Abstract
Objective
To assess how the COVID-19 outbreak has affected emergency general surgery (EGS) care during the pandemic, indications for surgery, types of procedures, perioperative course, and final outcomes.
Methods
This is a retrospective study of EGS patients during the pandemic period. The main outcome was 30-day morbidity and mortality according to severity and COVID-19 infection status. Secondary outcomes were changes in overall management. A logistic regression analysis was done to assess factors predictive of mortality.
Results
One hundred and fifty-three patients were included. Half of the patients with an abdominal ultrasound and/or CT scan had signs of severity at diagnosis, four times higher than the previous year. Non-COVID patients underwent surgery more often than the COVID group. Over 1/3 of 100 operated patients had postoperative morbidity, versus only 15% the previous year. The most common complications were septic shock, pneumonia, and ARDS. ICU care was required in 17% of patients, and was most often required in the SARS-CoV-2-infected group, which also had a higher morbidity and mortality. The 30-day mortality in the surgical series was of 7%, with no differences with the previous year. The strongest independent predictors of overall mortality were age > 70 years, ASA III–IV, ESS > 9, and SARS-CoV-2 infection.
Conclusions
Non-operative management (NOM) was undertaken in a third of patients, and only 14% of operated patients had a perioperative confirmation of -CoV-2 infection. The severity and morbidity of COVID-19-infected patients was much higher. Late presentations for medical care may have added to the high morbidity of the series.
Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is still ongoing and has hit with the utmost intensity the Madrid area in Spain, straining hospital resources and manpower to limits unknown to our generation. As the population lockdown was being implemented and scheduled surgical procedures were cancelled, the relentless influx of patients affected by the disease overwhelmed our ED and critical-care bed capacity. We expected this to have an impact on our non-trauma-related emergency general surgery (EGS) cases during the surge, but could only guess about the real consequences of it. The increased postoperative morbidity and mortality that were being reported in infected patients [1,2,3], together with the risks incurred by professionals caring for these patients [4], prompted several groups and surgical societies to issue early warnings and recommendations regarding emergency surgery [5,6,7,8,9,10,11]. These have been mainly directed at limiting surgical exposure whenever a non-operative management could be envisioned, recommending that surgery be done by the most experienced, limiting or altogether avoiding the laparoscopic approach, and performing the procedures under regional anesthesia when possible.
The aim of the study was to assess how the COVID-19 outbreak has affected EGS care during the surge of the pandemic at our institution, with the focus on indications for surgery, types of procedures, perioperative course, and final outcome according to severity and COVID-19 status of our patients. Our hypothesis was that infection by SARS-CoV-2, and late presentations, was responsible for the high overall and postoperative morbidity of the series.
Methods
Observational retrospective study of EGS patients included in a prospectively maintained database. All patients referred to our Emergency General Surgery Unit during this period of time were assessed. The 9-week period of time considered for the study goes from March 9th, 2020, to May 15th, 2020. The COVID-19 outbreak at our center in Madrid was considered to have started during the first week of March. This study period was assigned a categorical variable according to our center’s critical-care bed capacity, and the Pandemic Critcon-2020 Surge Levels criteria (12): CRITCON 1(from March 9th to March 13th), CRITCON 2 (from March 14th to March 20th, and from May 3rd to May 15th), and CRITCON 3 (from March 21st to May 2nd).
The main outcome measure was the morbidity and mortality of patients according to their severity and COVID-19 infection status. This severity was retrospectively assessed by the Emergency Surgery Score (ESS) [13,14,15,16]. Due to the lack of blood urea nitrogen (BUN) values in our lab, the ESS calculation had to be slightly modified according to the formula: BUN (mg/dl) = Urea (mg/dl)/2.1428. In patients with no urea values available, but with normal creatinine and urine output, a normal BUN was assumed. Secondary outcome measures were the appraisal of differences in morbidity and mortality with the same period of time of the previous year. We also assessed results according to the different Critcon-2020 Surge Levels; the percentage of procedures performed by residents as first surgeons, and the number of laparoscopic procedures were also registered; non-trauma EGS procedures routinely performed by laparoscopy in our Unit include appendicitis, cholecystitis, and GI tract perforations, with increasing selective indications in adhesive small bowel obstruction, and large bowel obstruction (for colostomy).
