previously defined groups (Figure 2). Ratio IL-33/IL-23 was higher in group II, but this difference did not reach statistical significance (Figure 2).
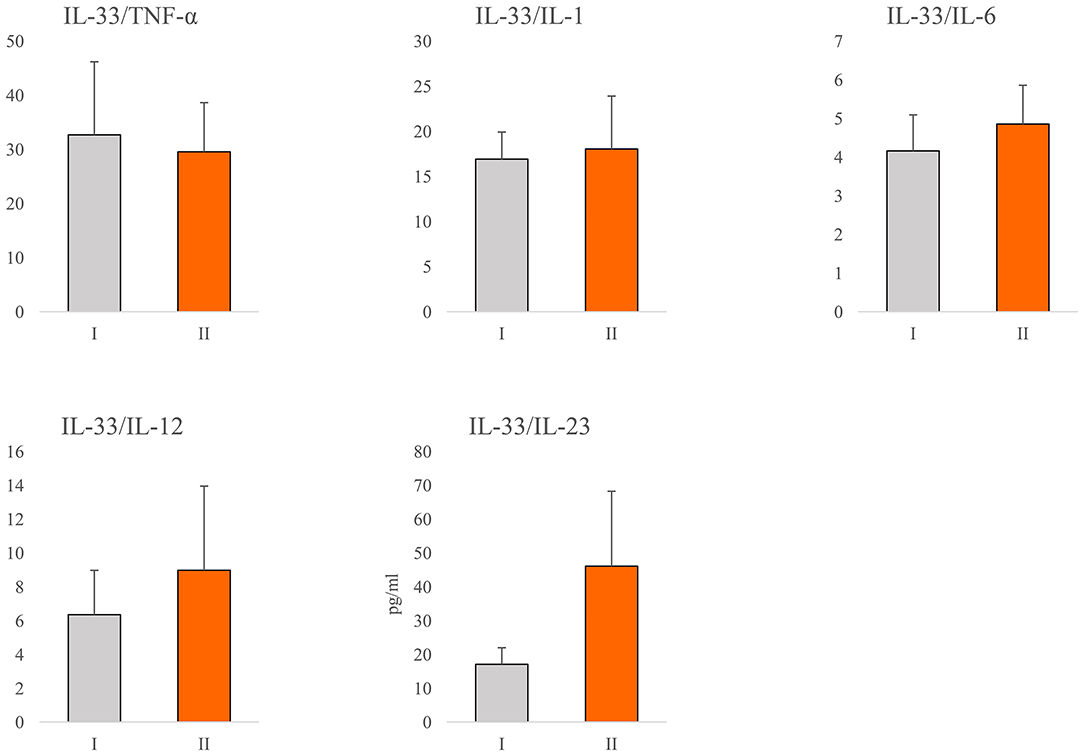
Figure 2. Ratio of IL-33 and proinflammatory cytokines. According to the disease severity, all COVID-19 patients were divided into two groups (I and II). The serum concentration of TNFα, IL-1β, IL-6, IL-12, IL-23, and IL-33 was determined by ELISA. The ratios of IL-33/TNF-α, IL-33/IL-1β, IL-33/IL-6, IL-33/IL-12, and IL-33/IL-23 were evaluated for each patient, separately. For statistical significance determination, Mann–Whitney Rank Sum test was used.
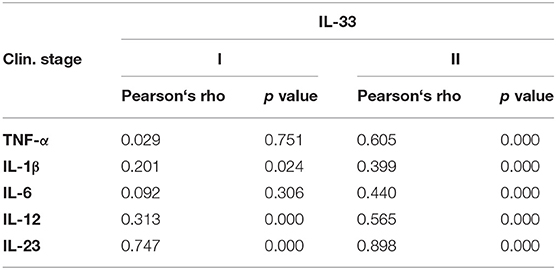
Further, in patients with milder stage of COVID-19, positive correlation was detected between IL-33 and IL-1β (p = 0.024), IL-12 (p = 0.000), and IL-23 (p = 0.000), respectively. Analysis revealed a stronger positive correlation between the serum values of IL-33 and TNF-α (p = 0.000), IL-1β (p = 0.000), IL-6 (p = 0.000), IL-12 (p = 0.000), and IL-23 (p = 0.000) in COVID-19 patients with severe disease (Table 3). Moreover, analysis revealed a positive correlation between the serum values of IL-33 and IL-12 (p = 0.001), IL-33 and IL-6 (p = 0.001), and IL-6 and IL-12 (p = 0.001) in patients with COVID-19 (Supplementary Table 1).
