Association of Time–Varying Intensity of Ventilation With Mortality in Patients With COVID−19 ARDS: Secondary Analysis of the PRoVENT–COVID Study
- 1Department of Intensive Care, Amsterdam UMC, Location AMC, Amsterdam, Netherlands
- 2Department of Clinical Medicine, Trinity Centre for Health Sciences, Multidisciplinary Intensive Care Research Organization (MICRO), St James's Hospital, Dublin, Ireland
- 3Department of Anaesthesiology, Hospital Universitario y Politécnico la Fe, Valencia, Spain
- 4Mahidol Oxford Tropical Medicine Research Unit (MORU), Mahidol University, Bangkok, Thailand
- 5Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
- 6ACHIEVE, Centre of Applied Research, Amsterdam University of Applied Sciences, Faculty of Health, Amsterdam, Netherlands
- 7Australian and New Zealand Intensive Care Research Centre (ANZIC–RC), Monash University, Melbourne, VIC, Australia
- 8Department of Intensive Care, Austin Hospital, Melbourne, VIC, Australia
- 9Data Analytics Research and Evaluation (DARE) Centre, Austin Hospital, Melbourne, VIC, Australia
- 10Department of Critical Care, Melbourne Medical School, University of Melbourne, Melbourne, VIC, Australia
- 11Department of Critical Care Medicine, Hospital Israelita Albert Einstein, São Pãulo, Brazil
Background: High intensity of ventilation has an association with mortality in patients with acute respiratory failure. It is uncertain whether similar associations exist in patients with acute respiratory distress syndrome (ARDS) patients due to coronavirus disease 2019 (COVID−19). We investigated the association of exposure to different levels of driving pressure (ΔP) and mechanical power (MP) with mortality in these patients.
Methods: PRoVENT–COVID is a national, retrospective observational study, performed at 22 ICUs in the Netherlands, including COVID−19 patients under invasive ventilation for ARDS. Dynamic ΔP and MP were calculated at fixed time points during the first 4 calendar days of ventilation. The primary endpoint was 28–day mortality. To assess the effects of time–varying exposure, Bayesian joint models adjusted for confounders were used.
Results: Of 1,122 patients included in the PRoVENT–COVID study, 734 were eligible for this analysis. In the first 28 days, 29.2% of patients died. A significant increase in the hazard of death was found to be associated with each increment in ΔP (HR 1.04, 95% CrI 1.01–1.07) and in MP (HR 1.12, 95% CrI 1.01–1.36). In sensitivity analyses, cumulative exposure to higher levels of ΔP or MP resulted in increased risks for 28–day mortality.
Conclusion: Cumulative exposure to higher intensities of ventilation in COVID−19 patients with ARDS have an association with increased risk of 28–day mortality. Limiting exposure to high ΔP or MP has the potential to improve survival in these patients.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier: NCT04346342.
Introduction
The coronavirus disease 2019 (COVID−19) is an infection caused by the highly contagious Severe Acute Respiratory Coronavirus−2 (SARS–CoV−2), of which the first outbreak was reported in Wuhan, China in the beginning of December, 2019 (1). Worldwide, healthcare workers are faced with surges of infected patients who need hospitalization and eventually admission to an intensive care unit (ICU) because of need for invasive ventilation. The care of coronavirus disease 2019 (COVID−19) patients receiving ventilation is challenging and the outcomes are poor (2). Mortality rates as high as 50% have been reported in invasively ventilated COVID−19 patients that develop acute respiratory distress syndrome (ARDS) (2).