Data regarding patients’ demographics and comorbidities, American Association of Anesthesiology (ASA) grading, lab values on admission, mean ESS, duration of symptoms, presence of peritonitis, final diagnoses, and COVID-19 infection status at the moment of diagnosis were recorded. COVID-19 Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR) screening test of a nasopharyngeal swab, or a chest X-ray or thoracic CT scan, were performed in every patient from April 4th to May 15th. Before that date, no established pre-operative mandatory screening protocol was in place, and most patients underwent either RT-PCR or a chest X-ray. COVID-19 RT-PCR test results were categorized as positive or negative, and radiolog-19 infection during the study period were categorized according to the confirmation test and the moment of infection, either pre- or postoperatively. No patient was considered as COVID-19 positive on the basis of clinical diagnosis alone. Data regarding specific medical and ICU treatments of the COVID-19 infection were also collected.
The different therapeutic options were categorized as: operative management (OM), non-operative management (NOM) (including interventional radiology procedures), and compassionate care (CC). This CC was decided upon by the surgery and anesthesia team, after detailed informed consent of the direct family or relatives of the patient, and after careful consideration of the evidence of futile care [17]. NOM failure was defined as the need of emergency surgery at any point during hospital follow-up. The decision to proceed with surgery or NOM in a confirmed or suspected COVID-19 patient was left to the discretion of the attending surgeon. In addition, the type of anesthesia, operative approach (open vs. laparoscopic), and intraoperative findings (no peritonitis, localized, or generalized peritonitis) were registered.
Patients were followed up for 30 days after diagnosis, and morbidity was determined according to the Clavien–Dindo classification [18]. In patients with more than one complication, we just considered the most clinically relevant, with the exception o‘‘‘f Acute Respiratory Distress Syndrome (ARDS), which could be secondary to abdominal sepsis or pneumonia, or both. Septic shock was defined as sepsis with persistent hypotension requiring vasopressors to maintain a mean arterial pressure (MAP) ≥ 65 mmHg, and lactate ≥ 2 mmol/L [19]. ARDS was defined as per the Berlin definition criteria [20]. The length of stay (LOS) at the SICU, and mortality at 24 h, 7 days, and 30 days, were also registered. A logistic regression analysis was done to assess factors predictive of mortality, and included the ESS (with a cutoff value of 9), age, gender, immunospression, ASA score, and COVID-19 status, among other factors. Data on signs of severity in the imaging techniques, morbidity and mortality were compared with that of the same period of time of the previous year. Statistical analysis and data management were done using SPSS version 23.0 (IBM). Means of continuous variables with normal distributions were compared using the two-tailed t test. Non-parametric tests (Mann Whitney U test, and Kruskal–Wallis test) were used to compare continuous variables without normal distributions or few cases. Categorical data were analyzed using Pearson’s Chi-square test or Fischer’s exact test. Due to the exceptional circumstances of the moment, we thought that the study was exempt from approval by the Ethics Committee of our center.
Results
The series included 153 patients. The descriptive analysis of patients’ demographics, comorbidities, ASA grading, mean ESS, RT-PCR screening, imaging techniques, lab values indicative of severity of infection, and hospital and ICU stays, is shown in Table 1. Almost half of the patients with an abdominal ultrasound and/or CT scan had signs of severity in those imaging procedures (i.e., perforation, abscess, free fluid, portal gas, and ischemic bowel). This severity was also reflected in lab values on admission. Table 2 shows the different diagnoses, number of operated patients, duration of symptoms, percentages of peritonitis, laparoscopic approach, surgery by residents, and type of anesthesia. A NOM was initially decided in 37% of patients, including conservative treatment in 33%, and percutaneous drainage in 4%. All patients with perforated appendicitis were subjected to OM, and there were no Hinchey III/IV cases of diverticulitis. Compassionate care was decided in four (2.5%) patients diagnosed of bowel obstruction, gastric perforation, sigmoid perforation, and acute cholecystitis, respectively. They were all very elderly (mean age of 88 ± 3 years), with multiple comorbidities, and presented in a dismal clinical condition in need of ICU care which was either unavailable at those days for lack of beds, or did not fulfill criteria for ICU admission. A laparoscopic approach was chosen in 76% of potentially eligible patients, vs. 82% the previous year. In patients with a non-mechanical, non-vascular, cause of acute abdomen the rate of intraoperative peritonitis was rather high. The majority of procedures (93%) were performed by residents (vs. 96.5% the previous year), and most were done under general anesthesia.