IL-33 Correlates With Clinical Parameters of COVID-19
Further, we have tested the correlation of the serum levels of proinflammatory cytokines with the clinical parameters of COVID-19. The results have shown a weak negative correlation between pO2 and serum IL-1β (p = 0.009), IL-12 (p = 0.018) and IL-33 (p = 0.018), and between SaO2 and serum IL-33 (p = 0.028) (Supplementary Table 2). The serum level of all cytokines of interest positively correlated with the CXR findings (Supplementary Table 2). The regression model for the independent variables, including the demographic features of the patients, clinical, biochemical parameters, and serum levels of innate immunity and dependent variable (COVID-19 severity) yielded significant results as shown in Supplementary Table 3. Male sex and advanced age are predictors of increased COVID-19 severity. Clinical findings such as dry cough, dyspnea, fatigue, and auscultation findings (attenuated breathing sound and crackles) are risk factors associated with developing severe COVID-19. When it comes to laboratory findings, lymphocyte and monocyte counts, levels of hemoglobin, and albumin were associated with decreased risk of disease progression. As expected, analyses of arterial blood gases were also associated with decreased risk of COVID-19 severity. Our results also illustrated that the serum levels of IL-1β, TNFα, IL-6, IL-12, IL-23, and IL-33 are risk factors associated with COVID-19 severity. The linear multiple regression model was calculated to predict the levels of IL-33 based on COVID-19 severity, IL-6, IL-1β, IL-12, TNF-α, and IL-23. A significant regression equation was established (Supplementary Table 4). The standardized beta values of COVID-19 severity (148.953, p = 0.049), IL-6 (−0.411, p = 0.003), IL-23 (0.896, p = 0.001), and IL-1β (0.858, p = 0.023) were significant, while beta values of IL-12 and TNF-α did not reach statistical significance (Supplementary Table 4). As shown in Figure 3, the serum values of IL-33 were significantly higher in patients with CXR score III, IV, and V compared with patients with CXR score I (p = 0.009; p = 0.002 and p = 0.001, respectively) and CXR score II (p = 0.01; p = 0.002, and p = 0.001, respectively). The ROC curve analysis of IL33 to differentiate mild from severe COVID-19 reviled that the optimum cut-off point for IL-33 was 332.08 pg/ml (AUC.634; Sensitivity 63.8%; Specificity 49.2%; 95% CI.562–0.705; p = 0.001).
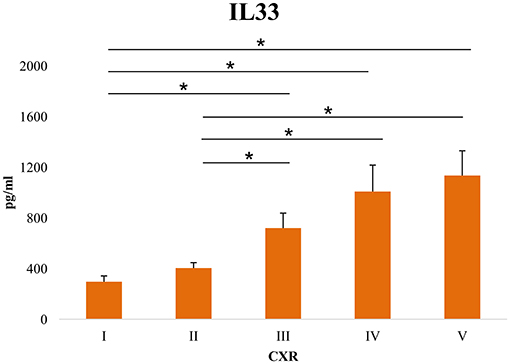
Figure 3. Level of serum IL-33 in patients sorted according to chest x-ray (CRX) findings. Using a digital portable anteroposterior (AP) technique, the chest X-ray findings were divided into five levels: (I) Normal finding (II) Interstitial thickening, {III) Focal consolidation, (IV) Multifocal consolidation (V) acute respiratory distress syndrome (ARDS). The serum concentration of IL-33 was determined by ELISA. For statistical significance determination, Mann–Whitney Rank Sum test was used. *P < 0.05.
