Multiethnic Investigation of Risk and Immune Determinants of COVID-19 Outcomes
- 1Division of Hematology and Medical Oncology, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 2Institute for Immunology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States
- 3Department of Systems Pharmacology and Translational Therapeutics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States
- 4University of California, Santa Barbara, Santa Barbara, CA, United States
- 5Scientific Computing, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 6Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 7Department of Genetics and Genomic Sciences, Center for Transformative Disease Modeling, Tisch Cancer Institute, Icahn Institute for Data Science and Genomic Technology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Background: Disparate COVID-19 outcomes have been observed between Hispanic, non-Hispanic Black, and White patients. The underlying causes for these disparities are not fully understood.
Methods: This was a retrospective study utilizing electronic medical record data from five hospitals within a single academic health system based in New York City. Multivariable logistic regression models were used to identify demographic, clinical, and lab values associated with in-hospital mortality.
Results: A total of 3,086 adult patients with self-reported race/ethnicity information presenting to the emergency department and hospitalized with COVID-19 up to April 13, 2020, were included in this study. While older age (multivariable odds ratio (OR) 1.06, 95% CI 1.05–1.07) and baseline hypoxia (multivariable OR 2.71, 95% CI 2.17–3.36) were associated with increased mortality overall and across all races/ethnicities, non-Hispanic Black (median age 67, interquartile range (IQR) 58–76) and Hispanic (median age 63, IQR 50–74) patients were younger and had different comorbidity profiles as compared to non-Hispanic White patients (median age 73, IQR 62–84; p < 0.05 for both comparisons). Among inflammatory markers associated with COVID-19 mortality, there was a significant interaction between the non-Hispanic Black population and interleukin-1-beta (interaction p-value 0.04).
Conclusions: This analysis of a multiethnic cohort highlights the need for inclusion and consideration of diverse populations in ongoing COVID-19 trials targeting inflammatory cytokines.
Background
Reports from the United States, the United Kingdom, and Brazil have highlighted racial disparities in the COVID-19 pandemic (Baqui et al., 2020; Price-Haywood et al., 2020; Oppel et al., 2020; Williamson et al., 2020). National studies from the United Kingdom and Brazil have found race to be an independent predictor of death (Baqui et al., 2020; Williamson et al., 2020). In the United States, Black and Hispanic individuals have disproportionately high rates of infection, hospitalization, and mortality (Hsu et al., 2020; Holtgrave et al., 2020; Price-Haywood et al., 2020; Oppel et al., 2020). These disparities have been attributed to greater representation of Black and Hispanic persons in essential services and a higher burden of comorbidities in minority communities, among others.
While Black and Hispanic individuals in the United States have been disproportionately affected by the pandemic, the majority of published studies investigating COVID-19 mortality risk factors have been in cohorts of individuals with predominantly European or Asian ancestry (Docherty et al., 2020; Gupta et al., 2020; Grasselli et al., 2020; Petrilli et al., 2020; Zhou et al., 2020). Few US studies have directly examined mortality risk factors and their effect sizes in Black or Hispanic as compared to White individuals (Gu et al., 2020; Price-Haywood et al., 2020). Rigorous analysis to establish risk factors and molecular predictors for each population is urgently needed.
We sought to identify race/ethnic-specific clinical and immune factors of mortality using a diverse cohort of White, Black, and Hispanic COVID-19 patients admitted to a single health system in New York. In addition to baseline characterization, we conducted stratified and interaction term analyses to identify risk and immune factors that may affect the outcomes of each patient population. The systematic analyses revealed population-specific effects of multiple risk factors that were previously unknown, highlighting the importance of including diverse patient populations and tailored consideration in precision medicine for COVID-19.
Methods
Study Setting
The study was conducted within the Mount Sinai Health System, which is an academic healthcare system comprising 8 hospitals and more than 410 ambulatory practice locations in the New York metropolitan area. This analysis involves patients who presented to five hospitals: The Mount Sinai Hospital (MSH) (1,134 beds), Mount Sinai West (514 beds), and Mount Sinai Morningside (495 beds) in Manhattan; Mount Sinai Brooklyn (212 beds); and Mount Sinai Queens (235 beds).
Data Sources
Data were captured by the Epic electronic health record (Epic Systems, Verona, WI, USA) and directly extracted from Epic’s Clarity and Caboodle servers. This de-identified dataset was developed and released by the Mount Sinai Data Warehouse (MSDW) team, with the goal of encompassing all COVID-19-related patient encounters within the Mount Sinai system, accompanied by selected demographics, comorbidities, vital signs, medications, and lab values. As part of de-identification, all patients over the age of 89 had their age set to 90.
This study utilized de-identified data extracted from the electronic health record and as such was considered non-human subject research. Therefore, this study was granted an exemption from the Mount Sinai institutional review board (IRB) review and approval process.
