Abstract
Aim
The COVID-19 pandemic is a global health emergency, and therefore the prevention and treatment of this disease is an important priority of world health. In the present study, some risk factors, including unhealthy nutrition, obesity, and physical inactivity, were assessed in patients infected with SARS-CoV-2, and their effects on the severity and duration of disease were evaluated.
Subject and methods
The present study was a cross-sectional study. Data was collected from all patients who visited the respiratory emergency department from March 20, 2020 to April 24, 2020 in the University Hospital. The outcome measures were body mass index, diet quality that was evaluated with a 16-item food intake questionnaire, and physical activity level that was assessed by the global physical activity questionnaire.
Results
Two hundred and six patients’ data was analyzed. The results investigated that patients with lower levels of physical activity or lower MET.min/week were affected by a more severe form of the disease (p = 0.05 and p = 0.03, respectively). We found that patients with a healthier dietary pattern were affected by lower severity of illness (p < 0.05).
Conclusion
It seems that increasing levels of physical activity may partly reduce the severity of COVID-19 disease. Some dietary patterns such as increasing fruit and poultry consumption as well as drinking less tea were correlated significantly with a less severe form of the disease. The results did not confirm previous concerns regarding a potentially harmful effect of smoking on the severity or duration of symptoms.
Similar content being viewed by others
Introduction
COVID-19 was first described in late 2019 in Wuhan, China, according to the assessment of multiple cases of acute respiratory infection (Liu and Saif 2020). In fact, the causative pathogen of this disease is a mutant and novel virus from the coronavirus family called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which spread worldwide in a short period (Gorbalenya et al. 2020). Accordingly, on January 30, 2020 the World Health Organization (WHO) notified that the outbreak of COVID-19 disease was a global health emergency and on March 11, the disease was announced as a global pandemic (Gralinski and Menachery 2020; WHO 2005a).
Additionally, the first case was identified in Iran on February 20, and more than 120,000 confirmed cases, including more than 7000 deaths, had been reported as of May 6, 2020 by the Ministry of Health and Medical Education of Republic of Iran, which shows the gravity of the disease.
Also, early reports suggested that some characteristics such as a past medical history of CVD and older age increase the severity of disease in COVID-19 patients. Moreover, researchers reported that patients who suffer from diabetes, hypertension, cardiovascular and pulmonary disease, had a higher risk of severe illness caused by the virus and a subsequently higher rate of hospitalization and death (Huang et al. 2020; Wang et al. 2020; Yang et al. 2020). However, many other characteristics of this novel coronavirus and other risk factors for severity remain unclear.
An unhealthy diet (excess energy intake, high consumption of saturated fats and trans-fatty acids, low intake of fruit and vegetables, eating salty foods, etc.) appears to be a major driver of increases in the prevalence of obesity and chronic diseases (Kant 2010). In fact, studies suggest that some dietary patterns may affect inflammatory markers linked to low-grade systemic inflammation (Casas et al. 2014; Del Chierico et al. 2014). Generally, low-grade inflammation appears to be associated with IL-17 and IL-10 ratios while IL-17 plays an important role in host defense against infections by absorbing neutrophils and producing antimicrobial peptides (Weber et al. 2014).
On the other hand, the world has been living with other pandemics for several years—obesity and physical inactivity, which have been identified as the main leading contributors to premature mortality (Kohl 3rd et al. 2012; Popkin et al. 2012; Pratt et al. 2019; Swinburn et al. 2011)—and their prevalence is increasing noticeably worldwide in both developed and developing countries (Klein et al. 2002). Moreover, they are the primary causes of the rising prevalence of type 2 diabetes and the important comorbid states such as hypertension, cardiovascular, renal, and gastrointestinal diseases along with increasing the burden of cancers (Blair and Brodney 1999; Cecchini et al. 2010). Above all, in terms of physiopathology, excess adiposity leads to an increase in the circulating level of pro-inflammatory protein, leptin protein, and a decrease in adiponectin, which is an anti-inflammatory factor. Also, excess body fat is accompanied by stress in the endoplasmic reticulum and hypoxemia, which occurs in hypertrophic adipose tissue, and consequently stimulates the expression of inflammatory genes and provokes a chronic inflammatory response (Chandra 1997). Physical inactivity is both directly (Laddu et al. 2020) and indirectly associated with poor immune response, through the vicious cycle between inactivity and obesity (Pietiläinen et al. 2008). Hence, an unhealthy diet, obesity, and inactivity have a negative impact on immune function and the host defense.
