
Ultrasound in COVID-19: only lung?
Ultrasound in COVID-19: only lung?
The COVID-19 pandemic has proven to be a test case for health services around the world. The need to respond as quickly and effectively as possible to the pressures arising from escalating numbers of critically ill patients has led to the development of new perspectives in the intensive care unit (ICU). Here, we underline the importance of an instrument that might best be described as sort of “medical compass”: the ultrasonic probe. In the ICU, ultrasonography can be used to explore the lung, heart, vessels, and diaphragm in patients with cardiorespiratory instability, in addition to looking for the typical ultrasound signs caused by COVID-19 associated complications. The use of the ultrasound system, both in the emergency room and the ICU, makes it possible to avoid moving the patient to other hospital departments, such as radiology for chest/abdomen computed tomography (CT), thus minimizing the risk of infecting the medical equips and spreading the contagion to other environments.
The main features of ultrasound are the following:
- Examinations can be performed at the bedside, sparing unstable patients unnecessary handling;
- It is cheap and fast to perform, easily to execute, non-invasive and repeatable;
- Unlike other diagnostic imaging methods, ultrasonography does not involve radiation usage;
- Well trained operators can surpass X-ray evaluation in terms of sensibility and specificity.
The possibilities and benefits offered by lung ultrasound (LU) as a state-of-the-art technique are steadily becoming more widely recognized and accepted compared with recent decades. As shown by Italian ICU teams, LU protocols can be applied with great success to reduce the number of serial X-rays required (1). Below, some of the most studied ultrasound applications and issues regarding its use in the isolation ward are described. How should the probe be used and cleaned in a COVID-19 Ward? The Italians Societies of Interventistic Radiology (SIRM), Ultrasounds in Medicine and biology (SIUMB), and the Italian Federation of Medical Scientific Societies (FISM) have all issued guidelines on ultrasound examinations with the scope of ensuring the safety of both operators and patients (2). The same rules should be followed independent of whether a patient is known or suspected to be COVID-19 positive or negative. For patients on a COVID-19 ward, the ultrasound system, including the probe when possible, should be covered with protective film. At the end of a procedure (i.e., after all sequential examinations), the protective film is to be removed and the ultrasound system and probe disinfected immediately, leaving them ready for a new procedure. Human coronaviruses can remain infectious on inanimate surfaces (such as metal, glass, or plastic) for up to nine days (presumably with a reduced charge, but this still requires confirmation). Surface disinfection with 0.1% sodium hypochlorite or 62–71% ethanol (ethyl alcohol) significantly reduces the infectivity of coronaviruses on surfaces within 1 minute of exposure, and a similar effect against SARS-CoV-2 (COVID-19) is expected (3). Each procedure must be carried out with all the personal protective equipment (PPE) foreseen for handling COVID-19 patients. High barrier precaution standards should be used: hand hygiene, sterile gloves, cap, surgical mask, sterile gown, sterile probe cover (4-6). The American Society of Echocardiography recently emphasized the need to limit echocardiography in the COVID-19 setting to urgent patients only. A single echocardiogram machine should be reserved for use in these patients, which would preferably be portable and small-sized in order to facilitate sanitation. Probe sheaths should be used whenever possible. The examination should be carried out by a single operator with sufficient experience in the field, and with a scanning mode that balances image quality and speed of execution (i.e., limited ultrasound sections; bimodal responses with high clinical impact; image recording enabling subsequent review off-line) (7).
