Morphea caused by COVID-19 infection, first polish case report
Hanna Cisoń , Rafał Białynicki-Birula
, Rafał Białynicki-Birula
Department of Dermatology, Venereology and Allergology, Wroclaw Medical University, Wroclaw, Poland
Citation tools:
Copyright information
© Our Dermatology Online 2024. No commercial re-use. See rights and permissions. Published by Our Dermatology Online.
ABSTRACT
Morphea, a localized scleroderma variant, is a rare dermatological condition characterized by fibrosis and thickening of the skin. While its etiology remains unclear, there have been reports of its association with viral infections, including COVID-19. We present a case of morphea in a 26-year-old female patient with a history of two episodes of COVID-19. The patient exhibited cutaneous lesions on her left forearm, bilateral axillae, and groin, accompanied by abdominal wall induration Laboratory investigations revealed no significant abnormalities, except for positive antinuclear antibodies (ANA) with various patterns. This case highlights the potential association between COVID-19 infection and the development of morphea, underscoring the importance of considering this relationship in patients presenting with dermatological manifestations following COVID-19. Further research is needed to elucidate the underlying mechanisms and optimize treatment strategies for such cases.
Key words: COVID-19, Morphea, Post-COVID-19 Morphea, Skin diseases
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was initially identified in December 2019, and by March, the World Health Organization declared the global pandemic of Covid-19 (Corona virus disease 2019) [1]. The virus rapidly disseminated among millions of individuals primarily through respiratory droplets, with an estimated mean incubation period of 6.4 days [2]. Viral infections can instigate autoimmune-mediated diseases either through direct viral-induced damage or by triggering immunological dysregulation due to inflammatory cascades [3].
Cases of post-COVID-19 infection systemic lupus erythematosus (SLE) have been reported in the literature [4,5], as well as a single case of atrophic lichen planus occurring after COVID vaccination in an individual with hepatitis C infection [6].
The integumentary system serves as a prominent organ system for the manifestation of COVID-19-related symptoms and complications, exhibiting a spectrum of dermatological manifestations including exanthems, urticaria, and mucocutaneous involvement [7].
CASE REPORT
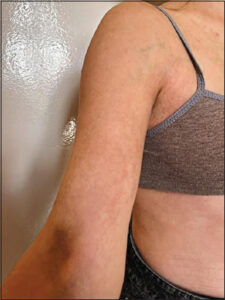
A 26-year-old female patient with no comorbidities or family history of autoimmune disorders was presented at the Department of Dermatology in Wrocław for the first time in December 2022. She had a history of two episodes of COVID-19 in December 2021 and January 2022. In March 2022, the patient developed cutaneous lesions on her left forearm, which were asymptomatic. She also had a longstanding history of acne vulgaris-like lesions on her back since the age of 12. Previous treatment with topical betamethasone with gentamicin yielded no improvement. Upon admission, the patient exhibited brown and porcelain-white indurations on her left forearm, bilateral axillae, and groin (Fig. 1). Subjectively, she reported abdominal wall induration. Additionally, open and closed comedones and inflammatory papules were observed on her back. Laboratory investigations revealed no significant abnormalities. Serological tests were negative for hepatitis B, hepatitis C, and HIV. Antinuclear antibodies (ANA) IgG (IIF) exhibited a speckled pattern at a titer of 1:320, a nucleolar pattern at a titer of 1:320, and a cytoplasmic pattern at a titer of 1:100 (reference range: 1:100). Furthermore, ANA profile 3 antibodies and DFS70 (anti-dense fine speckled 70) IgG were negative, except for weakly positive dsDNA (anti-double stranded DNA) antibodies. Due to the characteristic clinical picture of the lesions, histopathological examination was abandoned, and the patient was diagnosed with morphea. After obtaining written informed consent, the patient was initiated on PUVA therapy and oral isotretinoin treatment. Subsequently, in September 2022, a reduction in skin indurations and lesion size was noted on the left forearm, axillae, and groin, while post-inflammatory scars and hyperpigmentation were observed on the back. The patient provided written informed consent for a therapeutic intervention involving subcutaneous administration of 100 mg hydrocortisone via mesotherapy, resulting in satisfactory clinical outcomes.
DISCUSSION
Morphea After Vaccine Against Covid-19 Vaccine and/or After Covid -19 Infection
In the available literature, the majority of references focus on the occurrence of morphea following vaccination against COVID-19 [8,9]. Only a limited number of isolated studies or reports have highlighted the induction of morphea as a result of COVID-19 infection itself [10–12].
In a study conducted by Paolino G et al. [8] involving four patients, three individuals exhibited the development of multiple whitish and sclerotic plaques subsequent to receiving the first and/or second dose of the Comirnaty-Pfizer® SARS-CoV-2 vaccine, while one patient presented with similar lesions 20 days after the second dose of the Vaxzevria-Astrazeneca® vaccine. The number of lesions observed ranged from 5 to 10, with diameters varying between 5 and 12 cm. Notably, none of the patient’s displayed involvement of the vaccination site (arm). Subsequent evaluation led to the diagnosis of morphea in these individuals following COVID-19 vaccination.