In sum, it seems that important risk factors that have been mentioned earlier have potential effects on the likelihood of infection and the severity of the disease. Thus, in the present cross-sectional study, the risk factors including unhealthy nutrition, obesity, and physical inactivity were assessed under the title of “lifestyle” among patients infected with SARS-CoV-2, and their effects on the severity of COVID-19 disease and the duration of the symptoms were evaluated in patients referred to University Hospital.
Methods
Study design
The present study was a single-center, cross-sectional study. In the current study, data was collected from all inpatients and outpatients who were visited in the respiratory emergency department from March 20, 2020 to April 24, 2020. The study was approved by the review board and the Ethical Committee of Tehran University of Medical Sciences. The patient’s privacy was protected by an anonymous identification code, while the electronic data was stored in a locked, password-protected computer.
Population
The inclusion criteria were patients aged between 18 and 75 years and one of the following criteria: (1) positive real-time reverse transcriptase- polymerase-chain-reaction (RT-PCR) assay of nasopharyngeal or oropharyngeal swab specimens; (2) chest computed tomography scan (CT scan) confirming infection. In addition, the exclusion criteria were: (1) patients with any immune compromising conditions, including longstanding use of corticosteroids, chemotherapy, HIV positive, and organ transplantation; (2) patients who had underlying diseases, including cardiovascular diseases, hypertension, diabetes mellitus, and any other respiratory diseases; (3) do not consent to participate in the study.
Data collection
Initally, primary data was collected from medical records of all inpatients and outpatients, including information regarding demographical, clinical, laboratory, and radiological characteristics in addition to disease progression and symptoms duration. Next, further information was obtained by calling the patients. First, patients consented to participate in the project and then completed the questionnaires through answering the questions asked by the interviewer.
Severity
All patients were classified into mild to very severe cases based on the results from symptoms, clinical examinations, and chest radiology criteria (According to clinical classification of COVID-19 released by the National Health Commission of China). Therefore, patients with mild symptoms (i.e., fever, cough, expectoration, and other upper respiratory tract symptoms) and without abnormalities on chest CT scans were classified as mild types. In addition, fever or respiratory symptoms with changes in chest radiology were classified as moderate cases (lung involvement of less than 50%). Finally, severe disease was defined by the presence of any of the following conditions: (1) Significantly increased respiration rate (RR): RR ≥ 30 times/min; (2) Hypoxia: oxygen saturation (resting state) ≤ 93%; (3); Blood gas analysis: PaO2 /FiO2 ≤ 300 mmHg, or when lung involvement in CT scan was reported greater than 50%. Very severe cases had one of the following criteria: (1) Occurrence of the respiratory failure that requires mechanical ventilation; (2) Septic shock; (3) Other organ failures that require intensive care unit (ICU) monitoring and treatment.
Outcome measures
The primary outcome measures were body mass index (BMI), diet quality, and physical activity level. Patients’ nutrient intake was evaluated by the 16-Item Food Intake Questionnaire, whose validity has been previously established (Hemiö et al. 2014). Additionally, the assessment of physical activity was based on the Global Physical Activity Questionnaire, which is a standardized questionnaire recommended by the World Health Organization (WHO) as a physical activity surveillance instrument (Bull et al. 2009). Further, the MET-minute score was calculated for each participant by multiplying the reported average time spent at each activity per week by the typical energy expenditure requirements for the activity (expressed in MET-minutes) (Ainsworth et al. 2000). The secondary outcome measure was smoking, which was recorded based on patients’ self-declarations.
Measurements
16-item food intake questionnaire
The 16-item food intake questionnaire is a practical tool for evaluating and estimating diet quality (Hemiö et al. 2014). The questionnaire consists of 16 questions of three types: there are six questions about number of meals per day, the serving size and frequency of consumption of fast foods, fruits, vegetables, sugar-rich foods, and sweets. In addition, four questions are about the amount of fat used in the daily diet, and the remaining questions are open-ended, including the number of different dishes per week (fish, sausage, chicken, meat, vegetable), milk, cheese, and cold cut products, bread and breakfast cereals, and beverages.
