- 1Department of Epidemic Disease Research, Institute of Research and Medical Consultations (IRMC), Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 2Deanship of Scientific Research and Institute of Research and Medical Consultations (IRMC), Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 3Department of Basic Sciences, Preparatory Year Deanship, King Faisal University, Al Hofuf, Saudi Arabia
- 4Department of Public Health, Institute of Research and Medical Consultations (IRMC), Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 5Department of Obstetrics and Gynecology, Maternity and Children Hospital, Dammam, Saudi Arabia
- 6Biosciences, College of Health, Medicine and Life Sciences, Brunel University London, Uxbridge, United Kingdom
- 7Biology Department, College of Science and Institute of Research and Medical Consultations (IRMC), Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
The current coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome virus 2 (SARS-CoV-2), has resulted in a major global pandemic, causing extreme morbidity and mortality. Few studies appear to suggest a significant impact of gender in morbidity and mortality, where men are reported at a higher risk than women. The infectivity, transmissibility, and varying degree of disease manifestation (mild, modest, and severe) in population studies reinforce the importance of a number of genetic and epigenetic factors, in the context of immune response and gender. The present review dwells on several contributing factors such as a stronger innate immune response, estrogen, angiotensin-converting enzyme 2 gene, and microbiota, which impart greater resistance to the SARS-CoV-2 infection and disease progression in women. In addition, the underlying importance of associated microbiota and certain environmental factors in gender-based disparity pertaining to the mortality and morbidity due to COVID-19 in women has also been addressed.
Introduction
The coronaviruses belong to the subfamily Coronavirinae, which cause respiratory and gastrointestinal infections (1). First discovered in 1960, the coronaviruses were ascribed to causing a mild respiratory symptom; these viruses include human CoV 229E (HCoV-229E) and HCoV-OC43 (2). The present coronavirus disease 2019 (COVID-19) pandemic by severe acute respiratory syndrome virus 2 (SARS-CoV-2) initially emerged from Wuhan Province, China at the seafood market (3). Various studies on the innate and adaptive immune responses to coronaviruses have been carried out in recent years. The role of the immune responses is to initiate viral clearance, prevent viral replication, and help tissue repair. However, such immune responses play a crucial part in SARS-related pathogenicity. The SARS-CoV-2 is known to dysregulate cytokine-mediated inflammatory and immune responses (4). Innate immune humoral factors such as complement and coagulation-fibrinolysis systems, soluble proteins/naturally occurring antibodies, and cellular components (natural killer cells and other innate lymphocytes) seem to be fully engaged following viral infection. Dysregulation of these factors leads to viral replication in the lung airways and escalation of an adaptive immune response. Severity caused by SARS-CoV-2 infection thus may also be attributed to the degree of dysregulated immune and inflammatory response (5).
The virus has affected the global population; however, men seem to manifest more severe form of the disease than women, as per the onset of symptoms of the disease. The mortality in men is 2.4 times compared to women, although both gender have a similar susceptibility to transmission (6). One study that involved 425 COVID-19 patients reported 56% men (7), while another study reported 50.7% of 140 patients being men as infected individuals (8). Another study involving 1,019 COVID-19 patients revealed greater susceptibility of men compared to women to SARS-CoV-2, indicating that gender is as a risk factor for morbidity and mortality (6). One of the most noticeable differences is the mortality rates among men and women in the Western Europe, where 69% of men have died due to COVID-19. Even in the United States of America, a lesser percentage of women have died as compared to men (9). Similar patterns have been seen in China and other affected countries. According to one of the reports, the greatest sex disparity was seen in the death rate; it came to only 36.2% deaths in women as compared to men at the rate of 51.4%. Additionally, the analysis of COVID-19 cases documented in China showed a 2.8% case fatality in men as compared with a 1.7% rate in infected women (10) (Figure 1).
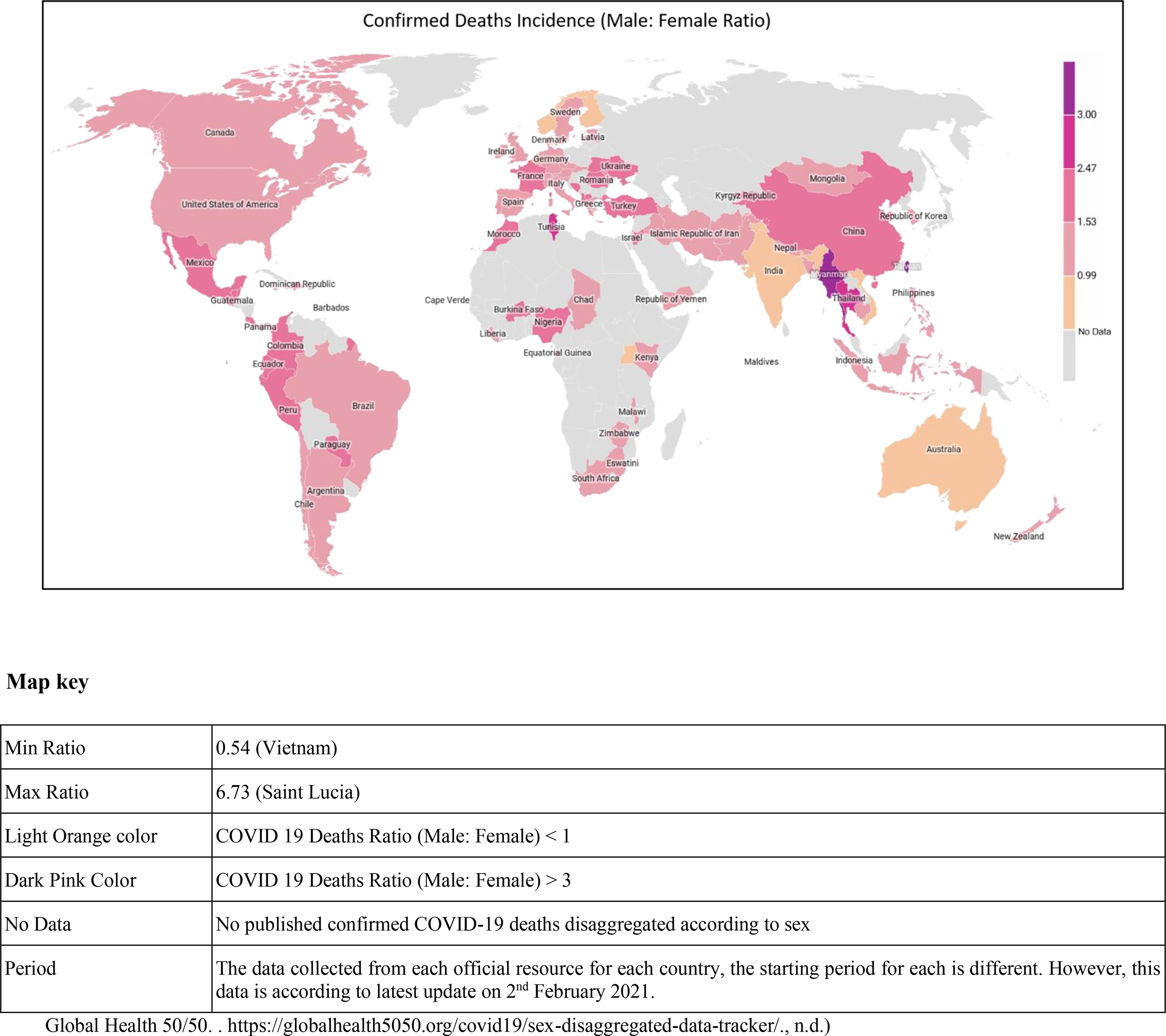
Figure 1 Global map showing the confirmed COVID-19 death incidence (male and female ratio) in various countries.
This is not the first time that coronaviruses have been found to affect women lesser than men. The epidemiological data from SARS-CoV (2003) and MERS-CoV (2012) epidemics also highlighted women at a lower risk of death from these deadly viruses (11). In Hong Kong, men were found to be affected more severely than women by the SARS-CoV (12). Furthermore, men had a significantly higher fatality rate than women (21.9% versus 13.2%) (13). In 2012, when MERS-CoV hit Saudi Arabia, the disease occurrence among men (62%) was considerably higher than in women (38%) of the total confirmed infected cases (14). Thus, gender seems to play an important role in severity and fatality in SARS-related diseases.
Estrogen Acts as an Immune-Stimulating Factor
As men are worse affected by SARS-CoV-2, they require longer hospital stay and have a higher mortality rate when compared to women (15). The observed resistance to SARS-CoV-2 in women can be attributed to sex hormones, specifically estrogen, which is known to enhance the immune activity of both B as well as T-helper cells (16). Estrogen receptor alpha (ERα) is a steroid hormone receptor that controls physiological functions, including immunity. ERα has an effect on the subsets of T cells that includes Th1, Th2, Th17, and T regulatory cells, as well as follicular helper T (TFH) cells. It has been established that induced immunization by NP-conjugated ovalbumin produces specific antibodies that are elevated in CD4-ERα knock-out mice, under sufficient estrogen environment (17). Therefore, estrogen, the primary female sex hormone, stands out as a key biological factor making women’s immune system more active against the virus (13).