In a study conducted by Safoura Shakoei et al. [9] skin manifestations were observed in twenty-two patients following administration of the Sinopharm vaccine, while three cases exhibited cutaneous changes after receiving the AstraZeneca vaccine. Among the observed cases, six patients developed newly onset lichen planus (LP), and one patient experienced LP exacerbation. Additionally, two individuals presented with new-onset psoriasis, and one case showed worsening of pre-existing psoriasis. One patient exhibited de novo pemphigus vulgaris (PV), while another case demonstrated PV exacerbation. Moreover, one patient experienced exacerbation of pityriasis lichenoides et varioliformis acuta (PLEVA). Other newly reported cases included toxic epidermal necrolysis (TEN), bullous pemphigoid (BP), alopecia areata (AA), pityriasis rosea, herpes zoster (shingles), cutaneous small-vessel vasculitis, erythema multiforme (EM), urticaria, and morphea.
Zahra Loft et al. [10], reported the initial case of post-COVID-19 pansclerotic morphea (PSM) in a previously healthy 57-year-old female patient. Following confirmation of COVID-19 infection, the patient presented with systemic skin stiffness, predominantly affecting the shins, arms, and abdomen, accompanied by areas displaying an orange-peel texture. Subsequent deep skin biopsy was performed, revealing sclerotic alterations. Based on clinical assessment, the definitive diagnosis of post-COVID-19 pansclerotic morphea was established.
Flavia Pigliacelli et al. [11] presented a case report describing a 61-year-old female patient who developed multiple brownish and purplish plaques on the forearms. The plaques exhibited a sclerotic appearance with mild erythematous borders, and some of them merged partially. The patient reported the onset of cutaneous symptoms approximately one month after being discharged from the hospital following COVID-19 pneumonia, confirmed by RT-PCR. Notably, the patient had no personal or family history of autoimmune or chronic inflammatory skin diseases. Histological analysis revealed a thin epidermis, moderate skin sclerosis, and thickening of collagen fibers, supporting the diagnosis of post-COVID-19 morphea.
Michael R. Stephens et al. [12] presented a case report describing a 61-year-old female patient with a medical history of arterial hypertension, chronic obstructive pulmonary disease, and hypothyroidism. The patient presented with diffuse skin thickening six months following a two-week illness characterized by fever, myalgia, dyspnea, and cough, accompanied by a positive COVID-19 nasopharyngeal swab test. A wedge biopsy of the left forearm was conducted, and histopathological analysis revealed features indicative of morphea profunda.
In the past, an association between Borrelia burgdorferi infection and the presence of morphea was postulated. However, current guidelines do not recommend routine IgM and IgG antibody testing for Lyme disease in patients with morphea. This position is corroborated by a study conducted by Anna Malewska-Wozniak et al. [13], which examined the prevalence of IgM and IgG antibody classes against Borrelia in 82 patients with morphea and 112 with psoriasis utilizing the conventional ELISA technique. The study revealed that IgM and IgG antibodies against Borrelia were identified only in 4% of blood samples obtained from patients diagnosed with morphea.
The infection caused by Hepatitis C virus (HCV) is considered as a potential etiological factor in the development of cutaneous dermatoses. Saleha Mohammada et al. [14] conducted a hospital-based study in Pakistan which indicated that, among patients with diagnosed HCV infection, pruritus was the most frequently observed cutaneous manifestation, with a prevalence of 33.96%, followed by lichen planus (LP) at a prevalence of 23.5%.
Anuradha Jindal et al. [15], documented a case report involving a 60-year-old female patient who presented with the sudden onset of pruritic fluid-filled lesions that persisted for a duration of 20 days. The patient provided a history of being bitten by a dog, following which intramuscular injections of 0.1 ml of inactivated rabies vaccine were administered on days 0, 3, and 7. Six days post-dog bite, the patient developed cutaneous vesicles and bullae. Histopathological examination confirmed the diagnosis of bullous pemphigoid (BP).
Recent literature also has reported cases suggesting an association between COVID-19 vaccinations and the development of BP [16–19].
CONCLUSIONS
In the case described in our study, the absence of a positive medical history for autoimmune diseases and the subsequent development of cutaneous symptoms following COVID-19 infection in the patient suggest a plausible association between autoimmune disease and SARS-CoV-2. Some authors have hypothesized that SARS-CoV-2 may act as a potential trigger for the development of organ-specific autoimmune disorders in genetically susceptible individuals. Further research is needed to elucidate the underlying mechanisms and explore the clinical implications of this potential link.
Consent
The examination of the patient was conducted according to the principles of the Declaration of Helsinki.