Different ventilation strategies have been studied in the setting of acute respiratory failure, and evidence from randomized clinical trials supports the use of ventilation with a low tidal volume (VT) and a low plateau pressure to decrease the risk of ventilator–induced lung injury (VILI) in patients with acute respiratory distress syndrome (ARDS) (3–5). There is increasing interest in limiting exposure to driving pressure (ΔP) and mechanical power (MP) in invasively ventilated patients (6–8). ΔP, the pressure applied by the ventilator to support the delivery of tidal volume, (7) is a surrogate for cyclic lung strain (9). MP, a parameter that combines several ventilatory variables, including respiratory rate (RR), VT, flow and ΔP, represents the amount of energy transferred from the ventilator to the respiratory system (8, 10). Associations of ΔP and MP with outcomes in patients receiving mechanical ventilation is well described (7, 8, 11–19).
A potential limitation of earlier studies is the inclusion of patients with spontaneous efforts during ventilation (18, 20). Even if appropriately adjusted for resistance, flow, and chest wall elastance, any estimate of these variables during spontaneous efforts would reflect both the ventilator's contribution and respiratory muscle activity, and thus would not represent the total energy imparted during inflation (20). Also, mechanical ventilation is a dynamic process, and thus far only one investigation studied the cumulative effect of ΔP and MP beyond baseline (12). To date, there have been no studies that assessed the impact of intensity of ventilation on outcome of COVID−19 patients with ARDS. To ascertain the effect of time–varying exposure to different levels of ΔP and MP on 28–day mortality in COVID−19 patients with ARDS, we conducted a secondary analysis of a national multicenter investigation, named the “PRactive of VENTilation in COVID−19” (PRoVENT–COVID) study. Our objectives were to estimate the effects of different levels of ΔP and MP over the first 4 days of ventilation on 28–day mortality in COVID−19 patients with ARDS, and whether there was a cumulative effect of exposure over time. We hypothesized that intensity of ventilation has an association with mortality in patients with COVID−19 ARDS, in a similar way as has been described in patients with ARDS from another cause.
Methods
Study Design and Participants
This is a preplanned secondary analysis of the “Practice of VENTilation in COVID−19” (PRoVENT–COVID) study, an investigator–initiated, multicenter, observational cohort study in patients with COVID−19 ARDS undertaken at 22 ICUs during the first 3 months of the pandemic in the Netherlands (21–23). The protocol of the PRoVENT–COVID study was prepublished, (21) and the statistical analysis plan for the current analysis, finalized before assessing the database, is available online (22). The institutional review boards of each participating center approved the study protocol, and need for individual patient informed consent was waived based on the observational nature of the study. Study sites were recruited through direct contact by members of the steering committee of the PRoVENT–COVID study. Study coordinators contacted the local doctors, trained and helped the data collectors, and monitored the study according to the International Conference on Harmonization Good Clinical Practice–guidelines. Integrity and timely completion of data collection was ensured by the study coordinators.
Consecutive patients aged 18 yr or older were eligible for participation if they were admitted to one of the participating ICUs and had received invasive mechanical ventilation for COVID−19 ARDS. The PRoVENT–COVID study itself had no exclusion criteria—for the current analysis we excluded patients if they had spontaneous breathing activity in more than half the observations, or when life status was unknown at day 28.
Procedures and Outcome
Demographics and data regarding premorbid diseases and home medication were collected at baseline. In the first hour of invasive ventilation, and every 8 h thereafter at fixed time points ventilator settings and parameters were collected up to day 4. Since plateau pressure was not recorded in the current study, all measurements of dynamic ΔP were calculated as peak inspiratory pressure (Ppeak) minus positive end–expiratory pressure. Dynamic MP was calculated as 0.098 * RR * VT * [Ppeak – (0.5 × dynamic ΔP)]. Both variables were calculated only considering moments without evidence of spontaneous breathing (additional information in eMethods in Supplementary Information). The Berlin definition for ARDS was used for classification of severity as mild, moderate and severe (24).
The primary outcome was 28–day mortality. In secondary analyses, we investigated whether the strength of association between intensity of ventilation and 28–day mortality changed over time. In addition, we quantified the effect of cumulative response, and we examined whether ARDS severity class changed the effects of time–varying ΔP and MP on 28–day mortality.