There is a growing interest in studying the role of sex hormones in the tissue renin-angiotensin system (RAS). The expression of ACE2 (angiotensin-converting enzyme) in some organs, such as uterus, kidney, and heart, is regulated by 17β-estradiol. This occurs by increasing the locally existing ACE2 effect on the cardiac tissue and suppressing the RAS through catalytic cleavage of a particular residue of angiotensin II to increase the release of cardioprotective angiotensin 1–7 and upregulate anti-oxidative and anti-inflammatory effects (18) Estrogen level is inversely related with the regulation of cardiac troponin secreted during ischemic or anoxic condition, leading to irreversible injury to the cardiac cells (19). In few studies conducted on COVID-19 patients, it was seen that 51% patients died due to cardiac injury (20). The death rate in COVID-19 patients was 7.6% having normal cardiac troponin levels and without any cardiovascular disease. Mortality of 13.3% was seen in patients with underlying cardiovascular disease and normal cardiac troponin levels, 37.5% cardiovascular disease but elevated cardiac troponin levels, and 69.4% patients having both the conditions. A higher proportion of men (65.4%) had increased cardiac troponin as compared to women (42.2%) with COVID-19 (20).
The effect of estrogenic hormones could justify these observations, as this hormone has been reported to reduce low-density lipoprotein cholesterol and increase the high-density lipoprotein (21). 17β-estradiol, an estrogenic hormone, is also known to mediate the activation of early and late endothelial nitric oxide synthase via estrogen receptor interaction (22). Cardiomyocytes also carry the functional estrogen receptors that regulate the expression of nitric oxide synthase to prevent the cardiovascular system from damage by some factors such as suppression of the formation of thrombus, platelet stimulation, and adhesion of leukocyte-endothelial cell. It has been reported that male mice are more vulnerable to SARS-CoV compared to females. However, when the ovaries (an endocrine gland producing and releasing estrogen) from female mice were removed, their mortality from the SARS-CoV sharply increased (23).
COVID-19 affects men and women differently likely due to the difference in genetic nature and influence of sex hormones. COVID-19 enters the host body via the upper respiratory system, through contacting droplets. Estrogen has a beneficial impact on the entire respiratory tract system (16). Estrogen activates the response of mucosa of the nose by regulating turbinate hypertrophy and boosting secretion of nasal mucus containing anti-viral, antibacterial, and immune factors such as IgA, lysozyme, mucins, lactoferrin, electrolytes, and oligosaccharides, which are important for restricting upper airway infections (24). Besides, estrogen stimulates the synthesis of hyaluronic acid that preserves a suitable tropism of the cilia and the mucosal membrane (Figure 2). Additionally, estrogen stimulates the local nasal immune system that acts directly by stimulating phagocytic cells, antigen-presenting cells, and natural killer cells (25). Once stimulated, they can kill the virus protecting the body before its access to its target cells in the part of the respiratory system, thus reducing the pathological effect of the virus (26). In a study, it was indicated that G protein-coupled estrogen receptor (GPER) specifically supports the diminishing nasal symptoms, serum OVA-specific IgE, and Th2 cell immune response, but boosts the Treg immune response in mice (27). In addition to its indigenous impact in the nasal cavity, estrogen provides the required level of hyaluronic acid secretion needed for the mouth’s hydration by promoting the function of the lower respiratory system as it acts directly on the bronchial epithelial membrane to secrete more mucus. At this stage, the effective role of estrogen is promoted by the progesterone (PG) physiological function as it upregulates amphiregulin (epidermal growth factor) to maintain the histological integrity of the lung tissue if the viral infection occurs. PG also improves the onset of the symptoms of respiratory disease, when given to women at menopause phase (28). Estradiol (E2) and PG support a reduced case of a naive immune-inflammatory reaction, via increasing the immune tolerance and synthesis of immunoglobulins. It has been reported that the combination of E2 and PG could enhance the anti-viral immunity, but downplay cytokine storms in COVID-19 (29).
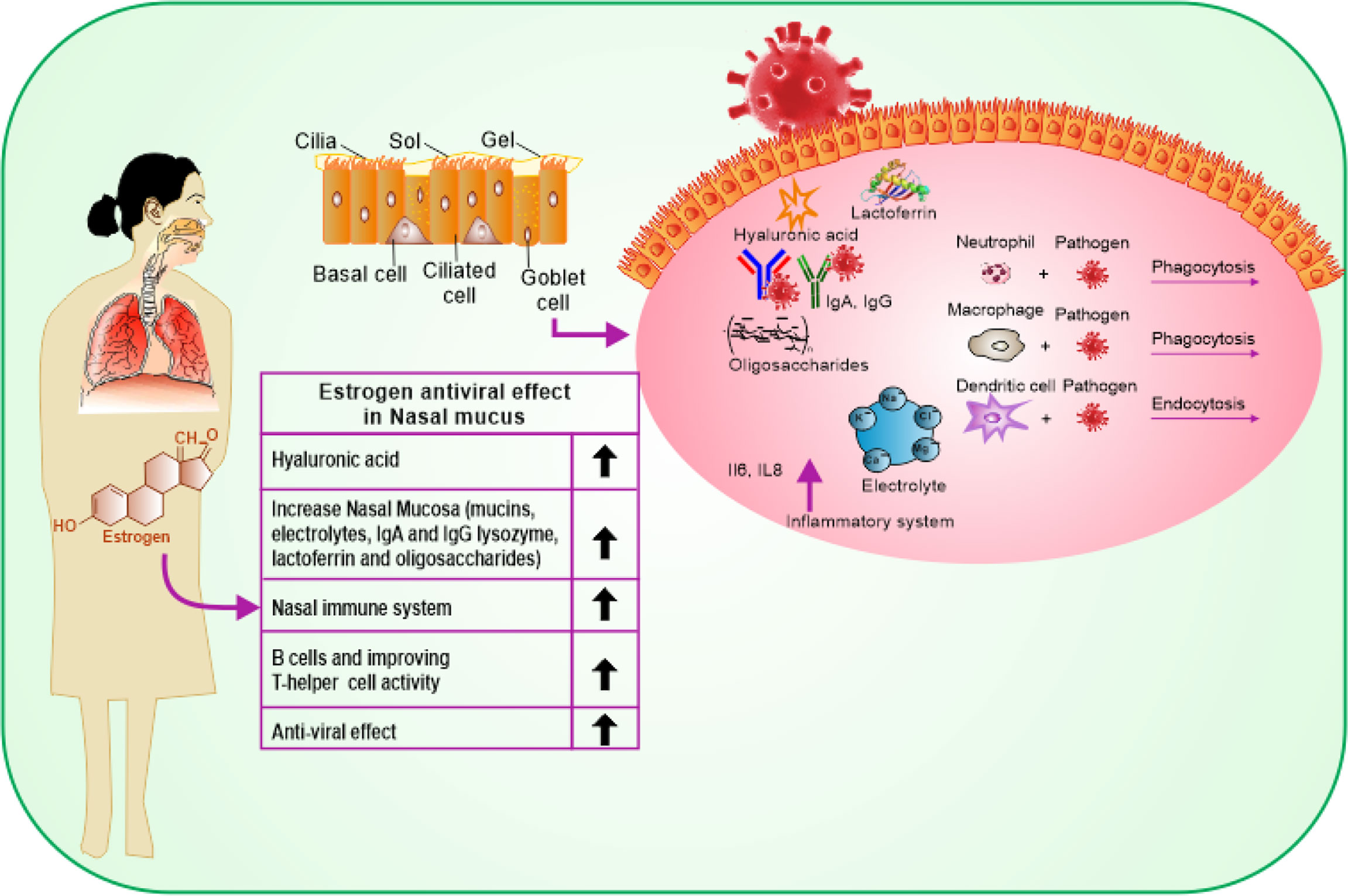
Figure 2 Schematic representation showing the protective effects of estrogen on the upper and lower respiratory tract cells and its benefits on the immune response.
E2 has been found to have a protective activity against the disease severity, as revealed by higher levels of cytokines such as IL-6 and IL-8 in severe cases. E2 corresponded to COVID-19 severity, because of the regulation of such cytokines associated with inflammation (30). Also, regulatory proteins (Cardiac troponin T and troponin I) play a key role in calcium regulation (7).
Estrogen has anti-inflammatory and anti-oxidative actions on the effectors of the renin-angiotensin system-like pro-oxidative LOX-1 and pro-inflammatory ICAM-1. Estrogen alters the homeostasis of the local RAS and offers protection in the atrial myocardium (31). Moreover, other studies have indicated the anti-viral activity of two selective estrogen receptor modulators against viral infection like Ebola. Primary differentiated human nasal epithelial cell cultures obtained from healthy men and women demonstrated the action of estrogenic receptors on the human cellular response to influenza A virus (IAV) infections. Nasal epithelial cells are the primary cell type infected with IAV, and these cultures allowed to investigate IAV infection and pathogenesis based on the sex and hormonal milieu of the donor cultures (32).