Table 3 shows the management of patients with confirmed SARS-CoV-2 infection. The eight patients who were diagnosed postoperatively had no pre-operative radiological abnormalities; RT-PCR was done preoperatively in only three of them, with a negative result. Nearly half of COVID-19-infected patients (13/27) were asymptomatic for the infection at the time of the surgical diagnosis. About two-thirds of patients received specific medical or ICU treatment, excluding the rest for lack of symptoms or of radiological abnormalities. During the study period, 20 surgeons and 23 surgical residents were involved in the surgical procedures, and their infection rate was of 20% (4/20) and 30.4% (7/23), respectively, for an overall infection rate of 25.5%. Only one of them had a clinical condition which required hospitalization for a few days, and most had mild-to-moderate, or no symptoms at all.
Table 4 shows that 35% of patients had postoperative morbidity, and 54% of them had at least one Clavien–Dindo class III or IV complication. The three most common complications in the overall and the surgical series were septic shock, pneumonia, and ARDS. Non-COVID patients underwent surgery more often than the COVID group, but without statistically significant differences. Postoperative ICU care was required more often in the SARS-CoV-2-infected group, which also had a higher than expected morbidity (according to the ESS), and also significantly higher overall and postoperative complication rates than the non-SARS-CoV-2 group. The overall ESS in the infected group was almost double that of the non-infected, and this difference was statistically significant when comparing the ESS of operated patients. No differences were found in ASA III–IV between both the groups. The 30-day mortality in the overall and the surgical series was of 9% and 7%, respectively, and was also higher in the SARS-CoV-2 group.
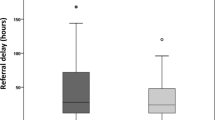
When comparing the number of patients who eventually underwent surgery with the 135 patients operated during the same period of time on the previous year, there was a considerable reduction (Table 5, Fig. 1). A fourfold increase in the signs of severity in abdominal CT scan and/or US in operated patients was noted, as compared to the previous year; in addition, the number of patients with postoperative complications was twice that of the comparative group.
Table 6 shows that the strongest independent predictors of overall mortality were age > 70 years, ASA III–IV, ESS > 9, and SARS-CoV-2 infection. Table 7 shows the management, complications and mortality analysis according to the Pandemic Critcon-2020 Surge Levels. Most patients were managed during the most critical period (Critcon III), which was also the longest in time. There were no differences in the mean ESS, rates of conservative management, or complications between Critcon III and Critcon II, but the mortality was lower in patients managed during Critcon III.
Discussion
The current COVID-19 pandemic is having a major impact in elective surgery, with massive cancellations, but also in emergency surgical procedures. The number of EGS interventions has dropped significantly, as confirmed in Italy [6, 21], the U.S.A. [7], Spain [5], and also in our series, when we compare it with the same period on the previous year. In addition, in a large survey from Italy, up to 40% of non-traumatic abdominal emergency cases had an unusual delayed treatment [6]. To what extent this has resulted from recommendations by health authorities encouraging patients to stay at home, or from fear of getting infected in hospitals’ EDs, or even from delayed in-hospital treatment, is difficult to assess. Concerns raised after isolated reports of postoperative complicated courses and unexpected fatalities in patients undergoing elective surgery [1,2,3,4] may have contributed. A very recently released international multicenter cohort study at 235 hospitals in 24 countries reports a high mortality and pulmonary complications in patients with perioperative SARS-CoV-2 infection; the majority (74%) were emergency procedures [2]. We have also experienced this delay, as attested by the long duration of symptoms before seeking urgent medical care, admission lab values and abdominal CT scan and/or US findings indicative of advanced infection, the 60.5% rate of peritonitis seen in non-mechanical, non-vascular acute abdomen, and the rate of postoperative sepsis. A high rate of peritonitis has also been reported from another hospital in Madrid [5].
In areas with a high incidence of COVID-19, all patients should be considered infected until proven otherwise [22]. This has prompted several groups to advocate limiting surgical exposure whenever a non-operative management could be envisioned, and is also consistent with our findings. NOM has been advocated for non-perforated appendicitis, with outpatient management and serial telephone follow-up when appropriate [8]. As for perforated appendicitis and other conditions, open surgery has been recommended by some as the access of choice during the peak of the pandemic in all COVID-19 + or suspected COVID-19 + patients [8, 22]. NOM of appendicitis and cholecystitis in our series was undertaken in 22% and 70% of cases, respectively, with no NOM failures. Our routine management of appendicitis is always surgical, with the exception of the appendiceal mass on palpation, but our usual NOM strategy in acute cholecystitis is of around 50%, due to the elderly population with multiple comorbidities that come to our ED on a regular basis. Overall, 65% of patients in the series underwent surgical treatment.