Analysis Plan
For the final assessment, patients receiving ventilation without evidence of spontaneous breathing at less than 50% of the available timepoints were identified and deselected (Supplementary Information eMethods). Continuous variables were reported as median (25th – 75th percentile) and compared with Wilcoxon rank–sum tests, and categorical variables as number and percentage, and compared with Fisher exact tests. The following variables were considered for adjustment in all models described below: age, gender, body mass index, PaO2 to FiO2 ratio, plasma creatinine, medical history of hypertension, heart failure, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, active hematological neoplasia and/or active solid tumor, use of angiotensin converting enzyme inhibitors, use of angiotensin II receptor blockers, use of a vasopressor or inotropes, fluid balance, arterial pH, mean arterial pressure, and heart rate. These baseline covariates were selected according to clinical relevance and as used in previous study (23). Finally, all continuous variables were standardized to interpret their effect on outcome in standard deviation units.
To estimate the association of subject–specific longitudinal profiles of either ΔP and MP with 28–day mortality, we used Bayesian joint models with shared random effects and adjusted for the covariates described above (25, 26). The repeated values of ΔP and MP (a maximum of 13 measurements) were included as time–varying exposure variables. Natural cubic splines were used in both the fixed–effects and random–effects models to account for the non-linearity of the longitudinal exposure profiles. To investigate whether the association between ΔP and MP and 28–day mortality changed over time, p–splines were included in an interaction term and presented in time–varying plots. Estimation of Bayesian joint model was done considering 28,000 iterations, 3,000 adapt, 3,000 burn–in, and 50 of thinning. Model diagnostics were done by visual inspection of the diagnostic plots. These results were presented as hazard ratios with corresponding 95% credible intervals (CrI).
To further expand the findings of the original model, 3 additional analyses were performed. First, the model described above was expanded to a multivariate joint model. In addition to the baseline covariates, the following time–varying covariates were included: daily use of prone positioning and daily use of inotropes or vasopressors. Second, the calculation of dynamic ΔP and MP were further restricted to moments when a neuromuscular blocking agent was administered, thereby decreasing the chance of including moments at which a patient was having spontaneous breathing activity. Third, patients with missing data in their 28–day vital status were not excluded but assessed in 2 different scenarios: i. best–case scenario (these patients were all considered alive at day 28); and ii. worst–case scenario (these patients were all considered to have died before or at day 28).
Three sensitivity analyses were added. First, to quantify the effect of cumulative exposure, we estimate the association between the percentage of moments with high ΔP and MP and 28–day mortality. The cut–off used to determine high ΔP was 15 cm H2O; the cut–off used for high MP was 17 J/min (7, 8, 12, 27). Second, we investigated the relationship between cumulative dose and 28–day mortality using the area under the ΔP and MP time curve above the thresholds described above divided by the number of hours of exposure, as a measure of dose. Using this definition, 1 cm H2O or 1 J/min of dose describes that a patient's average ΔP and MP were 1 cmH2O or 1 J/min per mL/cm H2O above the thresholds described for the duration of the exposure window. Third, we investigated the impact of time–weighted average ΔP and MP calculated as the area under the ΔP and MP time curve divided by the number of hours of exposure. For these 3 exposures, the impact on outcome was assessed using (shared–frailty) Cox proportional hazard models adjusted by the covariates described above.
The models were reassessed in a subgroup analysis according to ARDS severity at baseline (24). The models were repeated, considering an interaction between ΔP and MP and ARDS severity at baseline. The amount of missing data is <3% for the variables of interest, as shown in the Appendix (Supplementary Information Table 1). For the final models, missing data in covariates were imputed by the median due to the low number of missing, and in the repeated measurements of ΔP and MP over the days a linear imputation was used (Supplementary Information Figure 1). All analyses were performed using R version 4.0.2 (R Foundation for Statistical Computing), and a p < 0.05 was considered significant. P values for the Bayesian models were calculated as the tail probabilities using the formula 2 x min{P(θ > 0), P(θ <0)}, with θ denoting the corresponding regression coefficient from the survival submodel.
