The Potential Complementary Role of Using Chinese Herbal Medicine with Western Medicine in Treating COVID-19 Patients: Pharmacology Network Analysis
Abstract
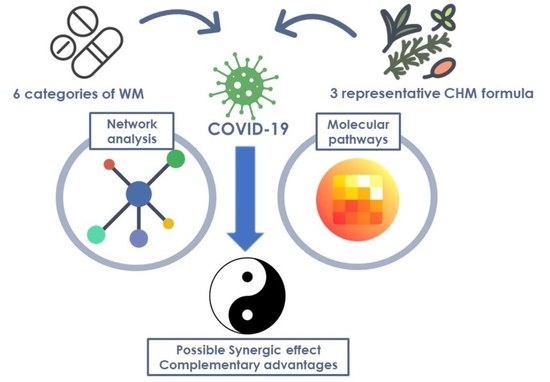
:1. Introduction
2. Results
2.1. Ingredients and Chemical Compounds of Commonly Prescribed CHM in COVID-19
2.2. Network Pharmacology of CHM and WM
2.3. COVID-Related Target Protein Covered by CHM and WM
2.4. Interaction and Relationships between CHM and WM
2.5. Proposed Molecular Pathways of the CHM and WM
3. Discussion
4. Materials and Methods
4.1. Data Collection and Study Design
4.2. Pharmacology Network Analysis
4.3. Pathway Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siemieniuk, R.A.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Izcovich, A.; Kum, E.; Pardo-Hernandez, H.; Rochwerg, B.; Lamontagne, F.; Han, M.A.; et al. Drug treatments for covid-19: Living systematic review and network meta-analysis. BMJ (Clin. Res. Ed.) 2020, 370, m2980. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ni, X.; Lin, J.; Zhang, Y.; Wu, L.; Huang, D.; Liu, Y.; Guo, J.; Wen, W.; Cai, Y.; et al. The add-on effect of Chinese herbal medicine on COVID-19: A systematic review and meta-analysis. Phytomedicine: Int. J. Phytother. Phytopharm. 2021, 85, 153282. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Bai, C.; He, F.; Xie, Y.; Zhou, H. Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID-19). Pharmacol. Res. 2020, 158, 104939. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, W.J.; Ni, Y.S.; Li, M.S.; Chen, J.K.; Liu, X.H.; Tan, X.H.; Li, J.Q. Therapeutic mechanism of Toujie Quwen granules in COVID-19 based on network pharmacology. BioData Min. 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wei, X.X.; Zheng, Y.J.; Zhang, L.L.; Wang, X.M.; Yang, H.Y.; Ma, X.; Zhao, L.H.; Tong, X.L. Potential mechanism prediction of Cold-Damp Plague Formula against COVID-19 via network pharmacology analysis and molecular docking. Chin. Med. 2020, 15, 78. [Google Scholar] [CrossRef]
- Mu, C.; Sheng, Y.; Wang, Q.; Amin, A.; Li, X.; Xie, Y. Potential compound from herbal food of Rhizoma Polygonati for treatment of COVID-19 analyzed by network pharmacology: Viral and cancer signaling mechanisms. J. Funct. Foods 2021, 77, 104149. [Google Scholar] [CrossRef]
- Yu, S.; Wang, J.; Shen, H. Network pharmacology-based analysis of the role of traditional Chinese herbal medicines in the treatment of COVID-19. Ann. Palliat. Med. 2020, 9, 437–446. [Google Scholar] [CrossRef]
- Zheng, S.; Baak, J.P.; Li, S.; Xiao, W.; Ren, H.; Yang, H.; Gan, Y.; Wen, C. Network pharmacology analysis of the therapeutic mechanisms of the traditional Chinese herbal formula Lian Hua Qing Wen in Corona virus disease 2019 (COVID-19), gives fundamental support to the clinical use of LHQW. Phytomedicine: Int. J. Phytother. Phytopharm. 2020, 79, 153336. [Google Scholar] [CrossRef]
- Pan, H.D.; Yao, X.J.; Wang, W.Y.; Lau, H.Y.; Liu, L. Network pharmacological approach for elucidating the mechanisms of traditional Chinese medicine in treating COVID-19 patients. Pharmacol. Res. 2020, 159, 105043. [Google Scholar] [CrossRef]