Triage of patients is of fundamental importance at a time of overload of the health system and when there is deficiency of healthcare resources. This we found especially during the pandemic from COVID-19. The greatest need for patients is to diagnose the disease as efficiently as possible and with the least waste of resources. Critically ill patients with cardiovascular risk factors presenting symptoms and signs of heart failure (STEMI patients, patients with out-of-hospital cardiac arrest) should quickly access medical or interventional treatment according to current evidence-based guideline recommendations. Echocardiography is a widely available and affordable tool for assessing cardiac structure and function. In critically ill individuals, a targeted assessment provides important information that can influence treatment decisions. Repeated transthoracic echocardiography (TTE) should not be performed, except for clear changes in the clinical status of patients. Therefore, they should be considered positive for SARS-CoV-2, until proven otherwise. Consequently, the healthcare professional should wear appropriate PPE, particularly in the triage phase. The recommendations formulated by the WHO state that contact precautions (through suitable face masks, eyeglasses, gowns and water-repellent gloves) are necessary from the very first phase of triage (8).
Diaphragmatic dysfunction
Critically ill patients often undergo long periods of hospitalization under assisted ventilation, which can give rise to various problems in terms of diaphragmatic dysfunction. In the weaning phase, two ultrasound indexes can help an expert operator to predict the probability of success or weaning failure: the diaphragmatic thickening fraction (DTF) and the diaphragmatic excursion (DE). These indexes are widely accepted as being the most studied and validated for this purpose.The DTF is the difference between end-inspiration and end-expiration thickness divided by the end-expiration thickness ×100, measured with a probe positioned in the 8th-to-11th intercostal space. DE is measured in millimeters and refers to the maximum range of the diaphragm’s movement, during a single breath in M-mode (9).
The Heart and Cardiovascular System
According to several reviews, a higher case fatality rate (CFR) could be found in cohorts of COVID-19 patients with pre-existing cardiovascular disease (CVD). This matter was first revealed in Chinese studies and has been confirmed by both American and Italian physicians (1). Several authors have indicated the transmitral E/E’ ratio as one of the most used Doppler imaging parameters for assessing diastolic dysfunction in patients (9). Indeed, echocardiography is widely recognized as a useful tool for identifying COVID-19-induced myocardial damage (Figures 1-4). Systemic inflammation combined with an aggressive therapeutic approach is widely recognized as a potential risk factor for myocardial damage (associated with a significant increase in troponin and/or ECG exam abnormalities) (10) as well as regional/global anomalies of the left ventricle or a possible acute overload of the right ventricle due to acute respiratory distress syndrome (ARDS) (11). Possible manifestations of direct damage may include dilated cardiomyopathy, global ventricular depression from myocardial infarction, or pericardial effusion.Cardiac involvement may vary depending on the type of ARDS considered. In the H-phenotype (high elastance), right heart impairment is expected the most, due to secondary alterations caused by the high impact of mechanical ventilation on the patient’s hemodynamics. In this case, a correct US examination should assess tricuspid functionality (annular plane excursion, regurgitation) and ventricular dimensions. On the other hand, considering the high percentage of COVID-19 pneumonia patients suffering from systemic arterial hypertension, several authors recommend studying left ventricle diastolic function, especially when combined with increased extravascular lung water (12,13). A cytokine storm spread by systemic disease may induce a pro-thrombotic state, with a heterogenous range of resulting dysfunctions. Deep venous thrombosis is a serious threat to long-term hospitalized patients, especially in COVID-19, where the inflammatory response can be extreme (Figure 5). On the other hand, arterial thrombosis (Figures 6,7) was reported in about 3.7% of COVID-19 patients, likely caused by the combination of the hyperinflammatory state with pre-existing conditions (14). Critically ill patients require the placement and periodic replacement of central venous catheters. In these circumstances, vascular ultrasound imaging not only guides the procedure (15,16), which is tricky due to the reduced comfort and difficult working conditions in an isolation ward (use of a protective suit and extra gloves causing loss of tactile sensitivity), but also allows for the venous vessels to be checked against the threat of thrombi. Right heart enlargement, severe tricuspid regurgitation, and the presence of thrombi in the venous system can be revealed by means of transesophageal echocardiography in the case of suspected pulmonary embolism (leading to acute pulmonary hypertension). Compression ultrasonography seems to be practicable in emergency situations, even with pocket ultrasound devices (PUD) (17) and should be performed under the effect of neuromuscular blocking agent infusion, especially before pronation or before vascular cannulation.