Results
From March 1, 2020, through June 1, 2020, 31 ICUs were invited for participation in the PRoVENT–COVID study, and 22 met inclusion criteria. A total of 1,340 individuals were screened. Of the 1,122 invasively ventilated COVID−19 patients, 734 (66.6%) were included in the current analysis (Supplementary Information Figure 2). Reasons for exclusion were evidence for presence of spontaneous breathing activity in more than 50% of available timepoints of data collection (n = 368), and unknown life status at day 28 (n = 20). Demographic characteristics and ventilation characteristics are presented in Table 1. The patients had a median age of 65 yr [IQR 57–72] and 194 (26%) of them were women. 8.9% of the patients had mild ARDS, and 57.8% and 33.4% moderate or severe ARDS, respectively. The most prevalent premorbid conditions were hypertension and diabetes. 29.2% of the patients died within the first 28 days of follow–up. Additional clinical outcomes and use of rescue therapies are presented in the Appendix (Supplementary Information Table 2).
In the first calendar day of ventilation, median ΔP was 14 (13–17) cm H2O, and median MP was 18.9 (15.7–22.8) J/min (Table 1). ΔP was > 15 cm H2O in 32.2% of the patients, and MP was > 17 J/min in 59.5% in 66.1% of the patients. ΔP and MP over the first 4 calendar days of ventilation are shown in Figure 1.
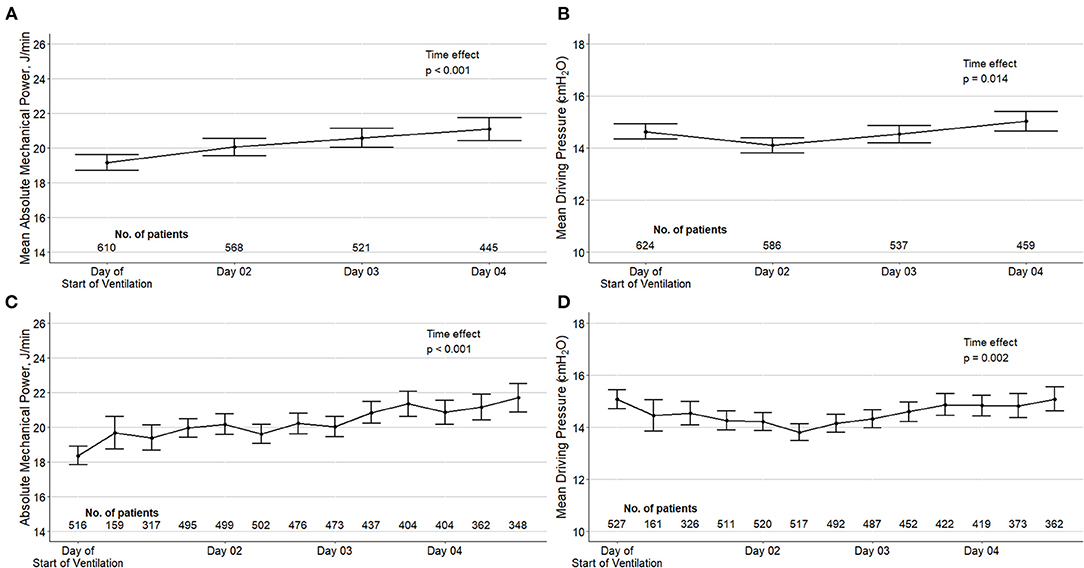
Figure 1. Driving pressure and mechanical power over the first four days of ventilation in the included patients. Top panels (A, B): mean daily values of ΔP and MP according to a maximum of four measurements in the day of start of ventilation and three measurements in the next days. Bottom panels (C, D): ΔP and MP per time point of assessment. Circles are means and error bars are 95% confidence intervals. Both variables were calculated using only measurements without spontaneous breathing activity. P values from a mixed-effect model with time as fixed effect (as continuous variable) and patients as random effect to account for repeated measurements.