Global physical activity questionnaire (GPAQ)
The global physical activity questionnaire (GPAQ) is a standardized questionnaire developed by WHO as an international standard questionnaire (Bull et al. 2009; WHO 2005b). In particular, GPAQ comprises 16 questions grouped to capture various aspects of physical activity undertaken in three domains: work, commuting, and recreational activities. Also, the intensity (i.e., vigorous or moderate) of activity in each domain and the time spent on sedentary behaviors (e.g., sitting, watching TV) are determined. The Persian version of GPAQ has been used in many national and local studies as a valid and reliable questionnaire (Esteghamati et al. 2011; Fattahi et al. 2019).
Body mass index (BMI)
Anthropometric measures, including standing height and body weight, were measured at admission with light clothing and no shoes. In addition, height was measured to the nearest 0.1 cm and weight was measured to the nearest 0.1 kg. BMI was calculated as weight divided by height squared (kg/m2) with underweight defined as BMI lower than 18.5 kg/m2, normal weight as BMI 18.5–24.9 kg/m2, overweight as BMI 25.0–29.9 kg/m2, and obesity as BMI ≥30 kg/m2.
Statistical analysis
Before doing data analysis, normal distribution was tested using the Kolmogorov–Smirnov test. Quantitative and categorical variables were presented as mean (standard deviation) and frequency (percentage), respectively. In addition, to estimate within-group and between-group mean differences, paired and independent sample T-tests were used, and the qualitative data was analyzed by the Chi-square test. Also, logistic regression adjusted by age was applied for assessing the relationship between physical activity and severity and duration of disease and odds ratio and p value were reported. The statistical significance was defined as p < 0.05, and all analyses were performed using SPSS version 21.
Results
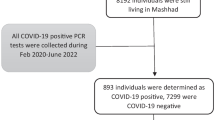
In the present study, data was collected from all inpatients and outpatients who visited the respiratory emergency department from March 20, 2020 to April 24, 2020. Among the 3870 patients’ profiles that were initially evaluated, 206 patients’ information was analyzed. The flowchart of the inclusion of the patients is shown in Fig. 1.
Table 1 shows the baseline demographic and characteristic of 206 patients with probable or definitive COVID-19 diagnosis. Also, the relationship between the parameters with the severity and duration of the disease is presented. To illustrate, women reported a longer duration of signs and symptoms than men. In addition, subgroup-analysis was done in the field of job and we did not find any differences between hospital workers and other jobs with regard to the severity or duration of disease (p = 0.50).
On the whole, the severity of the disease and duration of signs and symptoms were significantly related to each other (p = 0.00).
Correlation between physical activity according to METs.min/week and the level of physical activity (low and moderate to high) with intensity and duration of the disease is shown in Table 2.
Consumption frequency of confectionaries and sugar, sausage, fast foods, salad dressings (vegetable oil, mayonnaise, etc.), butter or cream with breakfast, cheese, type and amount of oils, as well as white or mixed grains was not different in moderate and severe groups. In addition, there was no difference between the groups in terms of consumption of low-fat or high-fat milk and dairy products (p ˃ 0.05). Also, the mentioned items had no significant effect on the duration of symptoms and signs. The details of the food questionnaire and differences between groups are shown in Table 3.
Discussion
This study involved 206 people with a probable or definitive diagnosis of COVID-19 in order to determine the respective effects of a healthy lifestyle, including physical activity and dietary pattern on the severity and duration of the signs and symptoms.
Because we used CT scan findings and positive PCR tests for inclusion criteria, mild cases were not included in the study. On the other hand, critical patients were not seen in our results probably due to exclusion of high-risk patients.
The results investigated that physical inactivity was significantly associated with the severity of COVID-19 disease. As an illustration, patients with lower levels of physical activity or lower MET.min/week were affected by a more severe form of the disease (mean 343.6 vs 779.3 MET.min/week) (p = 0.03) Table 2. Furthermore, the correlation between duration of signs and symptoms and physical activity suggested that patients with low physical activity suffered from the more prolonged illness compared with groups with moderate to a high level of physical activity, however, it was not significant between groups (666.4 vs 813.7 METs.min/week) (p ˃ 0.05). In addition to these associations, we found that patients with a healthier dietary pattern, including more consumption of poultry and fruits also less drinking of tea, had lower severity of the disease (p < 0.05). Also, it should be stressed that the results did not confirm previous concerns regarding a potentially harmful effect of higher BMI on the severity or duration of symptoms. Additionally, our results showed that women suffered from a longer duration of symptoms than men.