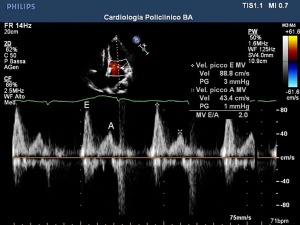
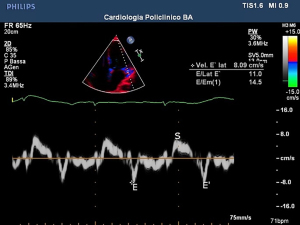
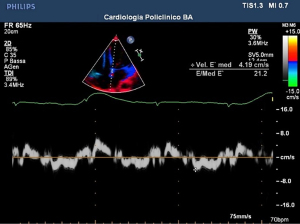

Echocardiography, in patients with ARDS, can signal overload of the right ventricle in presence of cardiocirculatory instability. In two analyses, a correlation between myocardial damage was found in more than 600 patients. On the contrary, a favorable prognosis was found in patients with chronic heart disease but without myocardial damage (18).
The procedure must be carried out by an experienced operator in selected cases of COVID infection, using PPE and a selected and portable echocardiograph, as suggested by the American Society of Echocardiography. The probe has to be covered and cleaned with each use (10).
Septic patients can develop: impairment of the diastolic function of the left ventricle; reduction of the ejection fraction of the left ventricle; possible impairment of the function of the right sections. Positive end-expiratory pressure (PEEP) can arise in increased lung vascular resistance with consequent increase in volume of the right ventricle and the reduction of compliance of the left ventricle. The hypoxia-induced vasoconstriction counters the growing pulmonary vascular resistance, who themselves increase the resistance of intrathoracic vein. This may cause reduction of venous return, worsening condition of real or relative hypovolemia. Peep in clinical practise has the side effect to reduce blood pressure due to reduction in the preload and in the afterload of the right ventricle in patients dependent on preload, so a volume filling can counterbalance this consequence (19,20).
Lung in COVID-19
Axial CT of affected patience shows a frosted glass opacification. This term indicates an interstitial pulmonary syndrome, associated with peripheral consolidation and the characteristic framework, the “Crazy-Paving pattern”.
The lung ultrasound score (LUS) is a useful tool made to assess lung involvement in case of systemic inflammation. This consists of topographical scanning of three different areas of the chest: anterior, lateral and posterior, then the upper and lower segments. Therefore, six specific regions for each lung are defined and classified by one of the four different ventilation models. A normal LU shows the so-called A lines, hyperechogenic (white) lines horizontal and parallel to each other and to the pleural line, sign of normal ultrasonic beam reverb artifacts on the pleural/pulmonary air interface and healthy lung.
The demonstration of B-lines is described as laser-like hyperechogenic artifacts that resemble a “comet tail”. They are pathological artifacts when typical of interstitial disease (from pulmonary edema to interstitial pneumonia); vertical hyperechogenic lines perpendicular to the pleural line and mask A lines. The more numerous, close and coalescent these lines are, the worse the interstitial involvement. Lines B1 are associated with interstitial syndrome and reduced pulmonary aeration. Lines B2 are confluent lines that appear as a “white lung” (also called glass rockets), equivalent to the frosted glass opacities of CT. This suggests a more severe loss of pulmonary aeration. Pulmonary consolidation (C) in which the ultrasound beam is able to penetrate the parenchyma highlighting a “consolidation” with an eco-structural pattern similar to that of the liver. They are associated with the hepatization of the pulmonary parenchyma with or without aerial bronchograms and suggest a greater loss of pulmonary aeration (atelectasis vs. pneumonia). Bedside LU allows differential diagnosis of barotrauma pneumothorax, pleural effusions, interstitial lung diseases and sub pleural consolidations, contributing to the integrated management of ARDS.