In the first 4 calendar days of ventilation, and after adjusting for confounders, both a high time–varying ΔP (HR, 1.04 [95% CrI, 1.01–1.07]) and a high time–varying MP (HR, 1.12 [95% CrI, 1.01–1.36]) had an association with increased risk of 28–day mortality (Table 2). The strength of the association of ΔP as well as of MP with 28–day mortality decreased slightly over the first 4 days (Figure 2). As shown in the Appendix, the effect of time–varying ΔP was more pronounced in patients with ARDS classified as severe (Supplementary Information Figure 3). The full multivariable model is reported in Supplementary Information Table 3. After adjustment for the daily use of prone positioning and inotropes or vasopressors, both higher ΔP (HR, 1.04 [95% CrI, 1.00–1.09]) and higher MP (HR, 1.06 [95% CrI, 1.00–1.15]) had an association with increased risk of 28–day mortality (Table 2). When restricting the measurements to moment at which neuromuscular blocking agents were administered, both ΔP (HR, 1.38 [95% CrI, 1.14–1.61]) and MP (HR, 1.35 [95% CrI, 1.06–1.73]) had an association with 28–day mortality (Table 2). The findings of the best– and worst–case scenario confirmed the findings of the primary analysis (Table 2).
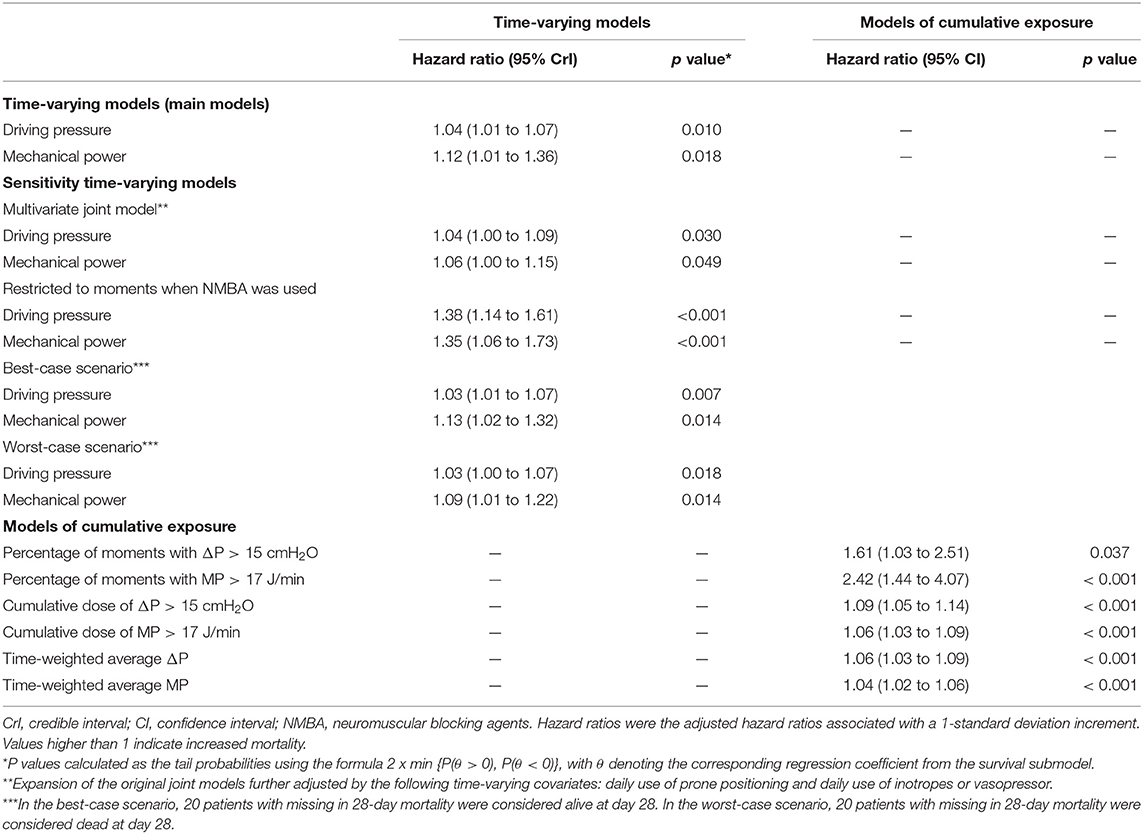
Table 2. Multivariable model assessing the association of time-varying driving pressure and mechanical power over the first four days of ventilation with 28-day mortality.