The authors certify that they have obtained all appropriate patient consent forms, in which the patients gave their consent for images and other clinical information to be included in the journal. The patients understand that their names and initials will not be published and due effort will be made to conceal their identity, but that anonymity cannot be guaranteed.
REFERENCES
1. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19):The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924.
2. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564-7.
3. SinanovićO, MuftićM, SinanovićS. COVID-19 Pandemia:Neuropsychiatric Comorbidity and Consequences. Psychiatr Danub. 2020;32:236-44.
4. Hosseini P, Fallahi MS, Erabi G, Pakdin M, Zarezadeh SM, Faridzadeh A, et al. multisystem inflammatory syndrome and autoimmune diseases following COVID-19:molecular mechanisms and therapeutic opportunities. Front Mol Biosci. 2022;9:804109.
5. Bonometti R, Sacchi MC, Stobbione P, Lauritano EC, Tamiazzo S, Marchegiani A, et al. The first case of systemic lupus erythematosus (SLE) triggered by COVID-19 infection. Eur Rev Med Pharmacol Sci. 2020;24:9695-7.
6. Sharma A, Bhandari A, Chatterjee D, Narang T. Atrophic lichen planus post-COVID vaccination in a hepatitis C positive individual. Dermatol Ther. 2022;35:15829.
7. Wollina U, KaradağAS, Rowland-Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID-19 patients:A review. Dermatol Ther. 2020;33:13549.
8. Paolino G, Campochiaro C, Di Nicola MR, Mercuri SR, Rizzo N, Dagna L, et al. Generalized morphea after COVID-19 vaccines:a case series. J Eur Acad Dermatol Venereol. 2022;36:e680-2.
9. Shakoei S, Kalantari Y, Nasimi M, Tootoonchi N, Ansari MS, Razavi Z, et al. Cutaneous manifestations following COVID-19 vaccination:A report of 25 cases. Dermatol Ther. 2022;35:15651.
10. Lotfi Z, Haghighi A, Akbarzadehpasha A, Mozafarpoor S, Goodarzi A. Pansclerotic morphea following COVID-19:a case report and review of literature on rheumatologic and non-rheumatologic dermatologic immune-mediated disorders induced by SARS-CoV-2. Front Med (Lausanne). 2021;8:728411.
11. Pigliacelli F, Pacifico A, Mariano M, D’Arino A, Cristaudo A, Iacovelli P. Morphea induced by SARS-CoV-2 infection:A case report. Int J Dermatol. 2022;61:377-8.
12. Stephens MR, Moore DF, Dau J, Jobbagy S, Neel VA, Bolster MB, et al. A case of generalized morphea profunda following SARS-CoV-2 infection. JAAD Case Rep. 2022;23:20-3.
13. Malewska-Woźniak A, Jałowska M, Lodyga M, Osmola-Mańkowska A, Adamski Z. Serological evidence of borrelia Burgdorferi in patients with morphea from west-central Poland:an original paper and review of literature. Vector Borne Zoonotic Dis. 2021;21:653-8.
14. Mohammad S, Chandio B, Soomro AA, Lakho S, Ali Z, Shaukat F. The frequency of cutaneous manifestations in hepatitis c:a cross-sectional study in a tertiary care hospital in Pakistan. Cureus. 2019;11:6109.
15. Jindal A, Nayak SUK, Shenoi SD, Rao R, Monappa V. Bullous pemphigoid triggered by rabies vaccine. Indian J Dermatol Venereol Leprol. 2020;86:66-8.
16. Pérez-López I, Moyano-Bueno D, Ruiz-Villaverde R. Bullous pemphigoid and COVID-19 vaccine. Med Clin (Engl Ed). 2021;157:333-4.
17. Agharbi FZ, Eljazouly M, Basri G, Faik M, Benkirane A, Albouzidi A, et al. Bullous pemphigoid induced by the AstraZeneca COVID-19 vaccine. Ann Dermatol Venereol. 2022;149:56-7.
18. Desai AD, Shah R, Haroon A, Wassef C. Bullous pemphigoid following the moderna mRNA-1273 vaccine. Cureus. 2022;14:24126.
19. Alshammari F, Abuzied Y, Korairi A, Alajlan M, Alzomia M, AlSheef M. Bullous pemphigoid after second dose of mRNA- (Pfizer-BioNTech) Covid-19 vaccine:A case report. Ann Med Surg (Lond). 2022;75:103420.
Notes
Request permissions
If you wish to reuse any or all of this article please use the e-mail (brzezoo77@yahoo.com) to contact with publisher.
| Related Articles | Search Authors in |
|
 http://orcid.org/0000-0003-3901-8876 http://orcid.org/0000-0003-3901-8876 http://orcid.org/0000-0002-2603-4220 http://orcid.org/0000-0002-2603-4220 |




Comments are closed.