Menopause is an individual risk factor for COVID-19 as it causes a sudden reduction in estrogen levels which could minimize the risk difference between men and women, although the case studies have revealed that the gender disparity still exists in elderly people. In postmenopausal women, the ovaries produce estrone, the inactive form of estrogen, in high quantities. Additionally, estrogen is no longer the only endocrine factor in the postmenopausal stage. A number of extragonadal tissues such as adipose tissue, bone chondrocytes and osteoblasts, aortic and endothelium, vascular smooth muscle cells, skin, skeletal muscle, and several brain regions produce estrogen, to act locally as a paracrine and intracrine factor (33). Therefore, circulating estrogen levels explain its effect in menopausal women because estrogen escapes from local metabolism and gets into the main circulation (33, 34). It is still unclear if the estrogen circulation and expression in the local tissue play a part in the reduced COVID-19 mortality in menopausal women compared to age-matched men (35). Therefore, the role of estrogen is fascinating.
Difference in Innate and Adaptive Immunity
Women show reduced susceptibility to viral infections due to their varying nature of innate immunity, hormones, and other factors associated with sex chromosomes. Sex-related hormones regulate the range of the immune responses distinctively in men and women (36). The estrogen and ER-α influence the activation and proliferation of T-lymphocytes and initiate elevation of IFN-γ level in Th1 lymphocytes. A gradual IFN reciprocation by mismatched dsRNA or exogenous IFN-α treatment has been found to inhibit SARS-CoV multiplication in the lungs of mice (37). Studies have reported that IFN-β and IFN-γ can significantly suppress the replication of SARS-CoV, and a symbiotic anti-SARS-CoV action was attained with the synthesis of the IFN-β and IFN-γ (38). As discussed earlier, treatment with estrogen suppresses the inflammatory response and reduces SARS-CoV load that leads to an increased survival in mice (23). Contrary to estrogen, testosterone have a general inhibitory action on the immune response, specifically to viral antigens (39). In a study, murine macrophage treatment with testosterone suppressed the nitrate oxidase synthetase (40).
Studies have shown suppressive effects of testosterone on the activation of dendritic cells, antigen presentation to T-lymphocytes, and initiation of immune response (41). Th1 cells have a crucial part to play in protection against viral infections by secreting IFN-γ (42). Androgens can influence the thymocyte response by suppressing the Th1 proliferation and reducing IFN-γ synthesis (43–45).
Among women, in order to reduce the duplication of X-linked genes, the second X chromosome is silenced via X chromosome inactivation (XCI), although many genes escape this inactivation. A location on Xp22.2, which is for the ACE2 gene, also bypasses X-inactivation, resulting in the phenotypic differences between the genders. The other XCI escaping regions are IRAK1 (Interleukin-1 receptor-associated kinase 1) and IKKγ (inhibitor of nuclear factor Kappa-B kinase subunit gamma) that might influence the immune response against the COVID-19 infection in women. Numerous genes are involved with the X chromosome. Mutations occurring in a single gene may lead to two different alleles with a distinct mechanism of response, suggesting that women could not only escape the outcome of deterrent mutations but also help to fight against infectious challenges such as SARS-CoV-2. Additionally, estrogen and estrogen receptor signaling confer an important potency to innate as well as adaptive immunity and the process of tissue repair during and after the viral infection (7, 36, 39) (Figure 3).
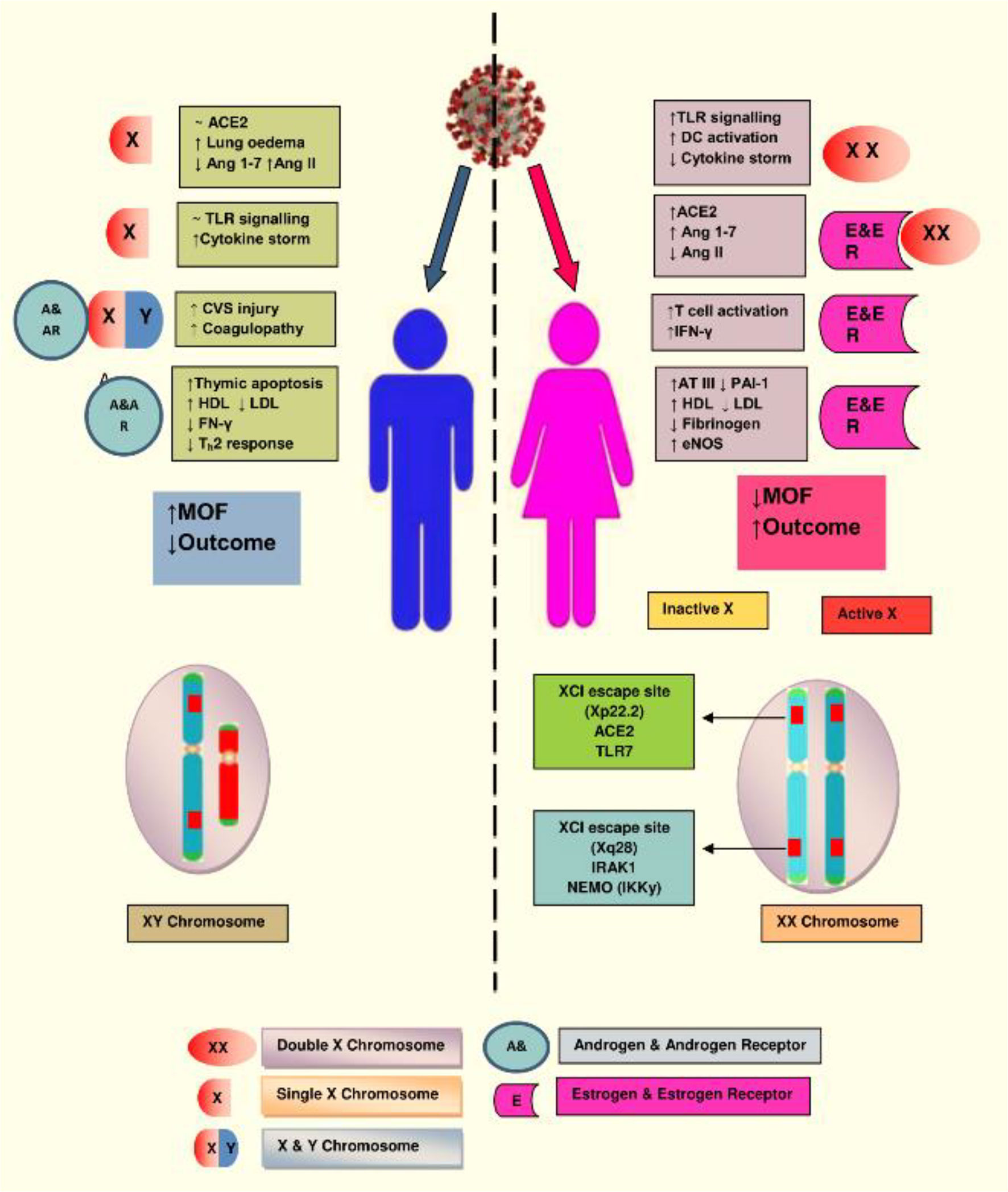
Figure 3 The X chromosome in females has various genes associated with immunity. Natural mutation in one copy of X gene may lead to two different alleles with distinct regulatory mechanism, which protect the women from implications of deleterious mutations and confers advantage in facing novel immunogens, like SARS-CoV-2. Illustration presents the genes encoded on the X-chromosome involved in the increased immune response in females during COVID-19. (Abbreviations used: TLR7, Toll-like receptor 7; DC, dendritic cell; CVS, cardiovascular system; IFN-γ, interferon gamma; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ATIII, antithrombin III; IRAK1, Interleukin-1 receptor-associated kinase 1; MOF, multiorgan failure).
In SARS-CoV-2 infected women, T cells, especially CD8+ T cells, were found much more activated. When their clinical trajectory was analyzed, it was revealed that elevated cytokine levels in women patients were related to the worsening condition of COVID-19 disease (36). Most COVID-19 affected patients have higher plasma levels of pro-inflammatory cytokines/chemokines (IL-6, IL-2, IL-8, IL-7, CCL2, CCL3, and TNF) (3). This may lead to tissue damage and subsequent organ failure. Elevated levels of plasma cytokines are correlated with a decrease in lymphocytes which leads to the progression of COVID-19 disease. Dysfunction of T cells with age is also associated with worse COVID-19 disease outcomes (46). However, even though elderly women develop a strong T-cell immune response, majority develop anti-Spike IgG at the initial stage of infection that helps in suppressing proinflammatory cytokines, and hence, worsening of disease does not occur (47).