Discussion
In this study, we analyzed the relationship between clinical features, laboratory features, radiographic findings, and innate immunity cytokine profile (IL-33, TNF-α, IL-1β, IL-6, IL-12, and IL-23) with disease severity. Patients with COVID-19 were divided into two groups (mild/moderate and severe/critical) according to the criteria described in the material and methods. We found that gender and age correlate with disease severity. Most patients in the severe/critical group were male (81.8%) and older (over 64.5 years). This is in accordance with the study of Hu et al., where the authors suggested that age and gender may be risk factors for severe clinical outcomes (24). In our study, patients in the severe/critical group have a significantly higher frequency of dyspnea, fatigue, and auscultatory attenuated breathing sound and diffuse cracks in comparison to the mild/moderate group (Table 1). Also, the higher values of the neutrophil count, CRP, D dimer, PCT, LDH, urea, creatinine, CK, Ferritin, TBIL, AST, as well as lower values of lymphocyte and monocyte count, and albumin were detected in the group of patients with severe COVID-19 (Table 2). The same phenomenon was reported in the studies of Wang et al. (25) and Sánchez et al. (26). We detected higher values of white blood cell count, ALT, and lower values of red blood cells, platelets count, K+, and Na+ concentrations in patients with severe COVID-19, but the differences did not reach statistical significance (Table 2). Given the vast range of immune, biochemical, and metabolic disruptions during severe COVID-19, multiple organ failure is inevitable. As far as it is known, the thrombosis of various organs, renal, hepatic, cardiac, and neurological injury occurs more frequently as the severity of COVID-19 increases (27–29). In addition, our study revealed that PCT sera levels were significantly higher in the severe/critical group in comparison to the mild/moderate group. Since PCT level is not elevated in viral infections per se, this finding might be a marker of amplified systemic inflammatory response (e.g., cytokine storm) or secondary bacterial or fungal infections (27, 30).
Further on, we analyzed the CXR findings of 220 patients divided in the mild/moderate and severe/critical groups. As mentioned before, CXR findings are divided into five groups, where CXR I, II, III are expected findings in the mild/moderate group, and CXR IV and V are expected to be found in severe/critical forms of the disease. Radiographic findings such as multifocal consolidation and diffuse alveolar damage, denoted as CXR IV and V, respectively, are significantly more frequent in severe forms of COVID-19 that require supportive modes of ventilation, such as high-frequency ventilation (HFV), non-invasive (NIV), or invasive mechanical ventilation.
It is believed that immunopathology is the main mechanism in the genesis and progression of COVID-19 (12, 14). The cellular composition of the lung infiltrates in patients with COVID-19 pneumonia changes with the progression of the disease. Infiltrates in patients with moderate pneumonia include mainly lymphoid and dendritic cells, while the severe form of the disease is characterized by massive infiltration of macrophages and neutrophils (31, 32). In line with this finding, our results revealed an increment in neutrophil count in patients with severe COVID-19 (Table 2). Interestingly, we detected a decrement in monocyte count in the same patients (Table 2), which we believe may be due to the accumulation of these cells in the lungs and differentiation into macrophages. The destruction of the alveolar epithelium, which is directly related to the severity of the disease, is due to an intense immune response (33, 34). There is ample evidence that both, innate and acquired immunity participate in the immunopathogenesis of COVID-19 (12, 14, 35). However, the main pro-inflammatory mediators that play a key role in tissue destruction belong to the innate immune response (36, 37). In order to clarify the correlation between COVID-19 and innate immunity cytokines of interest, we analyzed serum cytokines in patients with COVID-19. The increased systemic values of TNF-α, IL-1β, IL-6, IL-12, IL-23, and IL-33 (Figure 1) support the prevalence of innate pro-inflammatory mediators in the serum of subjects with a severe form of COVID-19. The obtained results are in line with similar studies, which confirm the strong correlation between disease severity and increased values of some innate immunity-associated proinflammatory cytokines (36–39).
Interleukin-33 is a member of the IL-1 cytokine family that is expressed in barrier tissues and exerts pleiotropic functions. Its role in immune response regulation after cellular stress or damage is crucial and is involved in the pathogenesis of COVID-19 (40). The analysis of the published scRNAseq data of bronchoalveolar lavage fluid (BALF) from patients with mild to severe COVID-19 revealed a population of IL-33-producing cells that increases with the disease. These findings show that IL-33 production is linked to SARS-CoV-2 infection (41). Our result revealed a significantly higher systemic IL-33 concentration in patients with severe COVID-19, positive association between IL-33 and innate immunity mediators, and clinical parameters of COVID-19 (Figure 1, Table 3, Supplementary Table 2), suggesting the important role of this cytokine in the progression of COVID-19. In line with these are the results of other studies, which showed that disease severity is associated with an increased level of IL-33 (41–43).