Patient Population and Definitions
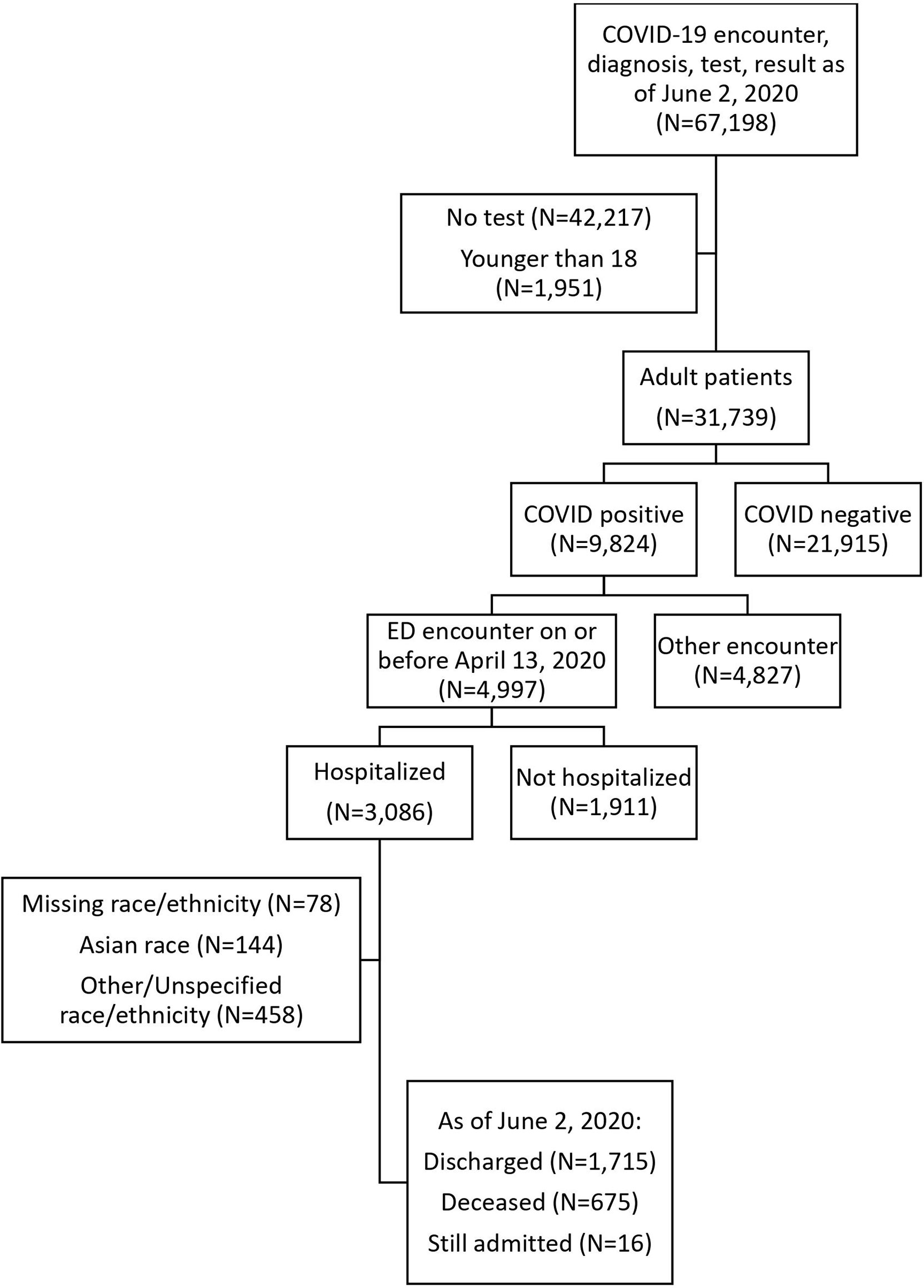
The MSDW dataset captured any patient encounters at a Mount Sinai facility with any of the following: a COVID-19-related encounter diagnosis, a COVID-19-related visit type, a SARS-CoV-2 lab order, a SARS-CoV-2 lab result, or a SARS-CoV-2 lab test result from the New York State Department of Health’s Wadsworth laboratory. For this study, patients with COVID-19-related visits to the emergency department (ED) on or before April 13, 2020, were identified, and patients who were admitted were selected. Their hospitalization outcomes through June 2, 2020, were observed.
Our analysis was limited to adults over 18 years old who were hospitalized for COVID-19 through a Mount Sinai ED. Self-reported race and ethnicity were classified into 3 mutually exclusive categories: non-Hispanic (NH) White (White), NH Black (Black), and Hispanic (Supplementary Table 1). COVID-19 positivity was determined by a positive or presumptive positive result from a nucleic acid-based test for SARS-CoV-2 in nasopharyngeal or oropharyngeal swab specimens. Baseline vital signs were the first documented vital signs for the encounter. Hypoxia was defined as oxygen saturation of less than 92%. Baseline labs were defined as the first lab value within 24 h of the start of the encounter.
University of Pennsylvania Cohort
Patients in the University of Pennsylvania cohort were identified based on a positive SARS-CoV-2 PCR test. Patients were screened and gave informed consent within 72 h of hospitalization. Clinical data were collected from electronic medical records into standardized case reports. Healthy donors (HDs) had no prior diagnosis or symptoms consistent with COVID-19. Recovered donors (RDs) were adults with a self-reported positive COVID-19 PCR test who recovered as defined by the Centers for Disease Control and Prevention. Cytokine levels were measured from peripheral blood plasma using a custom human cytokine 31-plex panel (EMD Millipore Corporation, Burlington, MA, USA; SPRCUS707), as described in Divij et al. (Mathew et al., 2020)
Logistic Regression
The primary outcome was in-hospital mortality. Univariable and multivariable logistic regression analyses were used to identify factors associated with death. Race/ethnicity-specific risk factors were identified by 1) constructing stratified models for each racial category and 2) constructing models including interaction terms between race/ethnicity and other covariates. Separate interaction models were created to test the interactions of either Hispanic ethnicity or Black race with other covariates. Interactions were compared against the White race as the reference group.
Demographic factors, comorbidities, initial vital signs, baseline lab values, and treatment facility site (Manhattan vs. Brooklyn/Queens) were analyzed as covariates. There was minimal clustering of outcomes by treatment site (ICC(ρ) = 0.026), and this was modeled as a fixed effect. Covariates were chosen a priori based on prior reports. The odds ratios (ORs) derived from the coefficients of each model were reported, along with the Wald-type confidence interval and p-values.
Laboratory Value Analysis
Markers of inflammation, such as C-reactive protein (CRP), ferritin, and D-dimer, have been proposed as being correlated with COVID-19 severity. However, the missingness of these lab values varied across sites. Given the possibility of confounding by indication (if providers ordered these labs in more acutely ill patients), the analyses involving lab tests were limited to those obtained at the largest site (MSH) and those that had less than 15% missing values at that site. The cytokines interleukin-1-beta (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-alpha (TNF-α) were exempted from this threshold because they were obtained on a subset of COVID-19 patients in the context of a study with broad inclusion criteria (Charney et al., 2020; Del Valle et al., 2020).
To test the associations of these lab values with mortality, each lab test was performed in race/ethnicity-stratified multivariable logistic regression models adjusting for age, sex, and hypoxia. The number of covariates in the model was limited due to the reduced sample size. Labs were standardized to a mean of 0 and an SD of 1 prior to regression analysis.
Statistical Analysis
Patient characteristics and baseline vitals and labs were described using medians and ranges for continuous variables and proportions for categorical variables. Continuous variables were compared using the Wilcoxon rank-sum test, and categorical variables were compared using Fisher’s exact test. All statistical analyses and data visualizations were carried out using R 4.0.0 (The R Foundation, Vienna, Austria), along with the tidyverse, ggpubr, forestplot, and Hmisc packages (Wickham, 2017; Kassambara, 2020; Gordon and Lumley, 2021; Harrell et al., 2022). Statistical significance was defined as p < 0.05.
Results
Study Population
There were 4,997 adult patients with COVID-19-related ED visits on or before April 13, 2020, of whom 3,086 (61.8%) were hospitalized. Hospitalization rates were significantly lower for NH Black patients compared to the other groups (NH Black 56.5%; NH White 63.4%; Hispanic 64.3%, Asian 62.6%; Other 63.6%; p < 0.001). Hospitalized patients were significantly older (median age 66 vs. 50, p < 0.001) and were more likely to have comorbidities such as hypertension (35.5% vs. 13.5%, p < 0.001), diabetes (24% vs. 7.8%, p < 0.001), and chronic kidney disease (11.9% vs. 3.1%, p < 0.001) (Supplementary Table 1). The clinical characteristics of non-hospitalized patients are summarized by race/ethnicity in Supplementary Table 2.