It is equally pertinent to mention that women are at no lesser risk of getting infected with coronavirus, especially during pregnancy, as women fall at higher risk of severe illness from other respiratory infections. COVID-19 infection in pregnant women did not differ much from non-pregnant women (48). Reports have suggested that pregnancy and childbirth do not seem to contribute to an increased risk of contracting SARS-CoV-2 infection; it also does not increase the severity of the clinical course of COVID-19, compared to non-pregnant women of the same age (49–51).
COVID-19 infection during pregnancy may have more unfavorable results in comparison with the non-pregnant group (52). Additionally, COVID-19 and pregnancy increase the chance of internal clotting that increases the risk of thrombosis (53). During the period of pregnancy, a large variety of immune cells, mostly natural killer (NK) cells, macrophages, and regulatory T cells (Treg), are activated. The accumulation of macrophages and NK cells takes place around trophoblastic cells during the first trimester of pregnancy protecting miscarriage of the allogeneic fetus (19). Hence, the maternal immune system shields fetus from the damage by environmental insults. Likewise, the fetus also modifies the maternal immune system. During pregnancy, PG has immunomodulatory effects that influence the Th1 response. In pregnancy, an enhancement in anti-inflammatory factors like interleukin-1 receptor antagonist (IL-RA) and TNF-α receptor (TNF-R) is recorded; conversely, a decrease in IL-1β and TNF-α is found (20).
Variations in the estrogen and PG levels during pregnancy may cause respiratory, cardiovascular, reversible degeneration in the thymus, with a reduction in CD4+ and CD8+ T cells that may lead to more susceptibility of women to SARS-CoV-2 infection. The PG on nasal mucosa acts as a facilitator in the attachment of the virus and prevents its elimination. Additionally, an increase in oxygen consumption due to vascular congestion and reduction in the capacity of the lung may increase the risk for severity of COVID-19 in pregnant women (54). Another risk factor is the higher ACE2 expression during pregnancy, and hence increased risk of complications from COVID-19 infection (55). An increase in ACE2 receptors in the kidneys during pregnancy may contribute to effective regulation of blood pressure, although it can favor the attachment and facilitate the virus entry into the host cells (54).
Androgens might lead to severe COVID-19 disease among men through raising neutrophil count and increasing the production of cytokines (IL-1β, IL-10, IL-2), altering TGF-β production by immune cells, and decreasing the antibody production (47). This event is crucial as the patients with severe COVID-19 exhibit cytokine storm syndrome due to neutrophils. One of the androgen pathways in COVID-19 infection is the transmembrane protease, serine 2 (TMPRSS2) gene that is expressed mainly in the adult prostate (56), and in metastatic prostate cancers; it is also found in tissues like lung, kidney, pancreas, colon, small intestine, and liver (56). The TMPRSS2 gene is transcribed and regulated by the androgen receptor, and the main target of TMPRSS2 expression in COVID-19 is the lungs, kidneys, and liver (57). In one retrospective study, increased levels of testosterone in most women (60%) having COVID-19 disease were recorded; a positive correlation between the levels of testosterone and pro-inflammatory cytokines among women with COVID-19 was also noted (58).
In view of a higher mortality in men from COVID-19 compared to women, it has recently been pointed out that testosterone may affect disease severity. This notion is supported by the evidence that the primer protease for SARS-CoV-2 spike protein, TIMPRSS2, as well as the virus entry receptor, ACE2, are upregulated by testosterone (59). Although debated, androgen-deprivation therapy in prostate cancer patients infected with SARS-CoV-2 has been suggested (60). However, hypogonadism can also be a risk factor for severe COVID-19 (61). It is worth noting that women suffering from polycystic ovarian syndrome (PCOS), which is characterised by heightened androgen levels (hyperandrogenism), have been found to be at a significantly higher risk of COVID-19 compared to non-PCOS women (62, 63).
Role of Angiotensin-Converting Enzyme 2 (ACE2)
The ACE2 gene that is found on the X chromosome (location: Xp22.2; nucleotides 15 494 402–15 602 148, GRCh38.hg38 version) has been reported to work differently in men and women (64). ACE2 carries out its important functions by dissociating angiotensin I into angiotensin II. Angiotensin II, being a small peptide, is of huge importance in the case of vasoconstriction and sodium balance. ACE2 breaks angiotensin I and II into dissociated peptides that possibly lead to vasodilatation and, hence, countering angiotensin II (65, 66). The entry route for SARS-CoV-2 is via ACE2, similar to the SARS-CoV virus, bearing a spike protein that binds with ACE2 to invade the cells (20, 46, 67). The location of the ACE2 gene on Xp22.2 is a site of genes that escapes XCI, leading to phenotypic dissimilarities between genders (68, 69). SARS-CoV-2 possesses 16 residues of receptor binding motif (RBM), and binds to 16 of the 20 ACE2 residues present in men. In women, the same RBM of SARS-CoV-2 may be detected by ACE2 on any of the two X chromosomes. The possibility becomes less for the similar residue sequences of ACE2 present on the second chromosome to bind efficiently to the RBM of SARS-CoV-2, leading to the breakdown of Ang II to form Ang 1–7 by unbound ACE2, and therefore might reduce the chance of respiratory edema during SARS-CoV-2 infection. Men, with only one X chromosome, are deficient in the alternative mechanisms that could impart cellular protection during COVID-19 infection (70, 71).
Several significant divergences in the prevalence of ACE2 variants have been reported among diverse races and ethnicity. Recently, a single-cell RNA sequencing (RNA-seq) study reported that Asian men could express tissue ACE2 at a higher level (72). During a study on the northeastern Chinese Han population, the serum ACE2 activity was found to have a negative correlation with body mass index, pulse pressure, and estrogen levels among hypertensive women (6). Such studies indicate a protective mechanism of circulating ACE2 and the participation of estrogens in the expression and upregulation of ACE2 activity levels (73).
ACE2 is present in epithelial cells of the lung, intestine, blood vessels, and kidney (74). The angiotensin system plays a vital role in cardiovascular homeostasis, acute inflammation, and autoimmune disorders (75). The presence of high ACE2 receptors may lead to a higher risk of contracting SARS-CoV2. It has been reported that men have elevated levels of circulating ACE2 than women, and also in patients having diabetes and cardiovascular ailments (76). People with cardiovascular failure have the plasma ACE2 elevated in men compared to women, which correlates with increased SARS-CoV infection (65, 77). Among the hypertensive women, blood pressure and body mass index inversely correspond to ACE2, whereas there is a direct correlation of blood sugar and estrogen levels to ACE2 level (65, 78). As mentioned above, estrogen also downregulates the renin-angiotensin system components acting as an anti-inflammatory and anti-oxidative agent (67, 78). Significant functional regulation of ACE2 by estrogen may explain the gender differences in COVID-19 associated morbidity and mortality (79).
Microbiota
Development of a pronounced innate and adaptive immune response is greatly influenced by the composition of the human gut microbiota. The human gut possesses a diverse and complex microbial consortium that reciprocates by establishing the persistent host immune homeostasis (80–82). The human gut harbors complex communities of microorganisms that includes holobiont (composite organism) and hologenome (collective genome of all bionts) (83). This complex composition offers a crucial genomic and metabolic capability that has an important impact on the initiation, development, and action of the host immune system, thereby protecting against infections and safeguarding the ecosystem of gut flora (84). The homeostatic cascades existing between the immune system and gut microbiota of the host play a crucial role in modulating the activation of host cells and tissues involved in response to infectious agents (85). The interaction of virus and microbiota has been studied in several viral infections. For example, surfactin, a molecule on a Bacillus subtilis surface, is known to disintegrate many viruses including influenza A (85). Thus, the gut microbiota is likely to influence COVID-19 pathogenicity, and conversely, SARS-CoV-2 may influence the gut microbiota leading to dysbiosis and other unpleasant consequences (86). Therefore, the alteration of the composition of existing microbiota and health conditions during SARS-CoV-2 infection is likely to have a major role in establishing the susceptibility and resilience of an individual to COVID-19. However, most of the COVID-19 severe symptoms and fatalities occur in individuals having some risk factors such as aging, preexisting comorbidities, and, to some extent, gender, which are indirectly characterized by disrupted microbiome status (87).
Like gastrointestinal system, the respiratory microbiome constitutes the community of differentiated bacterial phyla like Bacteroidetes, Firmicutes, and Proteobacteria and has a protective role in the host immunity (44, 88) (Figure 4A). Han et al. showed that the COVID-19 infection can alter the lung microbiome (89). A severe dysbiosis was found among COVID-19 patients, with a higher prevalence of pathogenic microbes such as Klebsiella oxytoca, Faecalibacterium prausnitzii, Lactic Acid Bacteria, and Tobacco mosaic virus (TMV) (Figure 4B). The serious inflammatory environment in the lungs correlated with Rothia mucilaginosa, TMV level, and SARS-CoV-2, suggesting a key role of respiratory microbiota in COVID-19 disease. Other studies also reported fecal microbial changes in 15 subjects infected with COVID-19 that correlated with high severity and abundance of Coprobacillus, Clostridium ramosum, and Clostridium hathewayi, and reduced levels of Faecalibacterium prausnitzii and Alistipes onderdonkii (90, 91) (Figures 4A, B).