The almost unchanged ratio between IL-33 and innate immunity mediators of interest during COVID-19 progression (Figure 2) indicates the stable dynamics of cytokine growth. Our results revealed a stronger positive correlation between serum values of IL-33 and innate immunity mediators TNF-α, IL-1β, IL-6, IL-12, and IL-23 in the severe form of COVID-19 (Table 3). Advanced stages of COVID-19 are characterized by high circulating and pulmonary concentrations of innate immunity cytokines (33, 44, 45). Signaling downstream of IL-1 family receptors, including IL-1β and IL-33 receptors, can activate MyD88 and elicit inflammation via production of high amounts of IL-1β, and NF-κB-induced cytokines such as TNF-α, IL-6, IL-12, and IL-23 (38, 46). In line with this, we found a positive mutual relation between the serum values of IL-33 and IL-12, IL-33 and IL-6, and IL-6 and IL-12 (Supplementary Table 1). The revelation of the positive correlation between the systemic IL-33, IL-6, and IL-12 levels in serum of COVID-19 patients suggested the synergistic effect of these cytokines in the pathogenesis of COVID-19 disease.
Significantly, disease severity was associated with age, neutrophil count, glucose, urea, creatinine, BIL T, BIL D, AST, LDH, CK, D dimer, CRP, PCT, TNF-α, IL-1β, IL-6, IL-12, IL-23, and IL-33 (Tables 1, 2; Supplementary Table 2). Among all the analyzed innate immunity cytokines, the strongest correlation was between IL-33 and disease severity (Supplementary Table 2). Serum levels greater than 332.08 pg/ml of IL-33 are associated with increased severity of COVID-19, indicating that IL-33 could be used as a prognostic biomarker in patients with a diagnosis of COVID-19. Interestingly, when CXR findings are compared with the sera levels of IL-33, a significant increase of IL-33 was detected in patients with CXR III, IV, and V findings. Focal consolidation, denoted as CXR III, is a CXR sign of mild to moderate cases of COVID-19. However, having in mind that elevated IL-33 is a marker of severe COVID-19 (19, 40), our result might imply that focal consolidation, alongside elevated IL-33 in the serum of patients might predict the progression of mild/moderate to severe/critical forms of the disease (Figure 3). As it is known, inflammation is closely related to the severity of COVID-19 (37). Increased inflammation consequently leads to more extensive tissue damage, and increased externalization of heat-shock proteins and alarmins. As IL-33 functions as both, alarmin and cytokine, it is possible that at first, as an alarmin, IL-33 facilitates inflammation, and then perpetuates it via interaction with other innate immunity cytokines or other immune cells, forming a circulus vitiosus of inflammation in the lungs.
Taking into account the results of this and similar studies, we believe that SARS-CoV-2 infection triggers IL-33 release from damaged epithelial cells in the lung (47). Released IL-33 initiates type 2 immune response that further leads to impaired antiviral activity and dysregulation of neutrophils (18). Dampened antiviral immunity results in delayed viral clearance and COVID-19 progression. In a more progressive stage of the disease, extensive injury of alveolar epithelial cells caused by strong interactions between the airway epithelium and activated immune cells is followed by overproduction of IL-33 (47). Under these circumstances, increased IL-33 facilitates lung inflammation by inducing the production of various innate proinflammatory cytokines (IL-1β, IL-6, TNF-α, IL-12, IL-23) in several target cells leading to the most severe forms of the disease (20, 48). Keeping in mind obtained data that IL-33 portrays an important role during severe form of COVID-19 followed by augmented inflammation, IL-33 might act as a promising therapeutic target which hinders COVID-19 severity.
The Limitations and Strengths of the Study
The first limitation of the study is its cross-sectional design. Additional limitation includes a small sample size and incomplete epidemiological and clinical data follow-up. Due to the absence of a unified national platform for COVID-19 data consolidation, we only used confirmed cases from the University Clinical Center of Kragujevac (Covid Center). Due to the excluding criteria, there is a possibility of selection bias, as the third limitation of our study. Future research should include the analyzes of the linkage of variables using longitudinal study design.