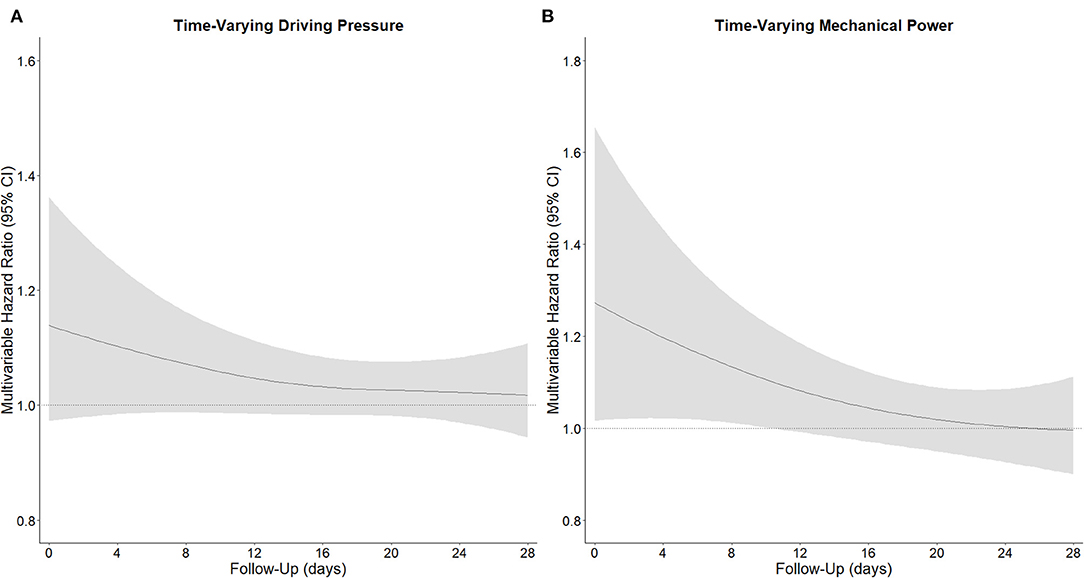
Figure 2. Time-varying hazard ratio of driving pressure and mechanical power for 28-day mortality. (A), Time-varying ΔP, and (B) time-varying MP. Time-varying hazard ratio obtained from a Bayesian joint model estimating the association between ΔP and MP and 28-day mortality, including p-splines in an interaction term. The strength of the association decreased over time. All models were adjusted for age, gender, body mass index, PaO2 to FiO2 ratio, plasma creatinine, medical history of hypertension, heart failure, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, active hematological neoplasia and/or active solid tumor, use of angiotensin converting enzyme inhibitors, use of angiotensin II receptor blockers, use of a vasopressor or inotropes, fluid balance, arterial pH, mean arterial pressure, and heart rate. Natural cubic splines were used in both the fixed-effects and random-effects models to account for the nonlinearity of the longitudinal exposure profiles.
The number and percentage of measurements per patient with ΔP > 15 cm H2O and MP > 17 J/min in the first 4 days of ventilation are shown in the Appendix (Supplementary Information Figure 4). A higher percentage of measurements with ΔP > 15 cm H2O (HR, 1.61 [95% CI, 1.03–2.51] p = 0.037) or with MP > 17 J/min (HR, 2.42 [95% CI, 1.44–4.07] p < 0.001) had an association with increased risk of 28–day mortality (Figure 3). Comparison of baseline characteristics and outcomes according to the median ΔP and MP in the first 4 calendar days is shown in Supplementary Information Table 4.