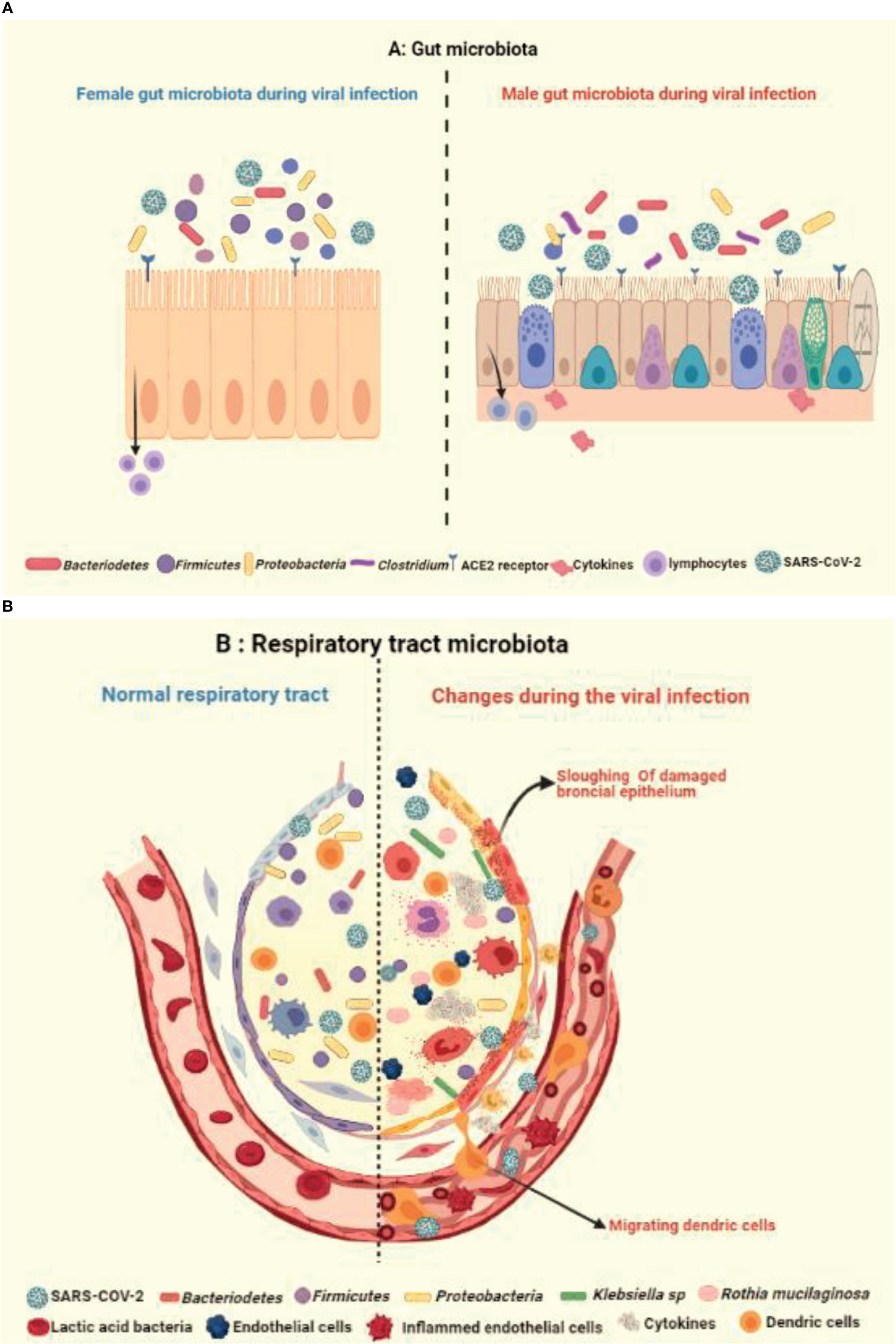
Figure 4 Illustration showing the impact of healthy and unhealthy microbiota on respiratory tract infection. The complex relationship via gut-lung axis might be crucial in determining the vulnerability of respiratory tract to COVID-19, as an outcome of potential variation and crosstalk between (A) healthy gut microbiota with occurrence of fewer Bacteriodetes and (B) respiratory microbiota with prevalence of more Klebsiella and Ruthia sps in virus infected alveolus.
The microbiota existing outside of the reproductive tract is significantly mediated by the sex steroid hormones. Many studies conducted on mice, fish, and humans have analyzed the sex difference in gut microbiota. This subject of whether the sex difference in gut microbiome in humans has any involvement in the disparity of viral infection is an interesting area to study (92). In a study, gender differences correlated with the overall composition of gut microbiota. The gut microbiome in women was found to have a lower occurrence of Bacteroidetes compared to men (93). An animal study evaluated gender-specific variations in the composition of gut microbiota (94, 95). The systemic estrogen levels may be influenced by dietary fiber, which is the main energy source of gut microbial fermentation and, hence, formulates the gut microbiota (94, 96).
Environmental Mediators
In addition to biological differences accounting for a significant gender disparity of COVID-19, the influence of environmental factors could also play a part (97).
Lifestyle
Lifestyle choices among the genders possibly makes a huge difference. Historically, it has been noticed that men are more habitual of smoking than women. Smokers tend to have weakened lungs leading to chronic lung and heart diseases that could be the worst outcome, if infected with COVID-19 (97–100). In China, the smoking prevalence in men is 57.6% which is nearly 10 times more than the women with 6.7% (101). The lower airways of smokers have shown a higher expression of ACE2, suggesting a higher risk for COVID-19 (102, 103). Such findings are an indication of one of the factors behind the increased mortality in men with COVID-19 which needs further validation.
Exercise
The decreased incidence rate of COVID-19 symptoms in women can be also related to the physical activity engaging from moderate-to-vigorous one. Women are considered to be physically more active when compared with men who prefer prolonged and intensive exercises (1, 104). Prolonged and vigorous exercise may lead to immunosuppression; on the contrary, mild and moderate exercise enhances immune response and significantly minimizes the risk and severity of respiratory viral infection. This is supported by a number of studies that explain a moderate level of exercise lowers inflammation and boosts the immune function. Regular mild physical activity influences the level of hormones related to stress, which downregulates intense inflammation of the respiratory tract and helps in activating the anti-viral innate immunity polarising the immune function towards a Th2 profile, extensive research is needed to study cellular and molecular cascades through which exercise regulates immune response (104–106).
Nutrition
A study shows nutritional environment during the post and prenatal period is associated with a reduced mortality rate among females in case of HIV, for example, high-fat diet and the micronutrients like Vitamin B, C, and E supplements have a reduction of 32% (107). Another study suggests the benefits of supplementary maternal micronutrients in women compared to men (108, 109).
Conclusions and Perspective
Immunity, X-chromosome associated genes, and sex hormones are the main distinguishing factors that are likely to offer greater resistance against SARS-CoV-2 in women. The evidence suggesting important decisive factors of gender-related disparity in immunity may impact on the onset of COVID-19 and vaccination outcomes.
Author Contributions
SR: conceptualized, drafted, figures, and revised. VN contributed to figure, environmental factors, and reference formatting. IN: contributed to epidemiology part. HA: contributed to environmental part. MS: contributed to mortality and map part. MA contributed to severity and manifestation part. UK: reviewed, edited, and revised the manuscript. EAl-S: conceptualized, drafted, revised, and edited. All authors read the article and approved the submitted version.
Funding
Institute for Research and Medical Consultation (IRMC) is highly acknowledged for the research facilities.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The figures have been created using Biorender software.