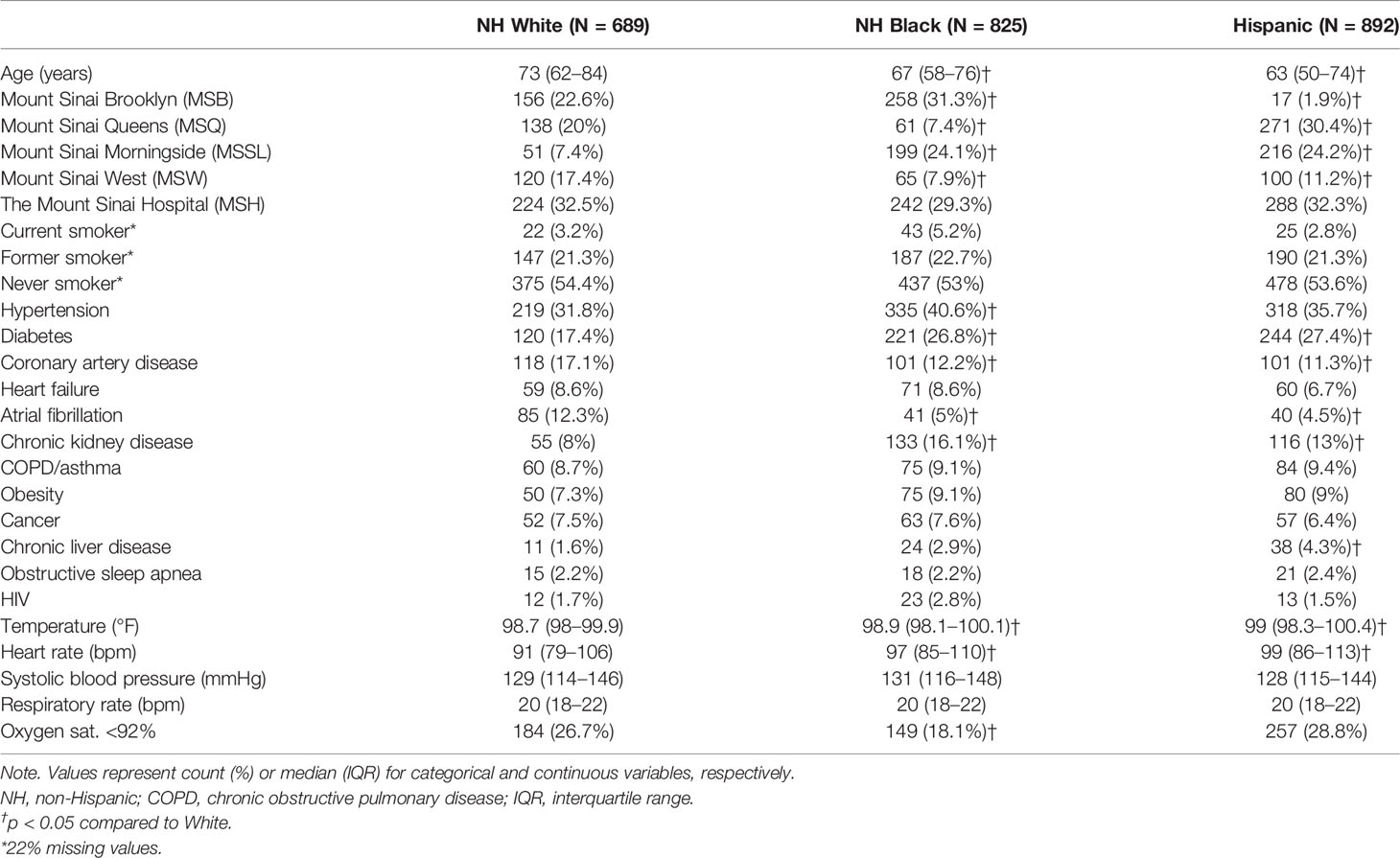
The hospitalized cohort included 3,086 adult patients. We excluded 78 patients with missing race or ethnicity data, 144 Asian patients, and 458 patients with other or unspecified race/ethnicity from the comparative/stratified analyses based on power considerations (Figure 1). The remaining 2,406 patients were 37.1% Hispanic, 34.3% NH Black, and 28.6% NH White.
Compared to NH White patients, NH Black and Hispanic patients were younger (median age 67 and 63 vs. 73, p < 0.001 for both) (Table 1). NH Black patients were more likely to have hypertension (40.6% vs. 31.8%, p < 0.001), diabetes (26.8% vs. 17.4%, p < 0.001), and chronic kidney disease (16.1% vs. 8%, p < 0.001) than NH White patients. Hispanic patients were more likely to have diabetes (27.4% vs. 17.4%, p < 0.001), chronic kidney disease (13% vs. 8%, p = 0.001), and chronic liver disease (4.3% vs. 1.6%, p = 0.003), compared to NH White patients. NH White patients were more likely to have coronary artery disease (17.1% vs. 12.2% and 11.3%, p = 0.008 vs. Black, p = 0.001 vs. Hispanic) and atrial fibrillation (12.3% vs. 5% and 4.5%, p < 0.001 for both) than NH Black or Hispanic patients.
These results demonstrate differences in the distributions of demographic and clinical COVID-19 mortality risk factors by race/ethnicity.
Population-Specific Clinical Factors Associated with Death
Unadjusted mortality rates were lower among NH Black (27.5% vs. 34.1%, p = 0.006) and Hispanic (23.9% vs. 34.1%, p < 0.001) patients compared to NH White patients. The rates of intensive care were not significantly different between NH Black and NH White (19.8% vs. 21.5%, p = 0.44) or Hispanic and NH White (23.4% vs. 21.5%, p = 0.36) patients.