Despite early recommendations against minimally invasive surgery [6,7,8,9,10,11, 22,23,24], there is little evidence regarding the aerosolization potential of laparoscopy and its effects on surgeon’s safety. The more recent policy is one of business-as-usual, with measures taken to minimize the free release of insufflated gas [25, 26]. This is the policy we have adhered to since the beginning of the pandemic, maintaining the usual overall rate of close to 80% of laparoscopic approach at our EGS Unit. Specific data about endoscopy, colonoscopy and bronchoscopy are not included in our data collection, as they are not performed by surgeons in Spain. Our experience is that they were not constrained, and the specialists and anesthetists doing them in the OR took the necessary precautions.
In addition, most procedures were performed under general anesthesia. Early reports also recommended that surgical procedures be preferentially performed by an experienced surgeon. Nevertheless, the vast majority of our procedures (93%) were still performed by residents assisted by the staff, in keeping with our usual policy. It is impossible to ascertain whether the 25% of general surgeons and residents who have become infected with the virus at our center got this exposure during the emergency procedure or elsewhere, in view of the overwhelming community transmission and the fact that many of those infected were involved in other hospital activities in close contact with COVID-19 + patients. Asymptomatic providers were not screened at our center, and this, together with some deficiencies in the availability of fully approved PPE at the beginning of the pandemic, may have contributed to this rather high rate of infection among surgeons. Regrettable as this situation was, only 1 of those 11 surgeons (staff and residents) had a clinical condition which required hospitalization for a few days, and most had mild-to-moderate, or no symptoms at all. A similar percentage of infection among surgeons has been reported from another hospital in Madrid [5]. After this initial scarcity, PPE (personal protective equipment) used during surgical procedures on patients with unconfirmed COVID-19 test was mainly based on FFP2 or FFP3 mask, protective glasses and face shields, two pairs of surgical gloves, and waterproof gown. Powered air-purifying respirator was used in the positive cases. For the negative cases, FFP2 masks were used and the rest of the usual equipment.
Only one-fifth (18%) of patients assessed had SARS-CoV-2 infection confirmed, and in 30% of them that confirmation was either during the postoperative course or in-hospital follow-up for those not operated. Half of them were asymptomatic. A chest CT scan was performed only in one-fifth of patients and in addition to the abdominal CT scan in symptomatic patients with a negative RT-PCR. Only 15% of those chest CT scans found the typical pattern described for the disease [27].
Surgery was more frequently indicated in COVID-19-negative patients, as expected. Overall, one-third of operated COVID-19-positive patients required ICU care vs one-seventh in the COVID-19-negative group. Although we did not consider the whole number of complications but the number of patients with complications, and the most relevant one, postoperative morbidity was high, and double that of the previous year. Half the patients had a complication classified as Clavien–Dindo class III or IV. Septic shock, pneumonia, and ARDS were the most frequent. It has recently been confirmed that ESS is a good predictor of EGS morbidity, even when some data may be missing [27]. This postoperative morbidity rate was three times higher in COVID-19-positive patients, and higher than the 50% rate expected for a mean ESS of 6.4. Morbidity in the COVID-19-negative group was within the expected range of 26–31% for a mean ESS of 2.9. The overall mortality was within the expected range, but was also higher in the COVID-19-positive patients. The 18.5% postoperative mortality rate of our EGS COVID-19-infected patients is lower than the 25.6% rate reported from the large COVIDSurg Collaborative study [2].
In hospitals throughout Spain, and in our institution in particular, pre-operative testing (PCR of a nasopharyngeal swab, or a chest X-ray or thoracic CT scan) was recommended and performed in every patient starting April 4th. Before that date, no established pre-operative mandatory screening protocol was in place, and pre-operative testing was conditioned by its low availability and the absence of a rapid test that would rule out COVID-19 active infection.
Visitors were strictly forbidden both in the emergency department and in the hospitalization areas. All health workers wore masks at all times, and these were surgical masks in low-risk areas, FFP2 masks when in contact with highly suspect or confirmed COVID patients, and FFP3 masks in case of airway manipulation.