References
1. Khan S, Tombuloglu H, Hassanein SE, Rehman S, Bozkurt A, Cevik E, et al. Coronavirus Diseases 2019: Current Biological Situation and Potential Therapeutic Perspective. Eur J Pharmacol (2020) 886:173447. doi: 10.1016/j.ejphar.2020.173447
2. Cleri DJ, Ricketti AJ, Vernaleo JR. Severe Acute Respiratory Syndrome (SARS). Infect Dis Clin North Am (2010) 24:175–202. doi: 10.1016/j.idc.2009.10.005
3. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
4. Hosseini A, Hashemi V, Shomali N, Asghari F, Gharibi T, Akbari M, et al. Innate and Adaptive Immune Responses Against Coronavirus. BioMed Pharmacother (2020) 132:110859. doi: 10.1016/j.biopha.2020.110859
5. Boechat JL, Chora I, Morais A, Delgado L. The Immune Response to SARS-CoV-2 and COVID-19 Immunopathology – Current Perspectives. Pulmonology (2021) 9:S2531–0437(21)00084-2. doi: 10.1016/j.pulmoe.2021.03.008
6. Jin JM, Bai P, He W, Wu F, Liu XF, Yang JK. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front Public Health (2020) 8:152. doi: 10.3389/fpubh.2020.00152
7. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med (2020) 382:1199–207. doi: 10.1056/NEJMOa2001316
8. Zhang J, Xiang D, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical Characteristics of 140 Patients Infected With SARS-CoV-2 in Wuhan, China. Allergy (2020) 75(7):1730–41. doi: 10.1111/all.14238
10. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi (2020) 41(2):145. doi: 10.46234/ccdcw2020.032
11. Aleanizy FS, Mohmed N, Alqahtani FY, El Hadi Mohamed RA. Outbreak of Middle East Respiratory Syndrome Coronavirus in Saudi Arabia: A Retrospective Study. BMC Infect Dis (2017) 17(1):23. doi: 10.1186/s12879-016-2137-3
12. Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W, et al. Coronavirus as a Possible Cause of Severe Acute Respiratory Syndrome. Lancet (2003) 361(9366):1319–25. doi: 10.1016/S0140-6736(03)13077-2
13. Karlberg J, Chong DSY, Lai WYY. Do Men Have a Higher Case Fatality Rate of Severe Acute Respiratory Syndrome Than Women Do? Am J Epidemiol (2004) 159(3):229–31. doi: 10.1093/aje/kwh056
14. Alghamdi IG, Hussain II, Almalki SS, Alghamdi MS, Alghamdi MM, El-Sheemy MA. The Pattern of Middle East Respiratory Syndrome Coronavirus in Saudi Arabia: A Descriptive Epidemiological Analysis of Data From the Saudi Ministry of Health. Int J Gen Med (2014) 7:417. doi: 10.2147/IJGM.S67061
15. Graziano O, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA (2020) 323(18):1775–6. doi: 10.1001/jama.2020.4683
16. Taneja V. Sex Hormones Determine Immune Response. Front Immunol (2018) 9:1931. doi: 10.3389/fimmu.2018.01931
17. Kim DH, Park HJ, Park HS, Jae UL, Che MK, Myung CG, et al. Estrogen Receptor α in T Cells Suppresses Follicular Helper T Cell Responses and Prevents Autoimmunity. Exp Mol Med (2019) 51(4):1–9. doi: 10.1038/s12276-019-0237-z
18. Joyner J, Neves LAA, Granger JP, Alexander BT, Merrill DC, Chappell MC, et al. Temporal-Spatial Expression of ANG-(1-7) and Angiotensin-Converting Enzyme 2 in the Kidney of Normal and Hypertensive Pregnant Rats. Am J Physiol Regul Integr Comp Physiol (2007) 293(1):R169–77. doi: 10.1152/ajpregu.00387.2006
19. Mingels AMA, Kimenai DM. Sex-Related Aspects of Biomarkers in Cardiac Disease. Adv Exp Med Biol (2018) 1065:545–64. doi: 10.1007/978-3-319-77932-4_33
20. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol (2020) 5:811–8. doi: 10.1001/jamacardio.2020.1017
21. Mebane S, Irma L. Effects of Estrogen or Estrogen/Progestin Regimens on Heart Disease Risk Factors in Postmenopausal Women: The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA (1995) 273(3):199–208. doi: 10.1001/jama.273.3.199
22. Fontaine C, Morfoisse F, Tatin F, Zamora A, Zahreddine R, Henrion D, et al. The Impact of Estrogen Receptor in Arterial and Lymphatic Vascular Diseases. Int J Mol Sci (2020) 21(9):3244. doi: 10.3390/ijms21093244
23. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J Immunol (2017) 198(10):4046–53. doi: 10.4049/jimmunol.1601896
24. Paulsson B, Gredmark T, Burian P, Bende M. Nasal Mucosal Congestion During the Menstrual Cycle. J Laryngol Otol (1997) 111(4):337–9. doi: 10.1017/s0022215100137259
25. Costa HO, de Castro Neto NP, Rossi LM, Millas I, Coelho F, da Silva L. Influence of Estradiol Administration on Estrogen Receptors of Nasal Mucosa: An Experimental Study on Guinea Pigs. Braz J Otorhinolaryngol (2014) 80(1):18–23. doi: 10.5935/1808-8694.20140006
26. Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, et al. Differential Induction of Mucosal and Systemic Antibody Responses in Women After Nasal, Rectal, or Vaginal Immunization: Influence of the Menstrual Cycle. J Immunol (2002) 169(1):566–74. doi: 10.4049/jimmunol.169.1.566
27. Wang YX, Gu ZW, Hao LY. The Environmental Hormone Nonylphenol Interferes With the Therapeutic Effects of G Protein-Coupled Estrogen Receptor Specific Agonist G-1 on Murine Allergic Rhinitis. Int Immunopharmacol (2020) 78:106058. doi: 10.1016/j.intimp.2019.106058
28. Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. Estradiol Increases Mucus Synthesis in Bronchial Epithelial Cells. PloS One (2014) 9(6):e100633. doi: 10.1371/journal.pone.0100633
29. Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, Progesterone, Immunomodulation, and COVID-19 Outcomes. Endocrinology (2020) 161(9):bqaa127. doi: 10.1210/endocr/bqaa127
30. Ting D, Zhang J, Wang T, Cui P, Chen Z, Jiang J, et al. A Multi-Hospital Study in Wuhan, China: Protective Effects of Non-Menopause and Female Hormones on SARS-CoV-2 Infection. medRxiv (2020), 20043943. doi: 10.1101/2020.03.26.20043943
31. Bukowska A, Spiller L, Wolke C, Lendeckel U, Weinert S, Hoffmann J, et al. Protective Regulation of the ACE2/ACE Gene Expression by Estrogen in Human Atrial Tissue From Elderly Men. Exp Biol Med (2017) 242(14):14. doi: 10.1177/1535370217718808
32. Peretz J, Pekosz A, Lane AP, Klein SL. Estrogenic Compounds Reduce Influenza A Virus Replication in Primary Human Nasal Epithelial Cells Derived From Female, But Not Male, Donors. Am J Physiol Lung Cell Mol Physiol (2016) 310:415–25. doi: 10.1152/ajplung.00398.2015.-Influenza
33. Simpson ER. Sources of Estrogen and Their Importance. J Steroid Biochem Mol Biol (2003) 86:225–30. doi: 10.1016/S0960-0760(03)00360-1
34. Murata Y, Robertson KM, Jones MEE, Simpson ER. Effect of Estrogen Deficiency in the Male: The ArKO Mouse Model. Mol Cell Endocrinol (2002) 193:7–12. doi: 10.1016/s0303-7207(02)00090-4
35. Di Stadio A, Della Volpe A, Ralli AM, Ricci G. Gender Differences in COVID-19 Infection. The Estrogen Effect on Upper and Lower Airways. Can It Help to Figure Out a Treatment? Eur Rev Med Pharmacol Sci (2020) 24:5195–6. doi: 10.26355/eurrev_202005_21298
36. Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, et al. Sex Differences in Immune Responses to SARS-CoV-2 That Underlie Disease Outcomes. Nature (2020) 588:315–20. doi: 10.1038/s41586-020-2700-3
37. Barnard DL, Craig WD, Bailey K, Heiner M, Montgomery R, Lauridsen L, et al. Evaluation of Immunomodulators, Interferons and Known In Vitro SARS-CoV Inhibitors for Inhibition of SARS-CoV Replication in BALB/c Mice. Antivir Chem Chemother (2006) 17:275–84. doi: 10.1177/095632020601700505
38. Sainz B Jr., Mossel EC, Peters CJ, Garry RF. Interferon-Beta and Interferon-Gamma Synergistically Inhibit the Replication of Severe Acute Respiratory Syndrome-Associated Coronavirus (SARS-CoV). Virology (2004) 329(1):11–7. doi: 10.1016/j.virol.2004.08.011
39. Torcia MG, Nencioni L, Clemente AM, Civitelli L, Celestino I, Limongi D, et al. Sex Differences in the Response to Viral Infections: TLR8 and TLR9 Ligand Stimulation Induce Higher IL10 Production in Males. PloS One (2012) 7(6):e39853. doi: 10.1371/journal.pone.0039853
