Golib Golib Tolibov / Alamy Stock Photo
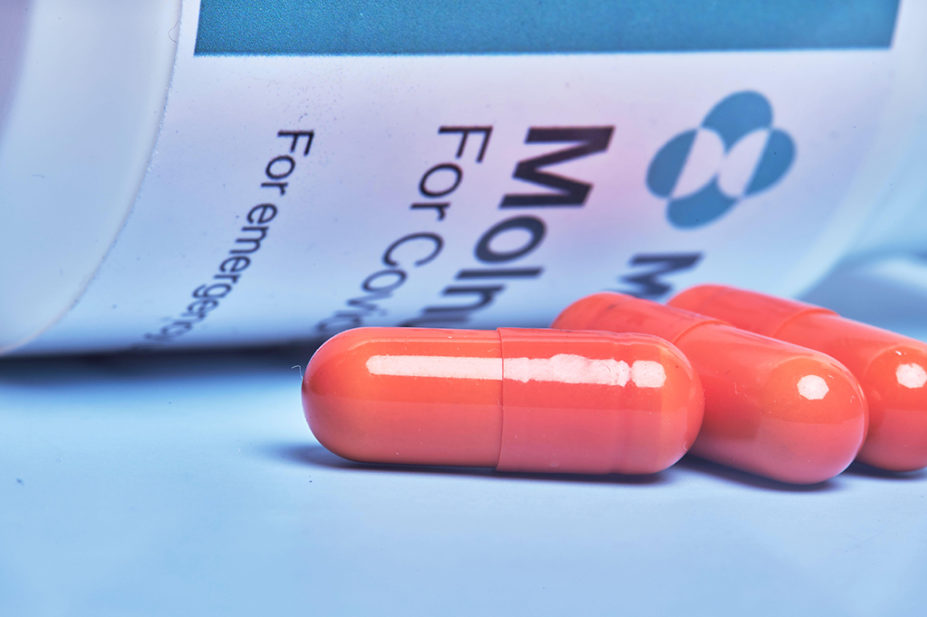
The UK government has order 4.3 million additional doses of COVID-19 antiviral treatments molnupiravir (Lagevrio; MSD) and PF-07321332/ritonavir (Paxlovid; Pfizer).
The contracts are for 1.8 million doses of molnupiravir and 2.5 million doses of PF-07321332/ritonavir and will be in addition to the procurement of 480,000 courses of molnupiravir and 250,000 courses of PF-07321332/ritonavir announced in October 2021.
The latest doses will be available from early 2022 and, according to a press release published by the Department of Health and Social Care on 22 December 2021, both are expected to be effective against the Omicron variant of COVID-19.
Sajid Javid, UK health and social care secretary, said: “Our COVID-19 booster programme continues at unparalleled pace and it’s vital we further bolster our national response to the virus by ensuring access to the world’s best treatments too.
“This is a mammoth deal for the UK government and for patients across the country that are set to benefit from these antivirals over the coming months.”
Molnupiravir is currently being rolled out to patients through the national ‘Platform adaptive trial of novel antivirals for early treatment of COVID-19 In the community’ (PANORAMIC) and, from 16 December 2021, NHS England began offering it to patients outside of hospitals through specially set up COVID-19 medicines delivery units.
As of 22 December 2021, more than 850 participants had been recruited to the PANORAMIC trial across 25 sites.
To be eligible for enrolment onto the trial, participants must be aged 50 years and over, or aged 18–49 years with underlying health conditions that make them clinically more vulnerable. All participants need to have recorded a positive PCR test within the past seven days and to have felt unwell with COVID-19 symptoms within the past five days.
Outside of the trial, molnupiravir is available to patients at very high risk of hospitalisation or death from COVID-19, such as people who are immunocompromised or people who have cancer.
In clinical trials, molnupiravir appeared to reduce the risk of hospitalisation or death for at-risk, non-hospitalised adults with mild-to-moderate COVID-19 by 30%.
Final results from phase III trials of PF-07321332/ritonavir suggested that it reduced the relative risk of COVID-19-associated hospitalisation or death by 89% in those who received treatment within three days of symptoms appearing.
Commenting on Pfizer’s results soon after their publication, Penny Ward, visiting professor in pharmaceutical medicine at King’s College London, said: “These results confirm the findings of the prior interim analysis and reiterate the high level of effectiveness of this agent, potentially conferring an additional approach to the management of COVID-19 at a time when vaccine effectiveness is under challenge.”
However, she highlighted that there was yet to be detailed information released on the side effect profile of the drug.
PF-07321332/ritonavir will be added to the PANORAMIC trial in the event that it is approved by the Medicines and Healthcare products Regulatory Agency.
Any questions about antivirals?
Let us know if you have any questions about antiviral treatment for COVID-19 and we will get experts to answer them. Please email us your name, place of work and your question to editor@pharmaceutical-journal.com.