- Ruan, X.; Du, P.; Zhao, K.; Huang, J.; Xia, H.; Dai, D.; Huang, S.; Cui, X.; Liu, L.; Zhang, J. Mechanism of Dayuanyin in the treatment of coronavirus disease 2019 based on network pharmacology and molecular docking. Chin. Med. 2020, 15, 62. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, J.; Wang, Y.; Liu, S. Exploring the potential therapeutic effect of traditional Chinese medicine on coronavirus disease 2019 (COVID-19) through a combination of data mining and network pharmacology analysis. Eur. J. Integr. Med. 2020, 40, 101242. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Wu, Y.; Gu, Y.; Lv, Q.; Qi, F.; Gong, S.; Chen, X. Analysis of the molecular mechanism of Pudilan (PDL) treatment for COVID-19 by network pharmacology tools. Biomed. Pharmacother. = Biomed. Pharmacother. 2020, 128, 110316. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Du, J.; Li, X.; Zeng, J.; Tan, B.; Xu, J.; Lin, W.; Chen, X.L. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19. Drug Dev. Ind. Pharm. 2020, 46, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin. Med. J. 2020, 133, 1087–1095. [CrossRef] [PubMed]
- Tsai, K.C.; Huang, Y.C.; Liaw, C.C.; Tsai, C.I.; Chiou, C.T.; Lin, C.J.; Wei, W.C.; Lin, S.J.; Tseng, Y.H.; Yeh, K.M.; et al. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: A bedside-to-bench study. Biomed. Pharmacother. = Biomed. Pharmacother. 2021, 133, 111037. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Hwang, K.-C.; Chao, C.-L.; Chang, S.G.N.; Ho, M.-S.; Lin, J.-G.; Chang, H.-H.; Kao, S.-T.; Chen, Y.-M.; Chou, P. An Evaluation of the Additive Effect of Natural Herbal Medicine on SARS or SARS-Like Infectious Diseases in 2003: A Randomized, Double-Blind, and Controlled Pilot Study. Evid. -Based Complementary Altern. Med. 2008, 5, 273504. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.H.; Hwang, K.C.; Chao, C.L.; Chang, S.G.; Ker, C.C.; Chien, L.C.; Ho, M.S.; Lin, J.G.; Chen, Y.M.; Chou, P. The lesson of supplementary treatment with Chinese medicine on severe laboratory-confirmed SARS patients. Am. J. Chin. Med. 2006, 34, 927–935. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.K.; Gao, Y.; Hu, L.S.; Yang, J.W.; Wang, J.R.; Sun, W.J.; Liang, Z.Q.; Cao, Y.M.; Cao, Y.B. Protection against COVID-19 injury by qingfei paidu decoction via anti-viral, anti-inflammatory activity and metabolic programming. Biomed. Pharmacother. = Biomed. Pharmacother. 2020, 129, 110281. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, X.; Bai, X.; Wang, Q.; Chen, B.; Wang, H.; Lu, J.; Hu, S.; Zhang, X.; Zhang, H.; et al. Association between use of Qingfei Paidu Tang and mortality in hospitalized patients with COVID-19: A national retrospective registry study. Phytomedicine: Int. J. Phytother. Phytopharm. 2021, 85, 153531. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, C.; Li, H.; Qi, W.; Ruan, L.; Bian, Y.; Shi, H.; Song, H.; Tu, S.; Zhang, Y.; et al. Efficacy and safety assessment of severe COVID-19 patients with Chinese medicine: A retrospective case series study at early stage of the COVID-19 epidemic in Wuhan, China. J. Ethnopharmacol. 2021, 277, 113888. [Google Scholar] [CrossRef]