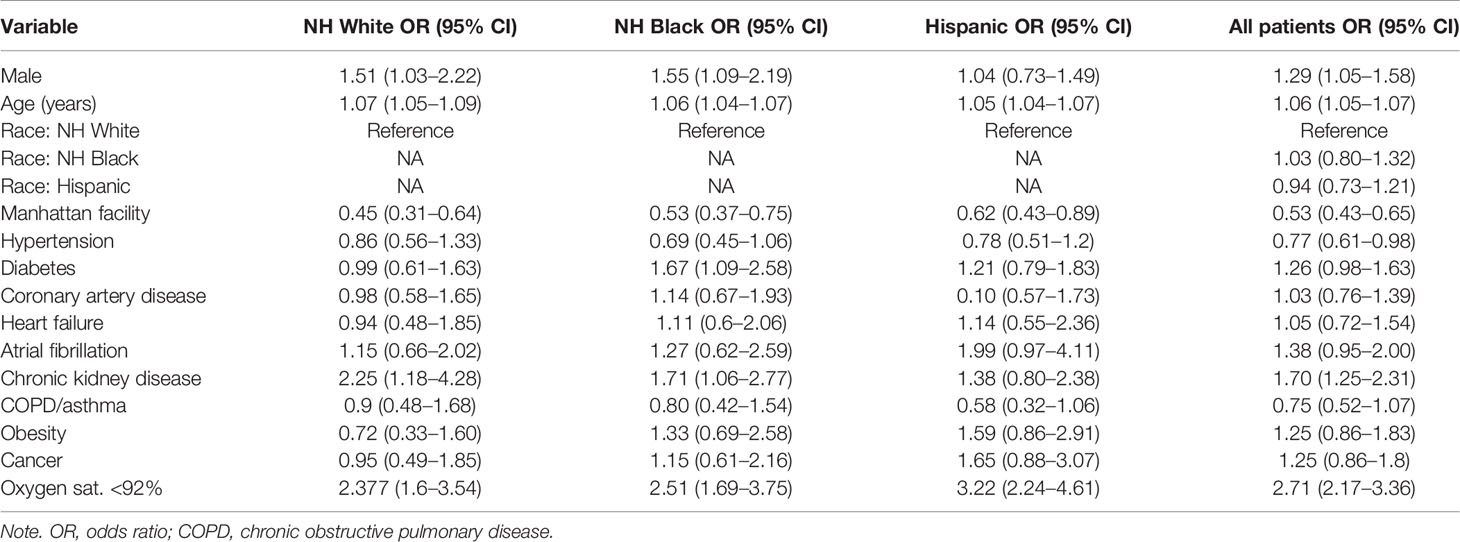
We first evaluated the association of demographic, clinical, and laboratory variables with in-hospital mortality (Table 2, Supplementary Tables 4, 5). In a univariate analysis, NH Black (OR 0.73, 95% CI 0.59–0.91) and Hispanic (OR 0.61, 95% CI 0.49–0.76) populations were associated with lower mortality as compared to NH White. However, race/ethnicity was not an independent predictor of mortality after adjusting for age, sex, comorbidities, and baseline hypoxia (oxygen saturation <92% at the first measurement of the clinical encounter) in this cohort (Black HR 1.03, 95% CI 0.80–1.32; Hispanic HR 0.94, 95% CI 0.73–1.21) (Figure 2A). Our finding is consistent with several cohort studies in the United States (Gold et al., 2020; Price-Haywood et al., 2020; Suleyman et al., 2020), although UK and Brazil studies have reported race as an independent predictor of mortality (Baqui et al., 2020; Williamson et al., 2020), possibly due to population differences.
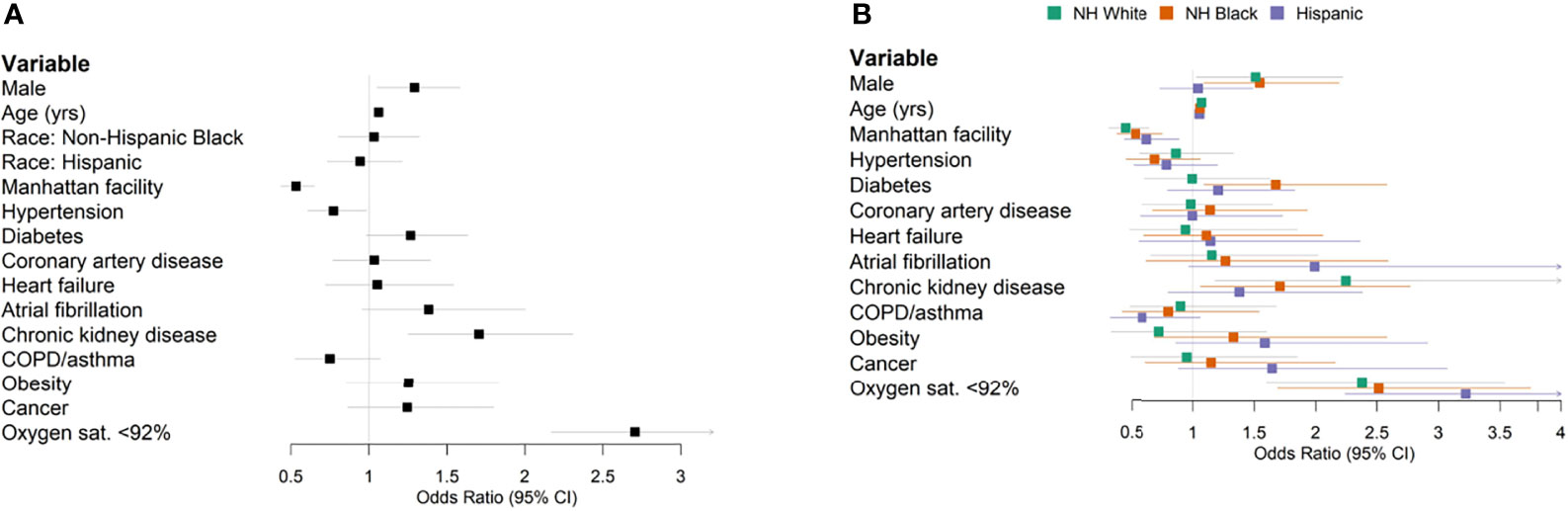
Figure 2 Forest plots of multivariable logistic regression results predicting in-hospital mortality. (A) All patients. (B) Stratified by race/ethnicity.
Previous patient cohort analyses rarely considered race/ethnic-specific risk factors, which require stratified modeling within each population cohort. We conducted stratified analyses within ethnic groups to determine the population-specific effect sizes of clinical factors and comorbidity. Diabetes was associated with an OR of 1.67 (95% CI 1.09–2.58) in the stratified NH Black population and an OR of 0.99 (95% CI 0.61–1.63) in the NH White population (Figure 2B). Obesity was associated with an OR of 1.33 (95% CI 0.69–2.58) in NH Black, compared to an OR of 0.72 (95% CI 0.33–1.60) in NH White (Figure 2B). Increased age and baseline hypoxia were consistently associated with increased mortality across all three populations (Supplementary Table 6). Altogether, these results highlight the shared and specific clinical risk factors of COVID-19 mortality across populations.
Baseline Laboratory Values
We analyzed baseline lab values among patients admitted to the largest site in our dataset, MSH. This site had the most complete records for routine and inflammatory lab values. We defined baseline labs as the first lab value within 24 h of the start of the encounter.
Among common lab values, Hispanic patients had higher baseline alanine aminotransferase values (median alanine transaminase (ALT), 33 vs. 28 U/L, p = 0.03) than NH White patients (Table 3), consistent with the increased prevalence of chronic liver disease among Hispanic patients.
