
Topical 5% Fluorouracil (5-FU) Prescribing Practices for the Treatment of Actinic Keratosis, Sun Damage and Superficial Non-Melanoma Skin Cancers at an Australian Tertiary Teaching Hospital
*Corresponding Author(s):
Charlotte VelikFlinders Medical Centre, Adelaide, Australia
Tel:+61 408826191,
Email:charlottevelik@bigpond.com
Abstract
Background/Objectives: Topical 5-FU is an effective treatment for Actinic Keratosis (AK) and Non-Melanoma Skin Cancer (NMSC) despite little evidence to support an optimum treatment regimen. This investigation reviewed 5-FU prescribing practices at an Australian tertiary teaching hospital to draw conclusions about clinical outcomes and guide future prescribing.
Methods: Retrospective review of 5-FU prescriptions dispensed at an Australian tertiary teaching hospital between April 1st 2018 and August 31st 2018.
Results: A total of 78 prescriptions were identified. The most common indication was AK (73%). The most common prescription was a twice daily dose (68%). There was great variation in treatment duration; the most common duration was two weeks (30%). Average follow-up timeframe was 11 weeks (σ = 13.2 weeks). Response to 5-FU was documented in 49% of follow-up appointments. Only 19.5% of patients had documented clinical resolution.
Conclusions: There was great variability in 5-FU prescribing practices, with very few prescriptions reflecting manufacturer recommendations. We suggest implementing a standardised 5-FU follow-up regimen to ensure earlier follow-up times based on expected clinical course of treatment. Less than half of patients had a clearly documented response, highlighting the need for standardised documentation practices.
Keywords
Actinic keratosis; Australia; Duration of therapy; Fluorouracil, Retrospective studies; Skin neoplasms, Treatment outcome
INTRODUCTION
Actinic Keratoses (AKs) are common skin lesions which arise as a result of chronic exposure to Ultraviolet (UV) radiation, and hence occur predominantly in older fair-skinned individuals [1]. Approximately “15-25% of [AKs] resolve spontaneously over a 12-month period” [2], however, “the risk of progressing to an invasive Squamous Cell Carcinoma (SCC) is in the range of 0.25-20% per year”. The uncertainty in determining which AKs will transform into invasive SCC in theory warrants treatment of all persisting or thickened lesions [2]. Topical 5-fluorouracil (5-FU) is an effective field therapy for AKs. It is also used in the setting of Non-Melanoma Skin Cancer (NMSC), Such as Squamous Cell Carcinoma (SCC) in situ, or Bowen’s disease. 5-FU induces premature cell death by interfering with DNA synthesis. In Australia, 5-FU is available as a 5% cream under the trade name Efudix [3]. The manufacturers recommend applying 5% cream once or twice daily until the ulceration stage [4], usually 3-4 weeks in total. In practice, however, there is great variability in treatment duration. Anticipated effects of 5-FU treatment range from application site erythema and crusting, to severe dermatitis, pain and ulceration, which may result in pa- tient non-compliance [2]. In fact, studies indicate that up to 97% of patients will experience at least one adverse effect [5], highlighting the importance of prescribing the correct dose and duration of treatment [6]. Evidence to support an optimum 5-FU treatment regimen that provides efficacy while minimising side-effects is lacking. The aim of this clinical investigation is to review 5-FU prescribing practices at an Australian tertiary teaching hospital to draw conclusions about optimal treatment regimens and guide future prescribing.
OBJECTIVE
To assess the topical 5% 5-FU prescribing practices at an Australian tertiary teaching hospital between April 1st 2018 and August 31st 2018 for the treatment of actinic keratosis, sun damage and superficial non-melanoma skin cancers in order to determine whether there is a treatment regimen that results in favourable patient outcomes.
METHOD PATIENTS
Adult patients were included in this investigation if they had been dispensed topical 5-FU from the hospital pharmacy between April 1st 2018 and August 31st 2018. This timeframe was selected to reflect the preference for prescribing 5-FU during the winter months in Australia, in order to minimise photosensitivity.
Prescriptions that were dispensed outside of the hospital pharmacy were not included. Prescriptions were used to identify paper-based patient case notes, which were reviewed by an investigator at the hospital. All recorded information was de-identified prior to statistical analysis. Ethics approval was sought.
Data extraction
The following data were analysed: demographics of the patient cohort, clinical indication for 5-FU, length of follow-up and details of the 5-FU prescription (prescriber, dosing frequency, application site, duration).
Outcome measures
The follow-up appointment documentation was analysed for mention of 5-FU response and resolution of lesions.
RESULTS
Patients
In all, 78 prescriptions of topical 5-FU were dispensed between April 1st 2018 and August 31st 2018. Of these prescriptions, nine were dispensed more than once during the five month period. Therefore, 69 patients were included in this investigation.
Demographics
The average patient age was 71 years. The male to female ratio was 2:1, consistent with previous studies indicating a higher prevalence of AKs in men than in women, likely attributable to differing degrees of sun exposure [5].