- Shi, N.; Guo, L.; Liu, B.; Bian, Y.; Chen, R.; Chen, S.; Chen, Y.; Chen, Y.; Cong, X.; Dong, G.; et al. Efficacy and safety of Chinese herbal medicine versus Lopinavir-Ritonavir in adult patients with coronavirus disease 2019: A non-randomized controlled trial. Phytomedicine: Int. J. Phytother. Phytopharm. 2021, 81, 153367. [Google Scholar] [CrossRef] [PubMed]
- Guney, E.; Menche, J.; Vidal, M.; Barábasi, A.L. Network-based in silico drug efficacy screening. Nat. Commun. 2016, 7, 10331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.Y.; Liu, J.C.; Liang, S.; Meng, X.H.; Greenbaum, J.; Xiao, H.M.; Tan, L.J.; Deng, H.W. Drug Repurposing for COVID-19 Treatment by Integrating Network Pharmacology and Transcriptomics. Pharmaceutics 2021, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.H.; Wu, F.; Cao, W.Y.; Wu, Z.G.; Chao, Y.C.; Liang, C. Network pharmacology for the identification of phytochemicals in traditional Chinese medicine for COVID-19 that may regulate interleukin-6. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yang, C.W.; Lin, Y.H.; Hsueh, J.Y.; Chen, J.L.; Yang, S.H.; Chen, Y.C.; Chen, H.Y. Identifying the Chinese Herbal Medicine Network and Core Formula for Allergic Rhinitis on a Real-World Database. Evid. Based Complement. Alternat. Med. 2020, 2020, 5979708. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370. [Google Scholar] [CrossRef]
- Chiang, C.C.; Korinek, M.; Cheng, W.J.; Hwang, T.L. Targeting Neutrophils to Treat Acute Respiratory Distress Syndrome in Coronavirus Disease. Front. Pharmacol. 2020, 11, 572009. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Nazari-Khanamiri, F.; Ghasemnejad-Berenji, M. A hypothesis that Notopterol may be effective in COVID-19 via JAK/STAT and other signaling pathways. J. Basic Clin. Physiol. Pharmacol. 2022. [Google Scholar] [CrossRef]
- Gong, L.; Wang, C.; Zhou, H.; Ma, C.; Zhang, Y.; Peng, C.; Li, Y. A review of pharmacological and pharmacokinetic properties of Forsythiaside A. Pharmacol. Res. 2021, 169, 105690. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, X.; Yang, L.; Zhao, Y.; Chew, Z.; Xiao, J.; Liu, C.; Zheng, X.; Zheng, Y.; Shi, Q.; et al. The Natural Compound Notopterol Binds and Targets JAK2/3 to Ameliorate Inflammation and Arthritis. Cell Rep. 2020, 32, 108158. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Sun, Y.W.; Ji, J.; Gan, L.; Zhang, C.F.; Wang, C.Z.; Yuan, C.S. Genkwanin ameliorates adjuvant-induced arthritis in rats through inhibiting JAK/STAT and NF-κB signaling pathways. Phytomedicine: Int. J. Phytother. Phytopharm. 2019, 63, 153036. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Ding, Z.; Li, Y.; Wang, J.; Chen, S.; Miao, J. Tanshinone I inhibits the growth and metastasis of osteosarcoma via suppressing JAK/STAT3 signalling pathway. J. Cell. Mol. Med. 2019, 23, 6454–6465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.X.; Zhong, S.; Meng, X.B.; Zheng, N.Y.; Zhang, P.; Wang, Y.; Qin, L.; Wang, X.L. Cinnamaldehyde Inhibits Inflammation of Human Synoviocyte Cells Through Regulation of Jak/Stat Pathway and Ameliorates Collagen-Induced Arthritis in Rats. J. Pharmacol. Exp. Ther. 2020, 373, 302–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Kumar, A.; Faiq, M.A.; Pareek, V.; Raza, K.; Narayan, R.K.; Prasoon, P.; Kumar, P.; Kulandhasamy, M.; Kumari, C.; Kant, K.; et al. Relevance of SARS-CoV-2 related factors ACE2 and TMPRSS2 expressions in gastrointestinal tissue with pathogenesis of digestive symptoms, diabetes-associated mortality, and disease recurrence in COVID-19 patients. Med. Hypotheses 2020, 144, 110271. [Google Scholar] [CrossRef]