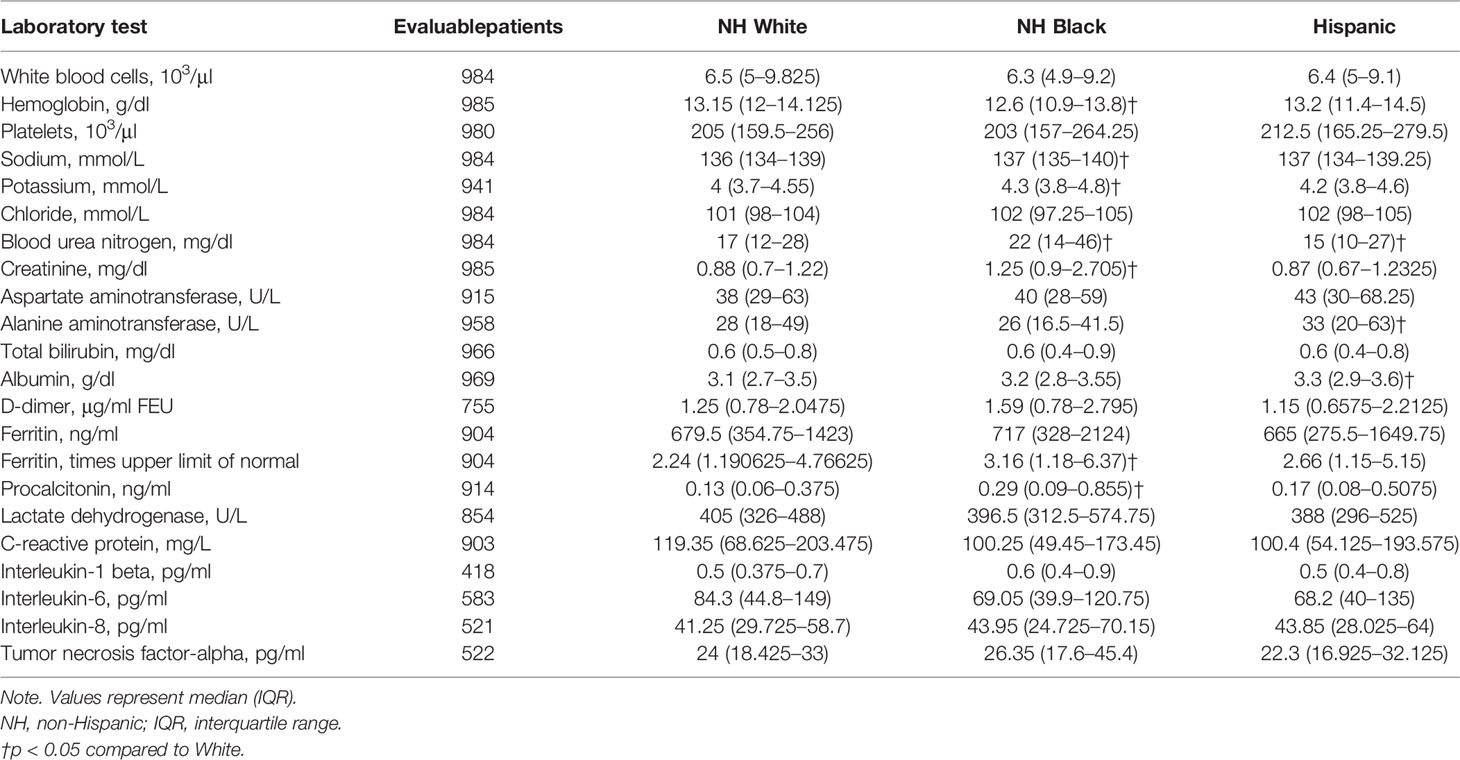
Table 3 Baseline laboratory values among patients admitted to The Mount Sinai Hospital, by race/ethnicity.
Among inflammatory lab markers, NH Black patients had higher initial levels of procalcitonin (0.29 vs. 0.13 ng/ml, p < 0.001) and more abnormal ferritin levels (3.16 vs. 2.24 times the upper limit of normal, p = 0.03) as compared to NH White patients (Table 3). There were no significant differences in baseline D-dimer, lactate dehydrogenase (LDH), CRP, IL-1β, IL-6, IL-8, or TNF-α levels. Thus, population-specific associations identified for these immune factors would indicate contributions from their relative differences between patients of different outcomes within a population rather than baseline differences across populations.
Population-Specific Immune Factors Associated with Clinical Outcomes
We next conducted a multiethnic analysis to identify immune markers associated with patient outcomes in MSH cohort. Using multivariate models adjusting for age, sex, and hypoxia, we identified CRP (OR 1.39, 95% CI 1.16–1.68), albumin (OR 0.75, 95% CI 0.61–0.91), IL-6 (OR 1.43, 95% CI 1.12–1.82), white blood cell (WBC) (OR 1.35, 95% CI 1.08–1.65), and LDH (OR 1.34, 95% CI 1.07–1.68) as independent predictors of mortality (Supplementary Table 7).
To identify immune markers showing population specificity in predicting COVID-19 outcomes, we applied the multivariate regression model in each population-stratified cohort. Elevated levels of IL-1β were associated with a higher risk of mortality in Black (OR 2.35, 95% CI 1.13–4.86) compared to White patients (OR 0.78, 95% CI 0.41–1.51) (Figure 3, Supplementary Table 7). Increased procalcitonin levels were associated with an OR of 2.65 (95% CI 0.88–7.96) in Hispanic patients, compared to an OR of 0.98 (95% CI 0.58–1.66) among NH White patients. Increased IL-8 levels were associated with an OR of 1.51 (95% CI 0.59–3.86) among Hispanic patients and an OR of 8.76 (95% CI 0.95–80.7) among White patients (Supplementary Table 7). To validate the population specificity of these COVID-19 mortality-associated immune markers, we further utilized a multivariate model including interaction terms, finding a significant interaction between NH Black and IL-1β (p = 0.04), and suggestive but non-significant interactions between Hispanic and procalcitonin (p = 0.07) and IL-8 (p = 0.09) as compared to NH White (Supplementary Table 6).