Physical activity
The relation between physical activity and respiratory tract infections is controversial and it is mostly correlated with exercise intensity (Baik et al. 2000; Chubak et al. 2006; Neuman et al. 2010; Nieman 2000; Nieman et al. 2011; Nieman et al. 2000). In fact, although high-intensity and long-duration exercise may increase the risk of upper respiratory tract infections (Murphy et al. 2008; Nieman 2000), data on moderate physical activity in the general population are limited (Selk-Ghaffari 2020). To illustrate, in a randomized controlled study involving 36 women, the risk of upper respiratory tract infections was reduced during moderate exercises (Nieman et al. 1990). Also, in another study that followed 1002 adults for 12 weeks during winter and fall seasons, the level of physical fitness and frequency of aerobic exercise program per week was important and correlated with reduced severity of upper respiratory tract infection (URTI) and the duration of symptoms (Nieman et al. 2011). Recently, a study with large sample size (n = 387,109) was published in favor of physical activity effect on reducing risk of hospital admission of COVID-19 patients, but the assessment was only in the last week before the disease (Mark et al. 2020). However, in a new large cross-sectional study, the authors concluded that regular sports participation may decrease the disease severity. In addition, hospitalization of athletes with regular sports participation was 33% lower than nonathletic group (Halabchi et al. 2020).
On the whole, consistent with the previously mentioned studies, the present results found a significant correlation between physical activity and decrease in severity of illness among COVID-19 patients. However, we did not observe a statistically significant association between moderate to high physical activity and duration of signs and symptoms, Table 2.
In fact, several physiological processes have been suggested to explain the relationship between physical activity and improved immune and lung function (Barrett et al. 2018; Chubak et al. 2006; Nieman et al. 2011; Obasi et al. 2013). In addition, although lymphocytosis is often associated with acute bouts of exercise, some studies reveal the little effect of training on lymphocyte concentrations (Nieman 2000; Nieman et al. 2000). Physical activity can affect different parts of the immune system in different ways. In particular, innate and acquired immunity based on cellular and humeral components are often measured using functional (e.g., natural killer cell cytotoxic activity) and non-functional (e.g., natural killer cell concentrations) indices; however, these indices do not always respond to the same exercise in a similar way (Nieman 2000). For instance, enhancing natural killer cell activity was reported following moderate physical activity (Nieman et al. 1990; Nieman 2000). Indeed, the improved immune function that is induced by moderate exercise intensity can reduce the influx of inflammatory cells into the lungs, decrease the pathogen load, improve the disease outcome, and decrease the chemokine and pro-inflammatory cytokines in the lungs or bronchoalveolar lavage fluid (Kohut et al. 2009; Lowder et al. 2006; Warren et al. 2015). Also, in some animal studies, the correlation between intensity of exercise and influenza infections was evaluated, for example, in two different studies in mice, both increase in susceptibility with intense exercise (Murphy et al. 2008) and decrease in susceptibility with moderate exercise were reported (Lowder et al. 2005).
In summary, it seems that moderate intensity of physical activity can reduce respiratory tract infections. In addition, according to the present results, increased level of physical activity and METs.min/week had a reverse correlation with COVID-19 disease severity.
Dietary pattern
In particular, it is probable that a healthy pattern of diet decreases the risk of severe COVID-19 as some previous studies on influenza patients showed that a healthy diet could decrease the hospitalization rate of the patients (Charland et al. 2013). On the other hand, an unhealthy diet could induce systemic inflammation due to changes in some inflammatory markers (Casas et al. 2014; Del Chierico et al. 2014). In fact, until now, the authors did not find research regarding the effect of nutritional habits on the severity or duration of COVID-19. However,, our results indicated that patients with lower consumption of poultry and fruit in the usual diet had a more severe form of the disease. In addition, the likelihood of having severe disease appeared to be higher in patients who drink more tea. Therefore, some parts of our results are in line with previous reports that were conducted on influenza patients (Charland et al. 2013), as Charland reported that the prevalence of low consumption of fruits and vegetables and physical inactivity was associated with more influenza hospitalization (Charland et al. 2013).
Consequently, although further work needs to be done on the relationship between the usual dietary pattern of COVID-19 patients with the severity and duration of the disease, according to results of the present study, healthy nutrition such as further consumption of fruits can be recommended to prevent the severe form of COVID-19.