Specific hospitalization areas were established for those who were tested positive, and a clean circuit for those patients who were not infected. Negative pressure operating rooms were not available in our center. Surgeons participated in critical-care COVID units, especially in CVP line placement, endothoracic drainage, and other procedures. All tracheostomies were performed by ENT specialists. ECMO was used only in one COVID patient during the time period of the study, but that number has increased significantly now.
Trauma cases all but disappeared from our ED during this time period, mainly due to the population lockdown. Despite being a referral center for severe trauma in Madrid, admitting between 3 and 4 cases with an ISS > 15 per week, we just had 2 cases of severe trauma in those 9 weeks.
This study has the limitations due to its retrospective nature and the small number of operated EGS cases with confirmed SARS-CoV-2 infection. In addition, the limitations of RT-PCR testing in the early phases may have excluded from the COVID-19 group some asymptomatic but infected patients. We could not compare lab values, the rate of NOM or ESS with that of the previous year for lack of that data in our registry.
With those limitations in mind, we still believe that several conclusions can be drawn from this analysis. During this COVID-19 period, there was a reduction in the number of EGS cases assessed and procedures performed, and the rate of NOM of acute cholecystitis and appendicitis was increased. We basically did not change our routines in terms of indications for laparoscopy, assisting our residents in doing most procedures, or the use of general anesthesia. Only 14% of operated patients had a perioperative confirmation of SARS-CoV-2 infection but, irrespective of the treatment given, the severity and morbidity of COVID-19-infected patients were much higher than the rest. Late presentations for medical care may have added to this high morbidity, as attested by lab values and signs of severity in imaging techniques. In our opinion, this important aspect of the EGS planning and response should be taken into account if and when a new outbreak of the disease or other similar pandemic occurs.
Data availability
All data and materials as well as software application or custom code support claims comply with field standards.
Code availability
Not applicable.
References
Aminian A, Safari S, Razeghian-Jahromi A, Ghorbani M, Delaney CP. COVID-19 outbreak and surgical practice: unexpected fatality in perioperative period. Ann Surg. 2020;272(1):e27–9.
COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020. https://doi.org/10.1002/bjs.11746.
Lepre L, Costa G, Virno VA, Dalsasso G, Campa RD, Clavarino F, et al. Acute care surgery and post-operative COVID-19 pneumonia: a surgical and environmental challenge. ANZ J Surg. 2020. https://doi.org/10.1111/ans.15962.
Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. E Clin Med. 2020;5(21):100331. https://doi.org/10.1016/j.eclinm.2020.100331.
Alvarez Gallego M, Cortazar de las Casas S, Pascual Miguelañez I, Rubio Perez I, Barragan Serrano C, Alvarez Peña E et al. SARS-CoV-2 pandemic on the activity and professionals of a general surgery and digestive surgery service in a tertiary hospital. Cir Esp. 2020; 98(6):320-327
Patriti A, Baiocchi GL, Catena F, Marini P, Catarci M. Emergency general surgery in Italy during the COVID-19 outbreak: first survey from the real life. World J Emerg Surg. 2020;15(1):36. https://doi.org/10.1186/s13017-020-00314-3.
Klein MJ, Frangos SG, Krowsoski L, Tandon M, Bukur M, Parikh M, et al. Acute care surgeons´ response to the COVID-19 pandemic: observations and strategies from the epicenter of the American crisis. Ann Surg. 2020. https://doi.org/10.1097/SLA.0000000000004028.10.1097/SLA.0000000000004028.
Di Saverio S, Khan M, Pata F, Ietto G, De Simone B, Zani E, et al. Laparoscopy at all costs? Not now during COVID-19 outbreak and not for acute care surgery and emergency colorectal surgery: a practical algorithm from a hub tertiary teaching hospital in Northern Lombardy. Italy J Trauma Acute Care Surg. 2020;88:715–8.
Coimbra R, Edwards S, Kurihara H, Bass GA, Balogh ZJ, Tilsed J, et al. European Society of trauma and emergency surgery (ESTES) recommendations for trauma and emergency surgery preparation during times of COVID-19 infection. Eur J Trauma Emerg Surg. 2020;46(3):505–10. https://doi.org/10.1007/s00068-020-01364-7.
Aranda-Narvaez JM, Tallón-Aguilar L, Pareja-Ciuró F, Martin-Martín G, Gonzáles-Sanchez AJ, Rey-Simó I et al. Emergency Surgery and trauma care during COVID-19 pandemic. Recommendations of the Spanish Association of Surgeons. Cir Esp 2020; S0009–739X(20)30168–8. doi: https://doi.org/10.1016/j.ciresp.2020.04.031.