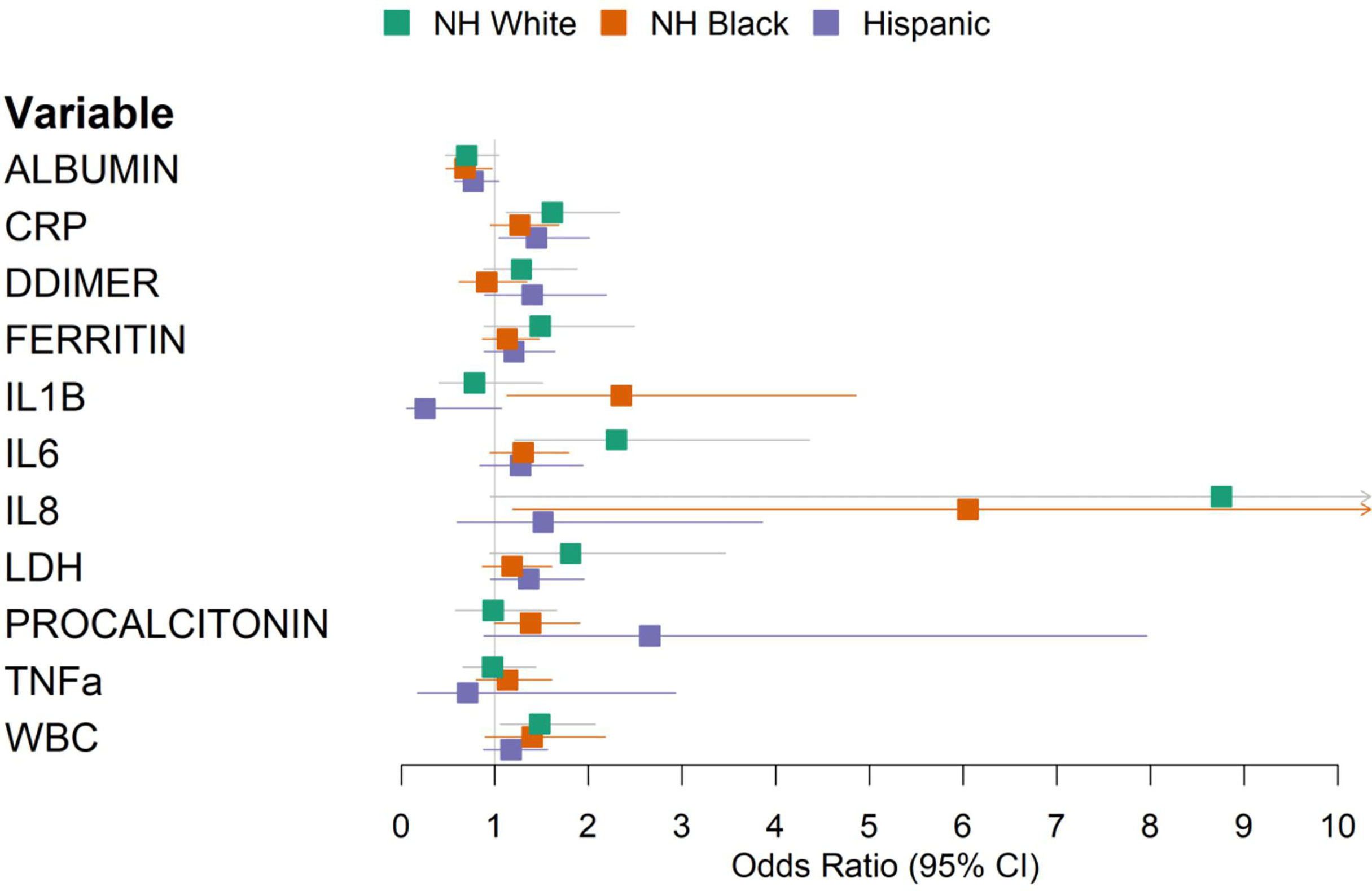
Figure 3 Forest plot of multivariable logistic regression results predicting in-hospital mortality using laboratory values, stratified by race/ethnicity. Models were adjusted for age, sex, and baseline hypoxia.
Next, we sought to validate the immune marker findings of MSH cohort in an independent dataset. We utilized immunoprofiling data from the University of Pennsylvania cohort (Mathew et al., 2020) to compare levels of serum cytokines and immunologic markers between diverse patient populations vs. HDs and RDs. Among the COVID-19 patients with available race/ethnicity data, eight were NH Black, three were NH White, and four were Asian. Additionally, there were ten HDs and twelve RDs with no available race/ethnicity data.
We used the non-parametric Mood’s median test to detect potential population differences in the median values of 13 measured immune markers against the combined cohort of HDs and RDs (HDs/RDs, Supplementary Table 8). NH Black patients had significantly higher median IL-6 (37.1 vs. 2.59, p = 0.003) and IP10 (227 vs. 55.6, p = 0.006) and lower median IL12p70 (1.98 vs. 3.41, p = 0.004) levels than HDs/RDs. IP10 was also found to be significant when comparing NH White or Asian patients with HDs/RDs (p < 0.05). Median IL-1β was 4.13 in NH Black patients compared to 2.66 in HDs/RDs (p = 0.115), providing a suggestive yet non-significant association to the finding in MSH cohort.
Discussion
Racial disparities in COVID-19 infections and outcomes have become apparent in both the United States and elsewhere (Baqui et al., 2020; Price-Haywood et al., 2020; Oppel et al., 2020; Williamson et al., 2020). The causes of these disparities are complex and multifactorial and must be considered in the context of the social determinants of health (Williamson et al., 2020; Nguyen et al., 2020).
In this study, set in New York City during the height of the initial COVID-19 surge, we describe the characteristics and outcomes of a diverse cohort including substantial numbers of NH White, NH Black, and Hispanic patients. The three groups differed significantly in demographic and clinical factors. White patients were older and showed higher rates of cardiovascular disease such as coronary artery disease and atrial fibrillation. NH Black and Hispanic patients were younger and had different comorbidity profiles, e.g., hypertension, diabetes, chronic kidney disease, and chronic liver disease.
Unadjusted in-hospital mortality was the highest in NH White patients, but multivariable analysis showed that race/ethnicity was not an independent predictor of mortality in this cohort. It remains unclear whether race and ethnicity are independent risk factors for COVID-19 mortality after adjusting for confounding factors. Large national-level studies in the United Kingdom and Brazil have reported race as an independent predictor of mortality (Baqui et al., 2020; Williamson et al., 2020), whereas smaller studies in the United States have not (Gold et al., 2020; Price-Haywood et al., 2020; Suleyman et al., 2020), possibly due to statistical power or population differences. Changes in clinical management and outcomes of COVID-19 over the course of the pandemic may also complicate comparisons of results from different time periods (Horwitz et al., 2020). In this New York City patient population, race/ethnicity was not an independent predictor for mortality.
In addition to describing this cohort, we aimed to test established COVID-19 risk factors for race/ethnicity-specific effects. Despite recapitulating several known risk factors, such as age, male sex, and hypoxia, we found only suggestive but non-significant interactions between Black race, diabetes, and obesity, and both diabetes and obesity tended to increase the mortality risk of Black patients to a greater degree than White patients. Notably, when analyzing inflammatory markers for their association with mortality, we found a significant interaction between the NH Black population and the inflammatory cytokine IL-1β.