Moreover, the effect of tea consumption especially green tea on the immune system, influenza, and upper respiratory tract infections has been evaluated in previous studies (Furushima et al. 2018; Furushima et al. 2019; Ide et al. 2017). However, there is some controversy about the effect of tea on immunity and influenza or common cold (Furushima et al. 2018). Therefore, in the present study, we evaluated black tea and the results showed that a history of drinking large amounts of this type of tea is related to an increase in severity of COVID-19.
Body mass index (BMI)
The results of the association between body mass index and acute respiratory infection in previous studies are inconsistent (Kornum et al. 2010). For instance, a few recent studies that assessed the effect of obesity on the severity of COVID-19 disease found an increased risk associated with obesity (Qingxian et al. 2020; Simonnet et al. 2020). Also, a study in China showed that obesity, particularly in men, could increase the risk of severe pneumonia in patients with COVID-19 (Qingxian et al. 2020). Therefore, the authors concluded that individuals with obesity were more likely to progress to severe pneumonia due to SARS-CoV-2 infection while they did not find significant differences in obese patients’ groups in terms of the duration of the disease. In addition, Petrilli found an association between obesity (BMI > 40) and hospitalization risk of COVID-19 patients in New York City (Petrilli et al. 2020). Indeed, such discrepancy between the results of the previous studies and the present work is probably due to the inclusion and exclusion criteria differences. More importantly, we did not include the high-risk patients according to WHO guidelines (high-risk patients’ classification), since BMI more than 40 kg/m2 was defined as a high-risk condition as well as other risk factors in the classification. Therefore, morbid obese patients with BMI more than 40 were not included in the study. In summary, it seems that obesity without a history of high-risk diseases should not be considered as an independent risk factor for COVID-19 severity. Then, probably other comorbidity conditions with obesity such as cardiovascular disease are the main reasons for COVID 19 severity in the previous studies.
Smoking
Though some have speculated that high rates of smoking in China explained some of the severe symptoms in patients with COVID-19, we did not find smoking status to be associated with an increased risk of severity or duration of the disease. In fact, This finding is consistent with a handful of other studies that have previously shown a lack of association between smoking and pulmonary disease associated ARDS (i.e., from pneumonia), as compared with non-pulmonary sepsis-associated ARDS (Calfee et al. 2015). On the other hand, in a new large observational study, the incidence and severity of COVID-19 in smokers were compared with non-smokers (Israel et al. 2020), and the results showed that smoking had some protective effects on new coronavirus infection risk. In addition, they did not find any relationship between current or past smoking history and disease severity (Israel et al. 2020). In sum, because of the lack of enough long-term evidence regarding tobacco or nicotine consumption and the prevention or treatment of COVID-19 to date (WHO Statement 2020), future studies are needed for further conclusions and recommendations.
Strength and limitation
In particular, we explored novel risk factors for COVID-19 severity and duration and compared them with risk factors in pneumonia or ARDS.
Our data is subject to several limitations. First, the generalizability of the results is limited by the race and type of dietary pattern. Secondly, the sample size is not large, and finally, we excluded high-risk patients with a past medical history of diseases such as hypertension, diabetes, cardiovascular disease, or immune-deficiency disorders that could respond differently to risk factors.
Most importantly, this study strongly suggests that public health guidance should focus on promoting physical activity alongside other interventions during the pandemic to protect against COVID-19 severe infection.
Conclusion
In sum, it seems that increasing levels of physical activity may partly reduce the severity of COVID-19 disease. Additionally, some dietary patterns, such as further consumption of fruits, poultry, and drinking less tea, probably reduce the severity of the disease. In addition, obesity and smoking were not related to the severity or duration of symptoms. Hence, future studies should focus on lifestyle as a modifiable risk factor for controlling COVID-19.