40. Friedl R, Brunner M, Moeslinger T, Spieckermann PG. Testosterone Inhibits Expression of Inducible Nitric Oxide Synthase in Murine Macrophages. Life Sci (2000) 68(4):417–29. doi: 10.1016/S0024-3205(00)00953-X
41. Trigunaite A, Dimo J, Jørgensen TN. Suppressive Effects of Androgens on the Immune System. Cell Immunol (2015) 294(2):87–94. doi: 10.1016/j.cellimm.2015.02.004
42. Micallef MJ, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, et al. Interferon-γ-Inducing Factor Enhances T Helper 1 Cytokine Production by Stimulated Human T Cells: Synergism With Interleukin-12 for Interferon-γ Production. Eur J Immunol (1996) 26(7):1647–51. doi: 10.1002/eji.1830260736
43. Kissick HT, Sanda MG, Dunn LK, Pellegrini KL, On ST, Noel JK, et al. Androgens Alter T-Cell Immunity by Inhibiting T-Helper 1 Differentiation”. Proc Natl Acad Sci USA (2014) 111(27):9887–92. doi: 10.1073/pnas.1402468111
44. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex Immune Dysregulation in COVID-19 Patients With Severe Respiratory Failure. Cell Host Microbe (2020) 27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009
45. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA (2020) 323(16):1574–81. doi: 10.1001/jama.2020.5394
46. Zhang X, Yun T, Yun L, Gang L, Feng L, Zhigang Y, et al. Viral and Host Factors Related to the Clinical Outcome of COVID-19. Nature (2020) 323(16):1574–81. doi: 10.1038/s41586-020-2355-0
47. Klein SL, Flanagan KL. Sex Differences in Immune Responses. Nat Rev Immunol (2016) 16:626–38. doi: 10.1038/nri.2016.90
48. Harman S. Ebola, Gender and Conspicuously Invisible Women in Global Health Governance. Third World Q (2016) 16(10):626–38. doi: 10.1080/01436597.2015.1108827
49. Breslin N, Baptiste C, Gyamfi-Bannerman C, Miller R, Martinez R, Bernstein K, et al. Coronavirus Disease 2019 Infection Among Asymptomatic and Symptomatic Pregnant Women: Two Weeks of Confirmed Presentations to an Affiliated Pair of New York City Hospitals. Am J Obstet Gynecol MFM (2020) 37(3):100118. doi: 10.1016/j.ajogmf.2020.100118
50. Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. Hospitalization Rates and Characteristics of Patients Hospitalized With Laboratory-Confirmed Coronavirus Disease 2019 - Covid-Net, 14 States, March 1–30, 2020. Morbid Mortal Wkly Rep (2020) 2(2):100118. doi: 10.15585/MMWR.MM6915E3
51. Sutton D, Fuchs K, D’Alton M, Goffman D. Universal Screening for SARS-CoV-2 in Women Admitted for Delivery. N Engl J Med (2020) 382(22):2163–4. doi: 10.1056/NEJMc2009316
52. Kotlar B, Gerson E, Petrillo S, Langer A, Tiemeier H. The Impact of the COVID-19 Pandemic on Maternal and Perinatal Health: A Scoping Review. Reprod Health (2021) 1(18):1–39. doi: 10.1186/s12978-021-01070-6
53. Guasch E, Brogly N, Gilsanz F. COVID in Obstetrics: Labor Analgesia and Cesarean Section. Curr Opin Anaesthesiol (2021) 34(1):62–8. doi: 10.1097/ACO.0000000000000949
54. Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, et al. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb Haemost (2020) 120(6):998–1000. doi: 10.1055/s-0040-1710018
55. Nabulsi AA, Folsom AR, White A, Patsch W, Heiss G, Wu KK, et al. Association of Hormone-Replacement Therapy With Various Cardiovascular Risk Factors in Postmenopausal Women. The Atherosclerosis Risk in Communities Study Investigators. N Engl J Med (1993) 328(15):1069–75. doi: 10.1056/NEJM199304153281501
56. Wambier CG, Goren A, Vaño-Galván S, Ramos PM, Ossimetha A, Nau G, et al. Androgen Sensitivity Gateway to COVID-19 Disease Severity. Drug Dev Res (2020) 81(7):771–6. doi: 10.1002/ddr.21688
57. Moradi F, Behnaz E, Ghadiri-Anari A. The Role of Androgens in COVIcD-19. Diabetes Metab Syndr (2020) 14(6):2003–6. doi: 10.1016/j.dsx.2020.10.014
58. Schroeder M, Tuku B, Jarczak D, Nierhaus A, Bai T, Jacobsen H, et al. The Majority of Male Patients With COVID-19 Present Low Testosterone Levels on Admission to Intensive Care in Hamburg, Germany: A Retrospective Cohort Study. medRxiv (2020) 14(6):20073817. doi: 10.1101/2020.05.07.20073817
59. Mohamed MS, Moulin TC, Schiöth HB. Sex Differences in COVID-19: The Role of Androgens in Disease Severity and Progression. Endocrine (2020) 71(1):3–8. doi: 10.1007/s12020-020-02536-6
60. Salciccia S, Del Giudice F, Eisenberg ML, Mastroianni CM, De Berardinis E, Ricciuti GP, et al. Androgen-Deprivation Therapy and SARS-Cov-2 Infection: The Potential Double-Face Role of Testosterone. Ther Adv Endocrinol Metab (2020) 11:2042018820969019. doi: 10.1177/204201882096901
61. Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, et al. Low Testosterone Levels Predict Clinical Adverse Outcomes in SARS-CoV-2 Pneumonia Patients. Andrology (2020) 9(1):88–98. doi: 10.1111/andr.12821
62. Subramanian A, Anand A, Adderley NJ, Okoth K, Toulis KA, Gokhale K, et al. Increased COVID-19 Infections in Women With Polycystic Ovary Syndrome: A Population-Based Study. Eur J Endocrinol (2021) 184(5):637–45. doi: 10.1530/EJE-20
63. Kyrou I, Karteris E, Robbins T, Chatha K, Drenos F, Randeva HS. Polycystic Ovary Syndrome (PCOS) and COVID-19: An Overlooked Female Patient Population at Potentially Higher Risk During the COVID-19 Pandemic. BMC Med (2020) 18(1):220. doi: 10.1186/s12916-020-01697-5
64. Culebras E, Hernández F. ACE2 Is on the X Chromosome: Could This Explain COVID-19 Gender Differences? Eur Heart J (2020) 41(32):3095. doi: 10.1093/eurheartj/ehaa521
65. Purdie A, Hawkes S, Buse K, Onarheim K, Aftab W, Low N, et al. Sex, Gender and Covid-19: Disaggregated Data and Health Disparities. BMJ Glob Health Blogs (2020) 41(32):3095.
66. Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JGF, et al. Circulating Plasma Concentrations of Angiotensin-Converting Enzyme 2 Inmen and Women With Heart Failure and Effects of Renin-Angiotensin-Aldosterone Inhibitors. Eur Heart J (2020) 41(19):1810–7. doi: 10.1093/eurheartj/ehaa373
67. Majdic G. Could Sex/Gender Differences in ACE2 Expression in the Lungs Contribute to the Large Gender Disparity in the Morbidity and Mortality of Patients Infected With the SARS-CoV-2 Virus? Front Cell Infect Microbiol (2020) 10:327. doi: 10.3389/fcimb.2020.00327
68. Carrel L, Willard HF. X-Inactivation Profile Reveals Extensive Variability in X-Linked Gene Expression in Females. Nature (2005) 434(7031):400–4. doi: 10.1038/nature03479
69. Talebizadeh Z, Simon SD, Butler MG. X Chromosome Gene Expression in Human Tissues: Male and Female Comparisons. Genomics (2006) 88(6):675–81. doi: 10.1016/j.ygeno.2006.07.016
70. Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature (2020) 581(7807):215–20. doi: 10.1038/s41586-020-2180-5
71. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A Crucial Role of Angiotensin Converting Enzyme 2 (ACE2) in SARS Coronavirus-Induced Lung Injury. Nat Med (2005) 11(8):875–9. doi: 10.1038/nm1267
72. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-Cell RNA Expression Profiling of ACE2, the Putative Receptor of Wuhan 2019-NCov. bioRxiv (2020) 11(8):919985. doi: 10.1101/2020.01.26.919985
73. da Silva JS, Gabriel-Costa D, Wang H, Ahmad S, Xuming S, Jasmina V, et al. Blunting of Cardioprotective Actions of Estrogen in Female Rodent Heart Linked to Altered Expression of Cardiac Tissue Chymase and ACE2. J Renin Angiotensin Aldosterone Syst (2017) 18(3):1470320317722270. doi: 10.1177/1470320317722270
74. Rehman S, Majeed T, Azam Ansari M, Ali U, Sabit H, Al-Suhaimi EA. Current Scenario of COVID-19 in Pediatric Age Group and Physiology of Immune and Thymus Response. Saudi J Biol Sci (2020) 27(10):2567–73. doi: 10.1016/j.sjbs.2020.05.024