- Zhu, L.; She, Z.G.; Cheng, X.; Qin, J.J.; Zhang, X.J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. (Lond. Engl.) 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Kappelmann, N.; Dantzer, R.; Khandaker, G.M. Interleukin-6 as potential mediator of long-term neuropsychiatric symptoms of COVID-19. Psychoneuroendocrinology 2021, 131, 105295. [Google Scholar] [CrossRef] [PubMed]
- Crooks, M.G.; Hart, S.P. Coagulation and anticoagulation in idiopathic pulmonary fibrosis. Eur. Respir. Rev.: Off. J. Eur. Respir. Soc. 2015, 24, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, M.; Letunic, I.; Jensen, L.J.; Bork, P. The SIDER database of drugs and side effects. Nucleic Acids Res. 2016, 44, D1075–D1079. [Google Scholar] [CrossRef]
- Chen, C.-C. TCM Database@Taiwan: The World’s Largest Traditional Chinese Medicine Database for Drug Screening In Silico. PLoS ONE 2011, 6, e15939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, F.; Yang, K.; Fang, S.; Bu, D.; Li, H.; Sun, L.; Hu, H.; Gao, K.; Wang, W.; et al. SymMap: An integrative database of traditional Chinese medicine enhanced by symptom mapping. Nucleic Acids Res. 2019, 47, D1110–D1117. [Google Scholar] [CrossRef] [Green Version]
- Meizlish, M.L.; Goshua, G.; Liu, Y.; Fine, R.; Amin, K.; Chang, E.; DeFilippo, N.; Keating, C.; Liu, Y.; Mankbadi, M.; et al. Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: A propensity score-matched analysis. Am. J. Hematol. 2021, 96, 471–479. [Google Scholar] [CrossRef]
- Hadid, T.; Kafri, Z.; Al-Katib, A. Coagulation and anticoagulation in COVID-19. Blood Rev. 2021, 47, 100761. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Lian, X.; Li, F.; Wang, C.; Zhu, F.; Qiu, Y.; Chen, Y. Therapeutic target database update 2022: Facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res. 2022, 50, D1398–D1407. [Google Scholar] [CrossRef]
- Hulsen, T.; de Vlieg, J.; Alkema, W. BioVenn - a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genom. 2008, 9, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabregat, A.; Korninger, F.; Viteri, G.; Sidiropoulos, K.; Marin-Garcia, P.; Ping, P.; Wu, G.; Stein, L.; D’Eustachio, P.; Hermjakob, H. Reactome graph database: Efficient access to complex pathway data. PLoS Comput. Biol. 2018, 14, e1005968. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Feng, X.; Stein, L. A human functional protein interaction network and its application to cancer data analysis. Genome Biol. 2010, 11, R53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabregat, A.; Sidiropoulos, K.; Viteri, G.; Forner, O.; Marin-Garcia, P.; Arnau, V.; D’Eustachio, P.; Stein, L.; Hermjakob, H. Reactome pathway analysis: A high-performance in-memory approach. BMC Bioinform. 2017, 18, 142. [Google Scholar] [CrossRef] [Green Version]