References
Ainsworth BE et al (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32:S498–S516
Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW (2000) A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med 160:3082–3088
Barrett B et al (2018) Meditation or exercise for preventing acute respiratory infection (MEPARI-2): a randomized controlled trial. PLoS One 13:e0197778
Blair SN, Brodney S (1999) Effects of physical inactivity and obesity on morbidity and mortality: current evidence and research issues. Med Sci Sports Exerc 31:S646
Bull FC, Maslin TS, Armstrong T (2009) Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health 6:790–804
Calfee CS et al (2015) Cigarette smoke exposure and the acute respiratory distress syndrome. Crit Care Med 43:1790
Casas R, Sacanella E, Estruch R (2014) The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets (Formerly Current Drug Targets-Immune, Endocr Metab Disord) 14:245–254
Cecchini M, Sassi F, Lauer JA, Lee YY, Guajardo-Barron V, Chisholm D (2010) Chronic diseases: chronic diseases and development 3 tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet 376:1775–1784
Chandra RK (1997) Nutrition and the immune system: an introduction. Am J Clin Nutr 66:460S–463S
Charland KM, Buckeridge DL, Hoen AG, Berry JG, Elixhauser A, Melton F, Brownstein JS (2013) Relationship between community prevalence of obesity and associated behavioral factors and community rates of influenza-related hospitalizations in the United States. Influenza Other Respir Viruses 7:718–728
Chubak J et al (2006) Moderate-intensity exercise reduces the incidence of colds among postmenopausal women. Am J Med 119:937–942 e935
Del Chierico F, Vernocchi P, Dallapiccola B, Putignani L (2014) Mediterranean diet and health: food effects on gut microbiota and disease control. Int J Mol Sci 15:11678–11699
Esteghamati A, Khalilzadeh O, Rashidi A, Kamgar M, Meysamie A, Abbasi M (2011) Physical activity in Iran: results of the third national surveillance of risk factors of non-communicable diseases (SuRFNCD-2007). J Phys Act Health 8:27–35. https://doi.org/10.1123/jpah.8.1.27
Fattahi MRJD, Halabchi F, Golsoorat-Pahlaviani F, Abouzari F, Sajedi-Monfared (2019) Physical activity, sedentary behavior and correlates among students of Tehran University of Medical Sciences. Acta Med Iran 57:663–671
Furushima D, Ide K, Yamada H (2018) Effect of tea Catechins on influenza infection and the common cold with a focus on epidemiological/clinical studies. Molecules 23:1795. https://doi.org/10.3390/molecules23071795
Furushima D et al (2019) Prevention of acute upper respiratory infections by consumption of Catechins in healthcare workers: a randomized, placebo-controlled trial. Nutrients 12:4. https://doi.org/10.3390/nu12010004
Gorbalenya AE, Baker SC, Baric RS, Groot RJD, Drosten C, Gulyaeva AA, Haagmans BL (2020) The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5:536–544
Gralinski LE, Menachery VD (2020) Return of the coronavirus: 2019-nCoV. Viruses 12:135
Halabchi F et al (2020) Regular sports participation as a potential predictor of better clinical outcome in adult patients with COVID-19: a large cross-sectional study. J Phys Act Health 1–5. https://doi.org/10.1123/jpah.2020-0392
Hemiö K, Pölönen A, Ahonen K, Kosola M, Viitasalo K, Lindström J (2014) A simple tool for diet evaluation in primary health care: validation of a 16-item food intake questionnaire. Int J Environ Res Public Health 11:2683–2697
Huang C et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506
Ide K, Kawasaki Y, Akutagawa M, Yamada H (2017) Effects of green tea gargling on the prevention of influenza infection: an analysis using Bayesian approaches. J Altern Complement Med 23:116–120. https://doi.org/10.1089/acm.2016.0094
Israel A, Feldhamer I, Lahad A, Levin-Zamir D, Lavie G (2020) Smoking and the risk of COVID-19 in a large observational population study. medRxiv. https://doi.org/10.1101/2020.06.01.20118877
Kant AK (2010) Dietary patterns: biomarkers and chronic disease risk. Appl Physiol Nutr Metab 35:199–206
Klein S, Wadden T, Sugerman HJ (2002) AGA technical review on obesity. Gastroenterology 123:882–932
Kohl HW 3rd et al (2012) The pandemic of physical inactivity: global action for public health. Lancet 380:294–305
Kohut ML, Sim Y-J, Yu S, Yoon KJ, Loiacono CM (2009) Chronic exercise reduces illness severity, decreases viral load, and results in greater anti-inflammatory effects than acute exercise during influenza infection. J Infect Dis 200:1434–1442
Kornum JB et al (2010) Obesity and risk of subsequent hospitalisation with pneumonia. Eur Respir J 36:1330–1336