Indication
The most commonly reported indication was AK (73%), followed by Non-melanoma Skin Cancers (NMSCs), comprising one fifth of prescriptions (21%). The remaining 6% were not clearly documented.
Of the NMSCs, the majority were SCC (8), followed by Bowen’s disease (5) and then BCC (1). No nodular BCC were reported. 3 scripts did not specify the subtype of NMSC.
Prescribers
62 of 78 prescriptions were prescribed by the Dermatology Unit. The next largest group were the Plastic Surgery Unit, with 13 scripts. The remaining three scripts were classed as outliers. All three were prescribed by different medical inpatient teams.
Prescription
The most common prescription was a twice daily dose (68%). 15% of patients were prescribed a once daily dose. The remaining prescriptions were either not specified, not documented or were unique dosing regimens, such as twice daily dosing Monday - Friday only, or a combination of daily and twice daily dosing to different body sites.
30% of prescriptions were for two weeks duration. The next most common were 4 weeks duration (18%). 16% of prescriptions were 6 weeks duration. 12% were either not specified or not documented. The remaining 22% contained variable durations (ie. 4-6 weeks, 2-4 weeks). The shortest duration was 5 days; the longest duration was 8 weeks.
Follow-up timeframe
The average timeframe from initial prescription to clinic review was 11 weeks +/- 13 weeks 2 days. The longest follow-up timeframe was 52 weeks, and the shortest was one week. The follow-up timeframe was determined by calculating the time from the date of the 5-FU prescription, which corresponded with the initial clinic appointment, to the next attended appointment.
5-FU response
Response to 5-FU was documented in only 49% of follow-up appointments. Of the documented responses, 54% were reported as ‘good’, 33% as ‘moderate’ and the remaining 13% utilised variable terminology. Some examples include ‘brisk’, ‘very brisk with ulceration’, and in some cases, commentary regarding the application was used as a marker for response ie., ‘too thick application’. Only 19.5% of patients had documented clinical resolution.
DISCUSSION
There was great variability in 5-FU prescribing practices, with very few prescriptions reflecting manufacturer recommendations. 30% of prescriptions were for a shorter duration than recommended, reflecting more conservative prescribing and a heavy reliance on clinical review at the two-week mark prior to prescribing further 5-FU. The average follow-up timeframe of 11 weeks (σ = 13 w, 2 d) coincides with the anticipated time for clinical resolution [7]. However, adverse effects usually arise one week after treatment commences and subside two weeks after application has ceased [1]. In fact, complete resolution of lesions may not be evident for up to 2 months following the cessation of active treatment. Askew, et al. suggest the minimum time period post-treatment to ascertain short-term benefit is 8 weeks. The critical follow up timeframes are therefore the 2 week mark for peak inflammatory response, 6 week mark for resolution of this responseand 2 month mark for absolute resolution, an ideal follow-up regimen would be as follows (Figure 1):
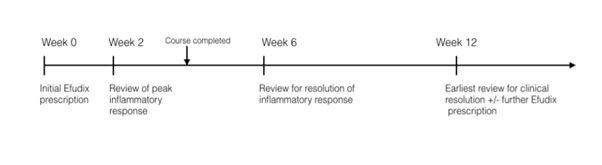 Figure 1: An example follow up timeline for a patient using 5-FU for duration of 4 weeks.
Figure 1: An example follow up timeline for a patient using 5-FU for duration of 4 weeks.
About two-thirds of patients can expect to require re-treatment after 1 year since completing a 5-FU course. Hence, most patients would require at least a 12 monthly review to assess for ongoing prescription or consideration of alternative treatments such as surgical intervention [8].
DOCUMENTATION
This investigation highlighted the arbitrary documentation of 5-FU response. It is difficult to assess whether patients achieved satisfactory treatment outcomes when less than half of responses were documented, and of the documented responses, there was a paucity of information regarding 5-FU response with only 19.5% of patients to have documented clinical resolution.
There appears to be insufficient information in the literature about how to describe an adequate response to 5-FU treatment. Terminology such as ‘good’, ‘moderate’ and ‘mild’ was employed by clinicians in this investigation to grade cutaneous responses, but there was no consensus regarding the reproducibility of these terms. A more objective measure would be to utilise clinical photography to document an individual’s response. This would also act as a visual reminder for the clinician that a patient had been prescribed 5- FU.
Inadequate clinical documentation is compounded further by delayed patient review and a lack of continuity of care. Some patients in this investigation had particularly prolonged review periods, some as long as 6-months to one year post-treatment, at which point a cutaneous response is expected to have completely resolved. There is also a high likelihood of the patient being reviewed by a clinician who was not involved in the initial 5-FU prescription. Clearly, there is a need for standardised documentation by way of a clinical pro forma.
Inpatient prescriptions
A surprising finding from this investigation was 5-FU being prescribed by inpatient medical subspecialty units. In examining these specific cases, a number of medication safety issues were identified.