Orthopoulos G, Fernandez GL, Dahle JL, Casey E, Jabbour N. Perioperative considerations during emergency general surgery in the era of COVID-19: a US experience. J Laparoendosc Adv Surg Tech A. 2020;30:481–4.
Pandemic Critcon Surge Levels. University Hospitals Birmingham, NHS. (issued 06.04.2020. (www.uhb.nhs.uk/coronavirus-staff/clinical-info-pathways/cc-operational.htm). Accessed April 7, 2020.
Nandan AR, Bohnen JD, Sangji NF, Peponis T, Han K, Yeh DD, et al. The emergency surgery score (ESS) accurately predicts the occurrence of postoperative complications in emergency surgery patients. J Trauma Acute Care Surg. 2017;83(1):84–9.
Bertsimas D, Dunn J, Velmahos G, Kaafarani H. Surgical risk is not linear. Ann Surg. 2018;268:574–83.
Kaafarani HMA, Napaporn Kongkaewpaisan MD, Aicher BO, Diaz JJ Jr, O´Meara LB, Decker C. et al. Prospective validation of the Emergency Surgery Score (ESS) in Emergency General Surgery: An EAST Multicenter Study. J Trauma Acute Care Surg. J Trauma Acute Care Surg. 2020 doi: https://doi.org/10.1097/TA.0000000000002658.
Naar L, El Hechi M, Kokoroskos N, Parks J, Fawley J, Mendoza AE, et al. Can the emergency surgery score (ESS) predict outcomes in emergency general surgery patients with missing data elements? A nationwide analysis Am J Surg. 2020;S0002–9610(20):30113–6. https://doi.org/10.1016/j.amjsurg.2020.02.034.
Maerz LL, Mosenthal AC, Miller RS, Cotton BA, Kirton OC. Futility and the acute care surgeon. J Trauma Acute Care Surg. 2015;78:1216–9.
Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6.336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Singer M, Deutschman CS, Seymour ChW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus definition for Sepsis and Septic shock (Sepsis-3). JAMA. 2016;315:801–10.
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33.
Patriti A, Eugeni E, Guerra F. What happened to surgical emergencies in the era of COVID-19 outbreak? Considerations of surgeons working in an Italian COVID-19 red zone. Updates Surg. 2020;23:1–2. https://doi.org/10.1007/s13304-020-00779-6.
Pata F, Khan M, Iovino D, Di Saverio S. Laparotomy represents the safest option during COVID-19 outbreak. J Trauma Acute Care Surg. 2020. https://doi.org/10.1097/TA.0000000000002791.
Gao Y, Xi H, Chen L. Emergency surgery in suspected COVID-19 patients with acute abdomen: case series and perspectives. Ann Surg. 2020;272(1):e38–9.
Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020. https://doi.org/10.1097/SLA.0000000000003924.
Campanile FC, Podda M, Arezzo A, Botteri E, Sartori A, Guerrieri M, et al. Acute cholecystitis during COVID-19 pandemic: a multisocietary position statement. World J Emerg Surg. 2020;15(1):38.
Yeo C, Yeo D, Kaushal S, Ahmed S. Is it too premature to recommend against laparoscopic emergency surgery in COVID-19 patients? Br J Surg. 2020;107:e202. https://doi.org/10.1002/bjs.11668.
Dai H, Zhang X, Xia J, Zhang T, Shang Y, Huang R, et al. High-resolution chest CT features and clinical characteristics of patients infected with COVID-19 in Jiangsu, China. Int J Infect Dis. 2020; 106–112.
Funding
No funding to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest to declare.
Ethics approval
It is an observational retrospective study and due to the exceptional circumstances of the moment, we thought that the study was exempt from approval by the Ethics Committee of our center. No ethics conflicts to declare.
Consent to participate
It is an observational retrospective study and no informed consent is needed.
Consent for publication
All the authors agree with the content and all give explicit consent to submit. Consent from the responsible authorities at the institute/organization where the work has been carried out has been obtained.
Rights and permissions
About this article
Cite this article
María, FM., Lorena, MR., María Luz, FV. et al. Overall management of emergency general surgery patients during the surge of the COVID-19 pandemic: an analysis of procedures and outcomes from a teaching hospital at the worst hit area in Spain. Eur J Trauma Emerg Surg 47, 693–702 (2021). https://doi.org/10.1007/s00068-020-01558-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-020-01558-z