A scoring system per region and ultrasound model is used as: A =0 points, B1 =1 point, B2 =2 points, C =3 points. Therefore, a LUS of 0 is normal framework and 36 would be the worst. The final LUS is the sum of points in all 12 regions. This allows the possibility of obtaining prognostic stratification by evaluating the degree of parenchymal commitment. Improving score in monitoring depone for improvement of lungs. Why do we use LUS in mechanically ventilated patients? The LUS has been shown to be a useful tool in ICU patients with adult respiratory distress syndrome (ARDS). LUS allows us to quantify: (I) the pulmonary recruitment induced by peep in poorly aerated lung regions; (II) extension and resolution of pulmonary edema; (III) diagnosis and monitoring ventilator-associated pneumonia (21).
Renal dysfunction
Acute kidney injury appears to be detectable in a large percentage of ARDS patients (23–50%). An Italian team studied the renal resistance indexes and the decrease in renal venous flow using pulsed and continuous Doppler ultrasound methods, respectively. The possible need to apply a high level of PEEP in critically ill patients with ARDS has a significant impact on hemodynamics, causing an increase in renal arterial resistance and a reduction in venous flow, which leads to glomerular congestion and an increase in the hydrostatic pressures of the renal interstitium. In this study, patients with COVID-19-related ARDS showed an increase in the arteriolar resistance index that was linearly correlated with PEEP (18).
Liver dysfunction
In a large number of critically ill patients, signs compatible with liver steatosis have been detected, but it has yet to be clarified whether these signs are induced by COVID-19 or whether they constitute collateral damage caused by drug therapy. The typical sign of steatosis is the so-called “bright liver”, so called for the organ’s abnormal brightness in images detected by ultrasound (19).
Conclusions
In all stages of hospitalization, the ultrasound probe constitutes a loyal ally to trained doctors for the care of critically ill patients. Indeed, evidence is now emerging showing how the method has contributed to improving the quality of care during the COVID-19 pandemic. Ultrasound is repeatedly demonstrated to be a reliable method for use in the emergency and intensive therapy departments. Its large number of possible uses, from monitoring patients’ hemodynamics via echocardiogram to the placement of venous and arterial catheters, underscore the need to train more and more personnel in the use of this resource. More resources need to be invested into the development of ultrasound machines to ensure improved image quality and a lower risk of misinterpretation due to the fact that it is operator-dependent. In particular, we hope an increasing number of clinicians will endeavor to deepen their knowledge on this subject within the setting of critical care.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Luigi Vetrugno and Cristian Deana) for the series “Management of COVID-19 in ICU: What’s New A Year Later?” published in Journal of Public Health and Emergency. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jphe.amegroups.com/article/view/10.21037/jphe-21-68/coif). The series “Management of COVID-19 in ICU: What’s New A Year Later?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guarracino F, Vetrugno L, Forfori F, et al. Lung, Heart, Vascular, and Diaphragm Ultrasound Examination of COVID-19 Patients: A Comprehensive Approach. J Cardiothorac Vasc Anesth 2021;35:1866-74. [Crossref] [PubMed]
- SIRM, SIUMB, and FISM guidelines on behavioral modalities for carrying out an ultrasound examination in this pandemic moment. Available online: https://sirm.org/2020/03/20/documento-intersocietario-sirm-siumb-fism-2020-utilizzo-della-diagnostica-per-immagini-nei-pazienti-covid-19/
- Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 2020;104:246-51. [Crossref] [PubMed]
- Infection prevention and control and preparedness for COVID-19 in healthcare settings. Third update – 31 March 2020. ECDC Technical Report. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Infection-prevention-control-for-the-care-of-patients-with-2019-nCoV-healthcare-settings_third-update.pdf
- Indicazioni ad interim per un utilizzo razionale delle protezioni per infezione da SARS-CoV-2 nelle attività sanitarie e sociosanitarie (assistenza a soggetti affetti da covid-19) nell’attuale scenario emergenziale sars-cov-2. Gruppo di Lavoro ISS Prevenzione e Controllo delle Infezioni. Rapporto ISS COVID-19 • n. 2/2020 Rev. Available online: https://www.iss.it/documents/20126/0/Rapporto+ISS+COVID+2_+Protezioni_REV.V6.pdf/740f7d89-6a28-0ca1-8f76-368ade332dae?t=1585569978473
- CDC-updated protocol March 19, 2020 on airborne precautions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
- Kirkpatrick JN, Mitchell C, Taub C, et al. ASE Statement on Protection of Patients and Echocardiography Service Providers During the 2019 Novel Coronavirus Outbreak: Endorsed by the American College of Cardiology. J Am Soc Echocardiogr 2020;33:648-53. [Crossref] [PubMed]
- V.Cianci: Handbook di ecografia in emergenza-urgenza, manuale operativo. C.G. Edizioni medico scientifiche. Book Ean (9788871102436).