All three patients had been prescribed 5-FU by an external provider, whether that be a general practitioner or specialist. Interestingly, on hospital admission, the 5-FU was charted as a continuing regular medication, despite the new prescriber lacking basic information about application site, dose and duration of treatment. It was particularly concerning to learn that an acutely confused patient with background chronic plaque psoriasis was prescribed 5-FU, but the directions read ‘apply to skin mane’ and did not specify the site. Of even more concern was the fact that this patient had completed her 5-FU course many weeks prior to hospital admission. Another patient was adamant that she no longer used 5-FU, however, the medication remained on her medication chart for the duration of her admission and the patient was left with little choice but to refuse each application. The third patient had 5-FU on his nursing home documentation, which was due to cease 4 weeks prior to hospital admission. Not only had the nursing home staff continued to apply 5-FU for an additional month past the anticipated cease date, but on hospital admission he was charted further 5-FU until this was identified by the pharmacist. These three inpatients have shone light on an otherwise overlooked source of 5-FU prescribers; inpatient units prescribing patient’s regular medications. Given the potential for error, particularly regarding medication safety, it would be beneficial for hospital based training to include education regarding safely prescribing topical agents, including 5-FU.
Limitations
One of the limitations of this investigation was the inability to track 5-FU prescriptions that were dispensed outside of the hospital pharmacy. Therefore, the data presented in this investigation may not be an accurate reflection of all patients being prescribed 5-FU at this hospital. Although, in considering the cost of 5-FU, this investigation is likely to have captured most patients in this socioeconomic group. It was also not possible to identify which patients had previously been prescribed 5-FU, which certainly would have impacted on patient adherence to treatment and self-reporting of adverse outcomes, if any, at follow-up appointments.
A further limitation of this investigation is that the lesions in most cases were diagnosed clinically without histological information, which may influence prescriber preferences regarding dose and duration of treatment. However, this investigation reflects a more common scenario of treating based on clinical judgement and is therefore more generalisable to current practice.
CONCLUSION
This investigation assessing the topical 5-FU prescriptions at an Australian tertiary teaching hospital found great variability regarding dose and duration, as well as follow-up timeframe. Very few prescriptions reflected manufacturer recommendations regarding duration. The average follow-up timeframe of 11 weeks, whilst appropriate for assessment of short-term resolution, does not allow for early monitoring of the inflammatory response or adverse reactions. We suggest implementing a standardised 5-FU follow-up regimen to ensure earlier follow-up times based on expected clinical course of treatment. It is yet to be determined whether more regular follow-up will lead to improved patient outcomes. This is an area that warrants further research. Furthermore, development of a clinical protocol would ensure adequate and clear documentation at these appointments. The use of topical 5-FU by inpatient medical teams also suggests the need for education directed at doctors who may not have specialist knowledge in this area. Despite its widespread use, there is a need for further studies correlating follow-up timeframes with long term 5-FU outcomes in order to determine an optimal treatment regimen for patients whilst minimising adverse events.
REFERENCES
- Dodds A, Chia A, Shumack S (2014) Actinic keratosis: rationale and management. Dermatol Ther (Heidelb) 4: 11-31.
- Askew DA, Mickan SM, Soyer HP, Wilkinson D (2009). Effectiveness of 5-fluorouracil treatment for actinic keratosis - a systematic review of randomized controlled trials. Int J Dermatol 48: 453-463.
- Jury CS, Ramraka-Jones VS, Gudi V, Herd RM (2005) A randomized trial of topical 5% 5?fluorouracil (Efudix® cream) in the treatment of actinic keratoses comparing daily with weekly treatment. Br J Dermatol 153: 808-810.
- Efudix (2018) In MIMS Online.
- de Oliveira EC, da Motta VR, Pantoja PC, Ilha CS, Magalhães RF, et al. (2019) Actinic keratosis-review for clinical practice. Int J Dermatol 58: 400-407.
- Love WE, Bernhard JD, Bordeaux JS (2009) Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: A systematic review. Arch Dermatol 145: 1431-1438.
- Gross K, Kircik L, Kricorian G (2007) 5% 5-Fluorouracil cream for the treatment of small superficial basal cell carcinoma: efficacy, tolerability, cosmetic outcome, and patient satisfaction. Dermatol Surg 33: 433-440.
- Askew DA, Mickan SM, Soyer HP, Wilkinson D (2009) Effectiveness of 5-fluorouracil treatment for actinic keratosis--A systematic review of randomized controlled trials. Int J Dermatol 48: 453-463.
Citation: Velik C, Marshman G (2020) A Topical 5% Fluorouracil (5-FU) Prescribing Practices for the Treatment of Actinic Keratosis, Sun Damage and Superficial Non-Melanoma Skin Cancers at an Australian Tertiary Teaching Hospital. J Clin Dermatol Ther 6: 059.
Copyright: © 2020 Charlotte Velik, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