Laddu DR, Lavie CJ, Phillips SA, Arena R (2020) Physical activity for immunity protection: inoculating populations with healthy living medicine in preparation for the next pandemic. Prog Cardiovasc Dis. https://doi.org/10.1016/j.pcad.2020.04.006
Liu S-L, Saif L (2020) Emerging viruses without borders: the Wuhan coronavirus. Viruses 12:130. https://doi.org/10.3390/v12020130
Lowder T, Padgett DA, Woods JA (2005) Moderate exercise protects mice from death due to influenza virus. Brain Behav Immun 19:377–380
Lowder T, Padgett DA, Woods JA (2006) Moderate exercise early after influenza virus infection reduces the Th1 inflammatory response in lungs of mice. Exerc Immunol Rev 12:97–111
Mark H, Mika K, Catharine RG, G. David Batty (2020) Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun 87:184–187. https://doi.org/10.1016/j.bbi.2020.05.059
Murphy E, Davis J, Carmichael M, Gangemi J, Ghaffar A, Mayer E (2008) Exercise stress increases susceptibility to influenza infection. Brain Behav Immun 22:1152–1155
Neuman MI, Willett WC, Curhan GC (2010) Physical activity and the risk of community-acquired pneumonia in US women. Am J Med 123:281. e287–281. e211
Nieman DC (2000) Exercise effects on systemic immunity. Immunol Cell Biol 78:496–501
Nieman D et al (1990) The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int J Sports Med 11:467–473
Nieman DC et al (2000) Immune function in female elite rowers and non-athletes. Br J Sports Med 34:181–187
Nieman DC, Henson DA, Austin MD, Sha W (2011) Upper respiratory tract infection is reduced in physically fit and active adults. Br J Sports Med 45:987–992
Obasi CN et al (2013) Advantage of meditation over exercise in reducing cold and flu illness is related to improved function and quality of life. Influenza Other Respir Viruses 7:938–944
Petrilli CM et al (2020) Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv. https://doi.org/10.1101/2020.04.08.20057794
Pietiläinen KH et al (2008) Physical inactivity and obesity: a vicious circle. Obesity 16:409–414
Popkin BM, Adair LS, Ng SW (2012) Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 70:3–21
Pratt M, Varela AR, Salvo D, Kohl HW III, Ding D (2019) Attacking the pandemic of physical inactivity: what is holding us back? Br J Sport Med 54:760–762
Qingxian C et al (2020) Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care 43:1392–1398
Selk-Ghaffari FHZAM (2020) COVID-19 epidemic: exercise or not to exercise; that is the question! Asian J Sports Med 11:e102630
Simonnet A et al (2020) High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 28:1195–1199
Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL (2011) The global obesity pandemic: shaped by global drivers and local environments. Lancet 378:804–814
Wang D et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama 323:1061–1069
Warren KJ et al (2015) Exercise improves host response to influenza viral infection in obese and non-obese mice through different mechanisms. PLoS One 10:e0129713
Weber A, Zimmermann C, Kieseier B, Hartung HP, Hofstetter H (2014) Bacteria and their cell wall components uniformly co-activate interleukin-17-producing thymocytes. Clin Exp Immunol 178:504–515
WHO statement: Tobacco use and COVID-19 (2020) https://www.who.int/news-room/detail/11-05-2020-who-statement-tobacco-use-and-covid-19. Accessed 4 Dec 2020
WHO (2005a) Statement on the second meeting of the International Health Regulations. Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov). Accessed 30 Jan 2020
WHO (2005b) WHO STEPS surveillance manual: The WHO STEPwise approach to chronic disease risk factor surveillance. World Health Organization, Geneva. https://apps.who.int/iris/handle/10665/43376
Yang J et al (2020) Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis
Funding
This research was supported by Tehran University of Medical Sciences and Health Services.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by Zahra Tavakol, Mastaneh Rajabian Tabesh, Malihe Hassan Nezhad, and Sahar Karimpour Reyhan. Data collection and analysis were performed by Zahra Alizadeh, Pardis Noormohammadpour, Samaneh Akbarpour, and Farzin Halabchi. The first draft of the manuscript was written by Shima Ghannadi and Zahra Alizadeh, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the ethical committee at Tehran University of Medical Sciences, Tehran, Iran (IRCTID: IR.TUMS.VCR.REC.1399.151).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tavakol, Z., Ghannadi, S., Tabesh, M.R. et al. Relationship between physical activity, healthy lifestyle and COVID-19 disease severity; a cross-sectional study. J Public Health (Berl.) 31, 267–275 (2023). https://doi.org/10.1007/s10389-020-01468-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10389-020-01468-9