- Tuinman PR, Jonkman AH, Dres M, et al. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients—a narrative review. Intensive Care Med 2020;46:594-605. [Crossref] [PubMed]
- Cameli M, Pastore MC, Soliman Aboumarie H, et al. Usefulness of echocardiography to detect cardiac involvement in COVID-19 patients. Echocardiography 2020;37:1278-86. [Crossref] [PubMed]
- Stefanini GG, Montorfano M, Trabattoni D, et al. ST-Elevation Myocardial Infarction in Patients With COVID-19: Clinical and Angiographic Outcomes. Circulation 2020;141:2113-6. [Crossref] [PubMed]
- Repessé X, Charron C, Vieillard-Baron A. Assessment of the effects of inspiratory load on right ventricular function. Curr Opin Crit Care 2016;22:254-9. [Crossref] [PubMed]
- Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007;28:2539-50. [Crossref] [PubMed]
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107-33. [Crossref] [PubMed]
- Revzin MV, Raza S, Warshawsky R, et al. Multisystem Imaging Manifestations of COVID-19, Part 1: Viral Pathogenesis and Pulmonary and Vascular System Complications. Radiographics 2020;40:1574-99. [Crossref] [PubMed]
- Buone Pratiche Cliniche SIAARTI per gli Accessi Vascolari. 04/10/2018 Available online: https://www.siaarti.it/news/370740
- Sanfilippo F, Noto A, Martucci G, et al. Central venous pressure monitoring via peripherally or centrally inserted central catheters: a systematic review and meta-analysis. J Vasc Access 2017;18:273-8.
- Fogagnolo A, Grasso S, Dres M, et al. Focus on renal blood flow in mechanically ventilated patients with SARS-CoV-2: a prospective pilot study. J Clin Monit Comput 2022;36:161-7. [PubMed]
- D'Andrea A, Martone F, Liccardo B, et al. Acute and Chronic Effects of Noninvasive Ventilation on Left and Right Myocardial Function in Patients with Obstructive Sleep Apnea Syndrome: A Speckle Tracking Echocardiographic Study. Echocardiography 2016;33:1144-55. [Crossref] [PubMed]
- Carrizales-Sepúlveda EF, Vera-Pineda R, Flores-Ramírez R, et al. Echocardiographic Manifestations in COVID-19: A Review. Heart Lung Circ 2021;30:1117-29. [Crossref] [PubMed]
- Bouhemad B, Dransart-Rayé O, Mojoli F, et al. Lung ultrasound for diagnosis and monitoring of ventilator-associated pneumonia. Ann Transl Med 2018;6:418. [Crossref] [PubMed]
Cite this article as: Maringelli G, Arcamone E, Brienza N. Ultrasound in COVID-19: only lung? J Public Health Emerg 2022;6:16.