The strength of our study is that it provides a detailed discussion regarding the pathophysiology of inflammatory markers in COVID-19. Well-defined inclusion criteria, data collection, and statistical interpretation were done.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study involving human participants was reviewed and approved by Ethics Committees of the University Clinical Center of Kragujevac, Serbia (Approval Number 01/20-406) Faculty of Medical Sciences, University of Kragujevac, Serbia (Approval Number 01-6776). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SM and IJ: conceptualization. SM, NG, and MilenaJ: methodology. MilenaJ: software and formal analysis. IJ and VM: validation. SM, MarinaJ (6th author), NG, and MilenaJ: investigation. NA: resources. SL, NZ, and MiodragJ: data curation. SM: writing—original draft preparation. IJ and MarinaJ (2nd author): writing—review and editing. VV, MilanJ, and JS: visualization. IJ: supervision. IJ and NA: project administration. NA and ZM: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Science Fund of the Republic of Serbia (CIBIRDS), Serbian Ministry of Education, Science and Technological Development (175069), project with PR China (06/2018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Dusan Tomasevic, Aleksandar Ilic, and Nikola Jankovic for their excellent technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.749569/full#supplementary-material
References
1. Rauf A, Abu-Izneid T, Olatunde A, Khalil AA, Alhumaydhi FA, Tufail T, et al. COVID-19 pandemic: epidemiology, etiology, conventional and non-conventional therapies. Int J Environ Res Public Health. (2020) 17:8155. doi: 10.3390/ijerph17218155
2. Rothan HA. Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID19) outbreak. J Autoimmun. (2020) 109:102433. doi: 10.1016/j.jaut.2020.102433
3. National Health Commission of the People's Republic of China. Notice on the novel coronavirus infection diagnosis and treatment plan (trial version sixth). In: National Health Commission of the People's Republic of China, editor. Beijing. (2020). Available online at: http://www.nhc.gov.cn/wjw/gfxwjj/list_5.shtml. Accessed 10 Mar 2020
4. Letelier P, Encina N, Morales P, Riffo A, Silva R, Requelme I, et al. Role of biochemical markers in the monitoring of COVID-19 patients. J Med Biochem. (2021) 40:115–28. doi: 10.5937/jomb0-29341
5. Lippi G, Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin Chem Lab Med. (2020) 58:1063–69. doi: 10.1515/cclm-2020-0240
6. Ramírez-Truque M, Herrera-Morice M. Rol del laboratorioclínico ante la epidemia del COVID-19: Revisión de los métodos diagnósticos disponibles y sus limitaciones. Revista médica de Costa Rica. (2020) 85:73–80.
7. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
8. Zeng H, Ma Y, Zhou Z, Liu W, Huang P, Jiang M, et al. Spectrum and clinical characteristics of symptomatic and asymptomatic coronavirus disease 2019 (COVID-19) with and without pneumonia. Front Med (Lausanne). (2021) 8:645651. doi: 10.3389/fmed.2021.645651
9. National Health Commission Stroke Prevention and Control Engineering Expert Committee. Expert consensus on prevention and control of novel coronavirus infection in neurology (version first). In: National Health Commission Stroke Prevention and Control Engineering Expert Committee, editor. Beijing. (2020). Available online at: http://www.sinosc.org/NewsInfo/News/NewsDetailWeb?Tid=2621. (Accessed Mar 10, 2020)
10. Wasilewski PG, Mruk B, Mazur S, Półtorak-Szymczak G, Sklinda K, Walecki J. COVID-19 severity scoring systems in radiological imaging - a review. Pol J Radiol. (2020) 85:e361–e68. doi: 10.5114/pjr.2020.98009
11. Yasin R, Gouda W. Chest X-ray findings monitoring COVID-19 disease course and severity. Egypt J Radiol Nucl Med. (2020) 51:193. doi: 10.1186/s43055-020-00296-x
12. Jacques FH, Apedaile E. Immunopathogenesis of COVID-19: Summary and Possible Interventions. Front Immunol. (2020) 11:564925. doi: 10.3389/fimmu.2020.564925
13. Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet. (2020) 395:1111. doi: 10.1016/S0140-6736(20)30691-7
14. Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. (2020) 20:269–70. doi: 10.1038/s41577-020-0308-3
15. Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: The anger of inflammation. Cytokine. (2020) 133:155151. doi: 10.1016/j.cyto.2020.155151
16. Xu Z, Shi L, Wang Y, Zhang J, Huang J, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–22. doi: 10.1016/S2213-2600(20)30076-X
17. Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, et al. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: The REPROGRAM consortium position paper. Front Immunol. (2020) 11:1648. doi: 10.3389/fimmu.2020.01648
18. Liang Y, Ge Y, Sun J. IL-33 in COVID-19: friend or foe? Cell Mol Immunol. (2021) 1602–04. doi: 10.1038/s41423-021-00685-w
19. Zizzo G, Cohen P. L Imperfect storm: is interleukin-33 the Achilles heel of COVID-19? Lancet Rheumatol. (2020) 2:e779–e90. doi: 10.1016/S2665-9913(20)30340-4
20. Kritas SK, Ronconi G, Caraffa A, Gallenga CE, Ross R, Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J Biol Regul Homeost Agents. (2020) 34:9–14.
21. Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol. (2020) 21:494–500. doi: 10.3348/kjr.2020.0132
22. Jovanovic M, Simovic Markovic B, Gajovic N, Jurisevic M, Djukic A, Jovanovic I, et al. Metabolic syndrome attenuates ulcerative colitis: Correlation with interleukin-10 and galectin-3 expression. World J Gastroenterol. (2019) 25:6465–82. doi: 10.3748/wjg.v25.i43.6465
23. World Health Organization. Clinical Management of COVID-19: Interim Guidance. New York, NY: World Health Organization. (2020) p. 13–15
24. Hu D, Lou X, Meng N, Li Z, Teng Y, Zou Y, et al. Influence of age and gender on the epidemic of COVID-19: Evidence from 177 countries and territories-an exploratory, ecological study. Wiener Klinische Wochenschrift. (2021) 133:321–30. doi: 10.1007/s00508-021-01816-z
25. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–69. doi: 10.1001/jama.2020.1585
26. Rubio-Sánchez R, Lepe-Balsalobre E, Viloria-Peñas M. Prognostic factors for the severity of SARS-CoV-2 infection. Advances in Laboratory Medicine / Avances en Medicina de Laboratorio. (2021) 2:253–58. doi: 10.1515/almed-2021-0017
27. Wang D, Li R, Wang J, et al. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: a descriptive study. BMC Infect Dis. (2020) 20:519. doi: 10.1186/s12879-020-05242-w
28. Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. (2020) 136:489–500. doi: 10.1182/blood.2020006520
29. Drake TM, Riad AM, Fairfield CJ, Egan C, Knight SR, Pius R, et al. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, multicentrecohort study. Lancet. (2021) 398:P223–37. doi: 10.1016/S0140-6736(21)00799-6
30. Hu R, Han C, Pei S, Yin M, Chen X. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. (2020) 56:106051. doi: 10.1016/j.ijantimicag.2020.106051
31. Meidaninikjeh S, Sabouni N, Marzouni HZ, Bengar S, Khalili A, Jafari R. Monocytes and macrophages in COVID-19: Friends and foes. Life Sci. (2021) 269:119010. doi: 10.1016/j.lfs.2020.119010
32. Miyazawa M. Immunopathogenesis of SARS-CoV-2-induced pneumonia: lessons from influenza virus infection. Inflamm Regener. (2020) 40:39. doi: 10.1186/s41232-020-00148-1
33. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. (2020) 26:842–44. doi: 10.1038/s41591-020-0901-9
34. Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: Current State of the Science. Immunity. (2020) 52:910–41. doi: 10.1016/j.immuni.2020.05.002
35. Hosseini A, Hashemi V, Shomali N, Asghari F, Gharibi T, Akbari M, et al. Innate and adaptive immune responses against coronavirus. Biomed Pharmacother. (2020) 132:110859. doi: 10.1016/j.biopha.2020.110859
36. Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 Viral Load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. (2020) 71:1937–42. doi: 10.1093/cid/ciaa449
37. Gong J, Dong H, Xia QS, Huang ZY, Wang DK, Zhao Y, et al. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19: a retrospective study. BMC Infect Dis. (2020) 20:963. doi: 10.1186/s12879-020-05681-5
38. Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. (2020) 369:718–24. doi: 10.1126/science.abc6027
39. Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. (2020) 26:1636–43. doi: 10.1038/s41591-020-1051-9