Excessive inflammation has emerged as an important aspect of COVID-19 pathophysiology, and the anti-inflammatory steroid dexamethasone has been shown to improve outcomes among those with severe disease (The RECOVERY Collaborative Group, 2020). The interaction between the NH Black population and IL-1β raises the possibility that differences in immunity may contribute to worse outcomes in some patients. Black Americans are at higher risk of autoimmune conditions such as systemic lupus erythematosus and lupus nephritis as compared to White Americans, with differences that can be linked in some cases to specific polymorphisms, which are more common in African Americans (Ness et al., 2004; Clatworthy et al., 2007; Richman et al., 2012; Freedman et al., 2014).
IL-1β is a pro-inflammatory cytokine that plays a role in both physiologic and pathologic inflammation. IL-1 inhibitors such as anakinra and canakinumab have been developed to target IL-1 in autoimmune diseases such as rheumatoid arthritis and Still’s disease. These agents are also being actively investigated as COVID-19 treatments. Thus far, two randomized studies have found no clinical benefit from IL-1 inhibitors in COVID-19 [Novartis Provides Update on CAN-COVID Trial in Hospitalized Patients With COVID-19 Pneumonia and Cytokine Release Syndrome (CRS) (Novartis); Tharaux et al., 2021]. However, these studies have not reported analyses taking race/ethnicity into account.
The strengths of our database include its size and the inclusion of 37.1% Hispanic patients, a vulnerable population in this pandemic, which has been underrepresented in the literature to date. Additionally, our near-complete follow-up of the cohort’s hospital outcomes (99.3%) strengthens the validity of our findings.
Our study has limitations that warrant specific mention. The cytokine analysis was limited to only a subset of the population and should be considered exploratory. We were not able to control for other comorbidities, which may have influenced cytokine levels (e.g., diabetes and IL-1β) (Dinarello et al., 2010). The dataset was derived from the electronic health record database without manual review, which may limit the completeness of comorbidity labels. Race and ethnicity were self-reported and were missing or unspecified in 17.4% of the initial cohort. The subset of patients with cytokine data was limited in number and had limited models testing interactions. Causal mechanisms underlying the correlations identified in this retrospective analysis remain to be elucidated.
In conclusion, our analysis of a diverse cohort drawn from the New York metropolitan area highlights both similarities and important differences across racial/ethnic groups in risk factors for death among hospitalized COVID-19 patients. The findings identified across populations call for conscious inclusion in future cohort studies and clinical trials to ensure the efficacy of potential diagnostics and treatments across diverse individuals.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
KH, EW, and TJ conceived and designed the study. DM collected and generated the data from the University of Pennsylvania cohort. SN and PK collected and organized the clinical data from Mount Sinai. TJ, NS, and H-HH analyzed the data. TJ and K-lH drafted the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by NIGMS R35GM138113 to K-lH.
Conflict of Interest
EW has consulting agreements with and/or is on the scientific advisory board for Merck, Elstar, Janssen, Jounce, Related Sciences, Synthekine, and Surface Oncology. EW is a founder of Surface Oncology and Arsenal Biosciences. EW has a patent licensing agreement on the PD-1 pathway with Roche/Genentech. TJ is employed by and owns stock in Sema4.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We dedicate this work to the frontline health workers and staff of the Mount Sinai Healthcare System.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.933190/full#supplementary-material
References
Baqui, P., Bica, I., Marra, V., Ercole, A., van der Schaar, M. (2020). Ethnic and Regional Variations in Hospital Mortality From COVID-19 in Brazil: A Cross-Sectional Observational Study. Lancet Glob Health 8, e1018–e1026. doi: 10.1016/S2214-109X(20)30285-0
Charney, A. W., Simons, N. W., Mouskas, K., Lepow, L., Cheng, E., Le Berichel, J., et al. (2020). Sampling the Host Response to SARS-CoV-2 in Hospitals Under Siege. Nat. Med. 26, 1–2. doi: 10.1038/s41591-020-1004-3
Clatworthy, M. R., Willcocks, L., Urban, B., Langhorne, J., Williams, T. N., Peshu, N., et al. (2007). Systemic Lupus Erythematosus-Associated Defects in the Inhibitory Receptor Fcγriib Reduce Susceptibility to Malaria. Proc. Natl. Acad. Sci. U.S.A. 104, 7169–7174. doi: 10.1073/pnas.0608889104
Del Valle, D. M. D., Kim-schulze, S., Huang, H.-H., Beckmann, N. D., Nirenberg, S., Wang, B., et al. (2020). An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 26, 1636–1643. doi: 10.1038/s41591-020-1051-9
Dinarello, C. A., Donath, M. Y., Mandrup-Poulsen, T. (2010). Role of IL-1beta in Type 2 Diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 17, 314–321. doi: 10.1097/MED.0b013e32833bf6dc
Docherty, A. B., Harrison, E. M., Green, C. A., Hardwick, H. E., Pius, R., Norman, L., et al. (2020). Features of 20 133 UK Patients in Hospital With Covid-19 Using the ISARIC WHO Clinical Characterisation Protocol: Prospective Observational Cohort Study. BMJ 369, m1985. doi: 10.1136/bmj.m1985
Freedman, B. I., Langefeld, C. D., Andringa, K. K., Croker, J. A., Williams, A. H., Garner, N. E., et al. (2014). End-Stage Renal Disease in African Americans With Lupus Nephritis is Associated With APOL1. Arthritis Rheumatol. (Hoboken NJ) 66, 390–396. doi: 10.1002/art.38220
Gold, J. A. W., Wong, K. K., Szablewski, C. M., Patel, P. R., Rossow, J., da Silva, J., et al. (2020). Characteristics and Clinical Outcomes of Adult Patients Hospitalized With COVID-19 - Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 69, 545–550. doi: 10.15585/mmwr.mm6918e1
Gordon, M., Lumley, T. (2021) Forestplot: Advanced Forest Plot Using ‘Grid’ Graphics. Available at: https://CRAN.R-project.org/package=forestplot (Accessed June 13, 2022).
Grasselli, G., Greco, M., Zanella, A., Albano, G., Antonelli, M., Bellani, G., et al. (2020). Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med 180, 1345–1355. doi: 10.1001/jamainternmed.2020.3539
Gu, T., Mack, J. A., Salvatore, M., Prabhu Sankar, S., Valley, T. S., Singh, K., et al. (2020). Characteristics Associated With Racial/Ethnic Disparities in COVID-19 Outcomes in an Academic Health Care System. JAMA Netw. Open 3, e2025197. doi: 10.1001/jamanetworkopen.2020.25197
Gupta, S., Hayek, S. S., Wang, W., Chan, L., Mathews, K. S., Melamed, M. L., et al. (2020). Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern. Med 180, 1436–1447. doi: 10.1001/jamainternmed.2020.3596
Harrell, F. A., Jr., Dupont, C., contributed several functions and maintains latex functions (2022) Hmisc: Harrell Miscellaneous. Available at: https://CRAN.R-project.org/package=Hmisc (Accessed June 13, 2022).