- Berthold, M.R.; Cebron, N.; Dill, F.; Gabriel, T.R.; Kötter, T.; Meinl, T.; Ohl, P.; Thiel, K.; Wiswedel, B. KNIME—The Konstanz information miner: Version 2.0 and beyond. SIGKDD Explor. Newsl. 2009, 11, 26–31. [Google Scholar] [CrossRef] [Green Version]









| Chinese medicine | Source | Ingredients | Clinical Studies | Sample Size (n) | Citation |
|---|---|---|---|---|---|
| NRICM101 | Chinese Medicine Clinical Practice Guideline on COVID-19 in Taiwan | Ban Lan Gen, Yu Xing Cao, Huang Qin, Gua Lou, Jing Jie, Fang Feng, Sang Ye, Hou Po, Bo He, Gan Cao | Patients who had more risk factors and showing no improvement after 21 days of hospitalization achieved 3 consecutive negative results within a median of 9 days | 33 | Tsai KC et al. (2021) |
| QFPDT | Clinical Practice Guideline on COVID-19 in China | Ma Huang, Gan Cao, Ku Xing Ren, Shi Gao, Gui Zhi, Ze Xie, Zhu Ling, Bai Zhu, Fu Ling, Chai Hu, Huang Qin, Ban Xia, Sheng Jiang, Zi Wan, Kuan Dong Hua, She Gan, Xi Xin, Shan Yao, Zhi Shi, Chen Pi, Huo Xiang | ↓50% COVID-19 related mortality ↓incidences of acute liver injury and acute kidney injury | 8939 | Yang RC et al. (2020) |
| HSBDF | Ma Huang, Ku Xing Ren, Shi Gao, Gan Cao, Huo Xiang, Hou Po, Cang Zhu, Cao Guo, Ban Xia, Fu Ling, Da Huang, Huang Qi, Ting Li Zi, Chi Shao | ↓clinical remission time | 40 | Shi NN et al. (2021) | |
| ↓median time of SARS-CoV-2 RNA clearance ↑ ratio of nucleic acid negative conversion ↓the high sensitivity C-reactive protein and serum ferritin | 55 | Wang Y et al. (2021) | |||
| Western medicine | Reference | Category | Effect | ||
| Dexamethasone, | Western medicine from BMJ living review (until 6 APR 2021) and WHO guideline (until 24 September 2021) | Corticosteroid | ↓mortality, ↓mechanical ventilation, ↑ventilator-free days | ||
| Sarilumab, Tocilizumab | Anti-Interleukin-6 (Anti-IL6) | ↓ mechanical ventilation, ↓duration of hospitalization | |||
| Baricitinib, Ruxolitinib | Janus kinase inhibitor (JAKi) | ↓ mechanical ventilation, ↓ duration of mechanical ventilation | |||
| Aspirin | Hadid T et al. (2021) Meizlish ML et al. (2021) | Antiplatelet | prophylaxis of thrombosis | ||
| UFH, LMWH (Enoxaparin) | Anticoagulant | ||||
| Rivaroxaban | Non-vitamin K antagonist oral anticoagulants (NOAC) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-C.; Tseng, L.-W.; Huang, Y.-C.; Yang, C.-W.; Chen, Y.-C.; Chen, H.-Y. The Potential Complementary Role of Using Chinese Herbal Medicine with Western Medicine in Treating COVID-19 Patients: Pharmacology Network Analysis. Pharmaceuticals 2022, 15, 794. https://doi.org/10.3390/ph15070794
Lu Y-C, Tseng L-W, Huang Y-C, Yang C-W, Chen Y-C, Chen H-Y. The Potential Complementary Role of Using Chinese Herbal Medicine with Western Medicine in Treating COVID-19 Patients: Pharmacology Network Analysis. Pharmaceuticals. 2022; 15(7):794. https://doi.org/10.3390/ph15070794
Chicago/Turabian StyleLu, Yi-Chin, Liang-Wei Tseng, Yu-Chieh Huang, Ching-Wei Yang, Yu-Chun Chen, and Hsing-Yu Chen. 2022. "The Potential Complementary Role of Using Chinese Herbal Medicine with Western Medicine in Treating COVID-19 Patients: Pharmacology Network Analysis" Pharmaceuticals 15, no. 7: 794. https://doi.org/10.3390/ph15070794