40. Choi YS, Park JA, Kim J, Rho SS, Park H, Kim YM, et al. Nuclear IL-33 is a transcriptional regulator of NF-κB p65 and induces endothelial cell activation. Biochem Biophys Res Commun. (2012) 421:305–11. doi: 10.1016/j.bbrc.2012.04.005
41. Stanczak MA, Sanin DE, Apostolova P, Nerz G, Lampaki D, Hofmann M, et al. IL-33 expression in response to SARS-CoV-2 correlates with seropositivity in COVID-19 convalescent individuals. Nat Commun. (2021) 12:2133. doi: 10.1038/s41467-021-22449-w
42. Werder RB, Zhang V, Lynch JP, Snape N, Upham JW, Spann K, et al. Chronic IL-33 expression predisposes to virus-induced asthma exacerbations by increasing type 2 inflammation and dampening antiviral immunity. J Allergy Clin Immunol. (2018) 141:1607–19.e9. doi: 10.1016/j.jaci.2017.07.051
43. Burke H, Freeman A, Cellura DC, Stuart BL, Brendish NJ, Poole S, et al. Inflammatory phenotyping predicts clinical outcome in COVID-19. Respir Res. (2020) 21:245. doi: 10.1186/s12931-020-01511-z
44. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
45. Wang W, Liu X, Wu S, Chen S, Li Y, Nong L, et al. Definition and risks of cytokine release syndrome in 11 critically Ill COVID-19 patients with pneumonia: analysis of disease characteristics. J Infect Dis. (2020) 222:1444–51. doi: 10.1093/infdis/jiaa387
46. Rodriguez AE, Bogart C, Gilbert CM, McCullers JA, Smith AM, Kanneganti TD, Lupfer CR. Enhanced IL-1β production is mediated by a TLR2-MYD88-NLRP3 signaling axis during coinfection with influenza A virus and Streptococcus pneumoniae. PLoS ONE. (2019) 14:e0212236. doi: 10.1371/journal.pone.0212236
47. Cayrol C, Girard JP. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol Rev. (2018) 281:154–68. doi: 10.1111/imr.12619
Keywords: IL 33, COVID-19, disease severity, correlation, proinflammatory innate immune response
Citation: Markovic SS, Jovanovic M, Gajovic N, Jurisevic M, Arsenijevic N, Jovanovic M, Jovanovic M, Mijailovic Z, Lukic S, Zornic N, Vukicevic V, Stojanovic J, Maric V, Jocic M and Jovanovic I (2021) IL 33 Correlates With COVID-19 Severity, Radiographic and Clinical Finding. Front. Med. 8:749569. doi: 10.3389/fmed.2021.749569
Received: 29 July 2021; Accepted: 28 October 2021;
Published: 30 November 2021.
Edited by:
Abhilash Perisetti, University of Arkansas for Medical Sciences, United StatesReviewed by:
Abu Baker Sheikh, University of New Mexico, United StatesRupinder Mann, Saint Agnes Medical Center, United States
Copyright © 2021 Markovic, Jovanovic, Gajovic, Jurisevic, Arsenijevic, Jovanovic, Jovanovic, Mijailovic, Lukic, Zornic, Vukicevic, Stojanovic, Maric, Jocic and Jovanovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marina Jovanovic, marina_jovanovic@rocketmail.com
 Sofija Sekulic Markovic1
Sofija Sekulic Markovic1  Marina Jovanovic
Marina Jovanovic Nevena Gajovic
Nevena Gajovic Milena Jurisevic
Milena Jurisevic Nebojsa Arsenijevic
Nebojsa Arsenijevic Marina Jovanovic
Marina Jovanovic Vladimir Vukicevic
Vladimir Vukicevic Jasmina Stojanovic
Jasmina Stojanovic Miodrag Jocic
Miodrag Jocic Ivan Jovanovic
Ivan Jovanovic