75. Aztatzi-Aguilar OG, Uribe-Ramírez M, Montaño JA, Barbier O, De Vizcaya-Ruiz A. Acute and Subchronic Exposure to Air Particulate Matter Induces Expression of Angiotensin and Bradykinin-Related Genes in the Lungs and Heart: Angiotensin-II Type-I Receptor as a Molecular Target of Particulate Matter Exposure. Part Fibre Toxicol (2015) 12:17. doi: 10.1186/s12989-015-0094-4
76. Patel SK, Velkoska K, Burrell LM. Emerging Markers in Cardiovascular Disease: Where Does Angiotensin-Converting Enzyme 2 Fit in? Clin Exp Pharmacol Physiol (2013) 40(8):551–9. doi: 10.1111/1440-1681.12069
77. Leong HN, Earnest A, Lim HH, Chin CF, Tan C, Puhaindran ME, et al. SARS in Singapore - Predictors of Disease Severity. Ann Acad Med Singap (2006) 35(5):326–31. doi: 10.1111/1440-1681.12069
78. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 Expression and Sex Disparity in COVID-19. Cell Death Discov (2020) 6:37. doi: 10.1038/s41420-020-0276-1
79. Groban L, Wang H, Sun X, Ahmad S, Ferrario CM. Is Sex a Determinant of COVID-19 Infection? Truth or Myth? Curr Hypertens Rep (2020) 22(9):62. doi: 10.1007/s11906-020-01073-x
80. Raoult D, Hsueh PR, Stefani S, Rolain JM. COVID-19 Therapeutic and Prevention. Int J Antimicrob Agents (2020) 55(4):105937. doi: 10.1016/j.ijantimicag.2020.105937
81. Maslowski KM, Charles RM. Diet, Gut Microbiota and Immune Responses. Nat Immunol (2011) 12(1):5–9. doi: 10.1038/ni0111-5
82. Thaiss CA, Zmora N, Levy M, Elinav E. The Microbiome and Innate Immunity. Nature (2016) 535(7610):65–74. doi: 10.1038/nature18847
83. Postler TS, Ghosh S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab (2017) 26(1):110–30. doi: 10.1016/j.cmet.2017.05.008
84. Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D. Regulation of Inflammation by Microbiota Interactions With the Host. Nat Immunol (2017) 18:851–60. doi: 10.1038/ni.3780
85. Johnson BA, Hage A, Kalveram B, Mears M, Plante JA, Rodriguez SE, et al. Peptidoglycan-Associated Cyclic Lipopeptide Disrupts Viral Infectivity. J Virol (2019) 93(22):e01282. doi: 10.1128/JVI.01282-19
86. Donati Zeppa S, Agostini D, Piccoli G, Stocchi V, Sestili P. Gut Microbiota Status in COVID-19: An Unrecognized Player? Front Cell Infect Microbiol (2020) 10:576551. doi: 10.3389/fcimb.2020.576551
87. Belkaid Y, Harrison OJ. Homeostatic Immunity and the Microbiota. Immunity (2017) 18(46):562–76. doi: 10.1016/j.immuni.2017.04.008
88. Zhang D, Li S, Wang N, Tan HY, Zhang Z, Feng Y. The Cross-Talk Between Gut Microbiota and Lungs in Common Lung Diseases. Front Microbiol (2020) 11:301. doi: 10.3389/fmicb.2020.00301
89. Han Y, Jia Z, Shi J, Wang W, He K. The Active Lung Microbiota Landscape of COVID-19 Patients. medRxiv (2020) 11:20144014. doi: 10.1101/2020.08.20.20144014
90. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients With COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet (2020) 395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3
91. Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology (2020) 159(3):944–55. doi: 10.1053/j.gastro.2020.05.048
92. Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker B, et al. Gender Bias in Autoimmunity Is Influenced by Microbiota. Immunity (2013) 39:400–12. doi: 10.1016/j.immuni.2013.08.013
93. Dominianni C, Sinha R, Goedert JJ, Pei Z, Yang L, Hayes RB, et al. Sex, Body Mass Index, and Dietary Fiber Intake Influence the Human Gut Microbiome. PloS One (2015) 10:e0124599. doi: 10.1371/journal.pone.0124599
94. Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TK, et al. Sex Differences and Hormonal Effects on Gut Microbiota Composition in Mice. Gut Microbes (2016) 7(4):313–22. doi: 10.1080/19490976.2016.1203502
95. Kim YS, Unno T, Kim BY, Park MS. Sex Differences in Gut Microbiota. World J Mens Health (2020) 38:48–60. doi: 10.5534/wjmh.190009
96. Santos-Marcos JA, Haro C, Vega-Rojas A, Alcala-Diaz JF, Molina-Abril H, Leon-Acuña A, et al. Sex Differences in the Gut Microbiota as Potential Determinants of Gender Predisposition to Disease. Mol Nutr Food Res (2019) 63(7):e1800870. doi: 10.1002/mnfr.201800870
97. Oakes JM, Fuchs RM, Gardner JD, Lazartigues E, Yue X. Nicotine and the Renin-Angiotensin System. Am J Physiol Regul Integr Comp Physiol (2018) 315:R895–906. doi: 10.1152/ajpregu.00099.2018
98. Yue X, Basting TM, Flanagan TW, Xu J, Lobell TD, Gilpin NW, et al. Nicotine Downregulates the Compensatory Angiotensin-Converting Enzyme 2/Angiotensin Type 2 Receptor of the Renin-Angiotensin System. Ann Am Thorac Soc (2018) 15:S126–7. doi: 10.1513/AnnalsATS.201706-464MG
99. World Health Organization (WHO), Global Adult Tobacco Survey (GATS). Fact Sheet China 2018. Annals of the American Thoracic Society (2018).
100. Klein SL. The Effects of Hormones on Sex Differences in Infection: From Genes to Behavior. Neurosci Biobehav Rev (2000) 24:627–38. doi: 10.1016/S0149-7634(00)00027-0
101. Yang T, Barnett R, Jiang S, Yu L, Xian H, Ying J, et al. Gender Balance and Its Impact on Male and Female Smoking Rates in Chinese Cities. Soc Sci Med (2016) 154:9–17. doi: 10.1016/j.socscimed.2016.02.035
102. Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK, et al. ACE-2 Expression in the Small Airway Epithelia of Smokers and COPD Patients: Implications for COVID-19. Eur Respir J (2020) 55:2000688. doi: 10.1183/13993003.00688-2020
103. Nasiri MJ, Haddadi S, Tahvildari A, Farsi Y, Arbabi M, Hasanzadeh S, et al. COVID-19 Clinical Characteristics, and Sex-Specific Risk of Mortality: Systematic Review and Meta-Analysis. Front Med (2020) 7:459. doi: 10.3389/fmed.2020.00459
104. Martin SA, Pence BD, Woods JA. Exercise and Respiratory Tract Viral Infections. Exerc Sport Sci Rev (2009) 37:157–64. doi: 10.1097/JES.0b013e3181b7b57b
105. Brown AS, Davis JM, Murphy EA, Carmichael MD, Carson JA, Ghaffar A, et al. Gender Differences in Macrophage Antiviral Function Following Exercise Stress. Med Sci Sports Exerc (2006) 38:859–63. doi: 10.1249/01.mss.0000218125.21509.cc
106. Brown AS, . Davis MM, Murphy EA, Carmichael MD, Ghaffar A, Mayer EP. Gender Differences in Viral Infection After Repeated Exercise Stress. Med Sci Sports Exerc (2004) 36:1290–5. doi: 10.1249/01.MSS.0000135798.72735.B3
107. Kawai K, Msamanga G, Manji K, Villamor E, Bosch RJ, Hertzmark E, et al. Sex Differences in the Effects of Maternal Vitamin Supplements on Mortality and Morbidity Among Children Born to HIV-Infected Women in Tanzania. Br J Nutr (2010) 103:1784–91. doi: 10.1017/S0007114509993862
108. Prentice S. They Are What You Eat: Can Nutritional Factors During Gestation and Early Infancy Modulate the Neonatal Immune Response? Front Immunol (2017) 8:1641. doi: 10.3389/fimmu.2017.01641
Keywords: COVID-19, environment, estrogen, immunity, gender, hormones, microbiota
Citation: Rehman S, Ravinayagam V, Nahvi I, Aldossary H, Al-Shammari M, Amiri MSA, Kishore U and Al-Suhaimi EA (2021) Immunity, Sex Hormones, and Environmental Factors as Determinants of COVID-19 Disparity in Women. Front. Immunol. 12:680845. doi: 10.3389/fimmu.2021.680845
Received: 15 March 2021; Accepted: 29 July 2021;
Published: 18 August 2021.
Edited by:
Chaofeng Han, Second Military Medical University, ChinaReviewed by:
Leiliang Zhang, Shandong First Medical University, ChinaDegang Yang, Tongji University, China
Copyright © 2021 Rehman, Ravinayagam, Nahvi, Aldossary, Al-Shammari, Amiri, Kishore and Al-Suhaimi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suriya Rehman, surrehman@iau.edu.sa; Ebtesam A. Al-Suhaimi, ealsuhaimi@iau.edu.sa
 Suriya Rehman
Suriya Rehman Vijaya Ravinayagam
Vijaya Ravinayagam Insha Nahvi3
Insha Nahvi3 Uday Kishore
Uday Kishore Ebtesam A. Al-Suhaimi
Ebtesam A. Al-Suhaimi