Holtgrave, D. R., Barranco, M. A., Tesoriero, J. M., Blog, D. S., Rosenberg, E. S. (2020). Assessing Racial and Ethnic Disparities Using a COVID-19 Outcomes Continuum for New York State. Ann. Epidemiol. 48, 9–14. doi: 10.1016/j.annepidem.2020.06.010
Horwitz, L. I., Jones, S. A., Cerfolio, R. J., Francois, F., Greco, J., Rudy, B., et al. (2020). Trends in Covid-19 Risk-Adjusted Mortality Rates in a Single Health System. medRxiv 16, 90–92. doi: 10.1101/2020.08.11.20172775
Hsu, H. E., Ashe, E. M., Silverstein, M., Hofman, M., Lange, S. J., Razzaghi, H., et al. (2020). Race/Ethnicity, Underlying Medical Conditions, Homelessness, and Hospitalization Status of Adult Patients With COVID-19 at an Urban Safety-Net Medical Center - Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 69, 864–869. doi: 10.15585/mmwr.mm6927a3
Kassambara, A. (2020) Ggpubr: ‘Ggplot2’ Based Publication Ready Plots. Available at: https://CRAN.R-project.org/package=ggpubr (Accessed June 13, 2022).
Mathew, D., Giles, J. R., Baxter, A. E., Oldridge, D. A., Greenplate, A. R., Wu, J. E., et al. (2020). Deep Immune Profiling of COVID-19 Patients Reveals Distinct Immunotypes With Therapeutic Implications. Science 369, eabc8511. doi: 10.1126/science.abc8511
Ness, R. B., Haggerty, C. L., Harger, G., Ferrell, R. (2004). Differential Distribution of Allelic Variants in Cytokine Genes Among African Americans and White Americans. Am. J. Epidemiol. 160, 1033–1038. doi: 10.1093/aje/kwh325
Nguyen, A., David, J. K., Maden, S. K., Wood, M. A., Weeder, B. R., Nellore, A., et al. (2020). Human Leukocyte Antigen Susceptibility Map for Severe Acute Respiratory Syndrome Coronavirus 2. J. Virol. 94, e00510-20. doi: 10.1128/JVI.00510-20
Oppel, R. A., Jr., Gebeloff, R., Lai, K. K. R., Wright, W., Smith, M. (2020) The Fullest Look Yet at the Racial Inequity of Coronavirus. The New York Times. Available at: https://www.nytimes.com/interactive/2020/07/05/us/coronavirus-latinos-african-americans-cdc-data.html (Accessed Aug 1, 2020).
Novartis Provides Update on CAN-COVID Trial in Hospitalized Patients With COVID-19 Pneumonia and Cytokine Release Syndrome (CRS) (Novartis). Available at: https://www.novartis.com/news/media-releases/novartis-provides-update-can-covid-trial-hospitalized-patients-covid-19-pneumonia-and-cytokine-release-syndrome-crs (Accessed March 29, 2021).
Petrilli, C. M., Jones, S. A., Yang, J., Rajagopalan, H., O’Donnell, L., Chernyak, Y., et al. (2020). Factors Associated With Hospital Admission and Critical Illness Among 5279 People With Coronavirus Disease 2019 in New York City: Prospective Cohort Study. BMJ 369, m1966. doi: 10.1136/bmj.m1966
Price-Haywood, E. G., Burton, J., Fort, D., Seoane, L. (2020). Hospitalization and Mortality Among Black Patients and White Patients With Covid-19. New Engl. J. Med. 382, 2534–2543. doi: 10.1056/NEJMsa2011686
Richman, I. B., Taylor, K. E., Chung, S. A., Trupin, L., Petri, M., Yelin, E., et al. (2012). European Genetic Ancestry is Associated With a Decreased Risk of Lupus Nephritis. Arthritis Rheum 64, 3374–3382. doi: 10.1002/art.34567
Suleyman, G., Fadel, R. A., Malette, K. M., Hammond, C., Abdulla, H., Entz, A, et al. (2020). Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw. Open 3, e2012270. doi: 10.1001/jamanetworkopen.2020.12270
Tharaux, P.-L., Pialoux, G., Pavot, A., Mariette, X., Hermine, O., Resche-Rigon, M., et al. (2021). Effect of Anakinra Versus Usual Care in Adults in Hospital With COVID-19 and Mild-to-Moderate Pneumonia (CORIMUNO-ANA-1): A Randomised Controlled Trial. Lancet Respir. Med. 9, 295–304. doi: 10.1016/S2213-2600(20)30556-7
The RECOVERY Collaborative Group (2020). Dexamethasone in Hospitalized Patients With Covid-19 — Preliminary Report. New Engl. J. Med 384, 693–704. doi: 10.1056/NEJMoa2021436
Wickham, H. (2017) RStudio. Tidyverse: Easily Install and Load the ‘Tidyverse’. Available at: https://CRAN.R-project.org/package=tidyverse (Accessed Oct 9, 2019).
Williamson, E. J., Walker, A. J., Bhaskaran, K., Bacon, S., Bates, C., Morton, C. E., et al. (2020). OpenSAFELY: Factors Associated With COVID-19 Death in 17 Million Patients. Nature 584, 1–11. doi: 10.1038/s41586-020-2521-4
Keywords: COVID-19, interleukin-1beta, African American, electronic medical record, risk factors
Citation: Jun T, Mathew D, Sharma N, Nirenberg S, Huang H-H, Kovatch P, Wherry EJ and Huang K-l (2022) Multiethnic Investigation of Risk and Immune Determinants of COVID-19 Outcomes. Front. Cell. Infect. Microbiol. 12:933190. doi: 10.3389/fcimb.2022.933190
Received: 30 April 2022; Accepted: 20 June 2022;
Published: 22 July 2022.
Edited by:
You Zhou, Cardiff University, United KingdomReviewed by:
Yongfen Xu, Institut Pasteur of Shanghai (CAS), ChinaSreedhar Chinnaswamy, National Institute of Biomedical Genomics (NIBMG), India
Copyright © 2022 Jun, Mathew, Sharma, Nirenberg, Huang, Kovatch, Wherry and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuan-lin Huang, kuan-lin.huang@mssm.edu
 Tomi Jun
Tomi Jun Divij Mathew
Divij Mathew Navya Sharma4
Navya Sharma4  Kuan-lin Huang
Kuan-lin Huang