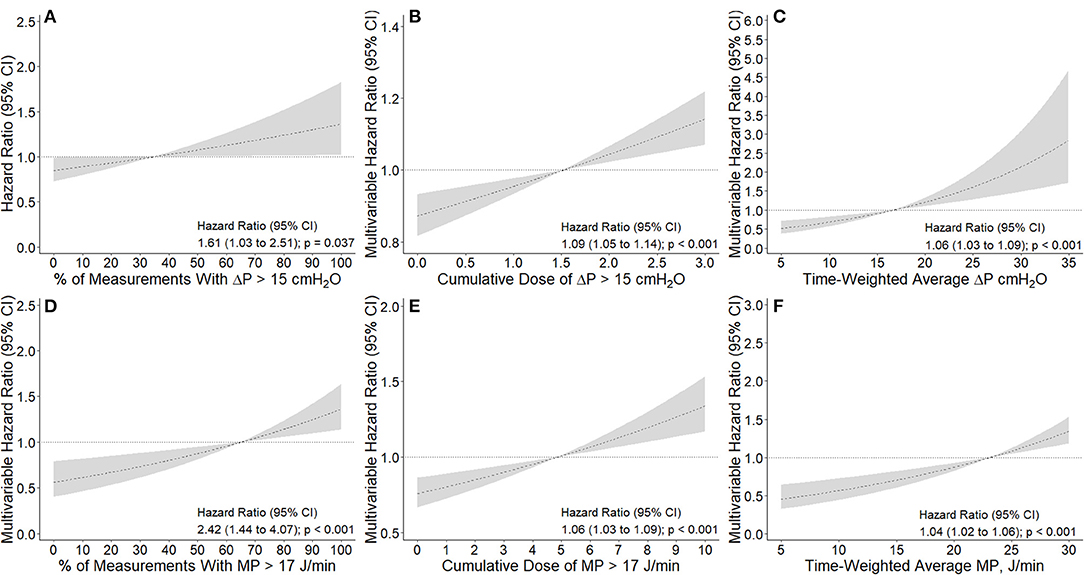
Figure 3. Association between intensity of exposure to higher driving pressure and mechanical power and 28-day mortality. (A, D): Association between percentage of measurements with ΔP > 15 cmH2O and MP > 17 J/min and 28-day mortality. Percentage calculated from a maximum of 13 measurements extracted every 8 h. (B, E): Association between cumulative dose of ΔP > 15 cmH2O and MP > 17 J/min and 28-day mortality. Cumulative dose calculated as area under the ΔP and MP time curve above the thresholds described above divided by the number of hours of exposure, as a measure of dose. Using this definition, 1 cmH2O or 1 J/min of dose describes that a patient's average ΔP or MP was 1 cmH2O or 1 J/min above the thresholds described for the duration of the exposure window, respectively. (C,F) Association between time-weighted average ΔP and MP and 28-day mortality. Time-weighted average calculated as the area under the ΔP and MP time curve divided by the number of hours of exposure. All models were adjusted for age, gender, body mass index, PaO2 to FiO2 ratio, plasma creatinine, medical history of hypertension, heart failure, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, active hematological neoplasia and/or active solid tumor, use of angiotensin converting enzyme inhibitors, use of angiotensin II receptor blockers, use of a vasopressor or inotropes, fluid balance, arterial pH, mean arterial pressure, and heart rate. Dashed lines and gray areas represent hazard ratio and 95% confidence interval for increasing values the variable analyzed as a continuous variable and centralized in the mean of each variable. ΔP is driving pressure and MP is mechanical power.
Time–weighted average and cumulative dose of ΔP and MP are shown in the Appendix (Supplementary Information Figure 5). Cumulative dose of ΔP > 15 cm H2O (HR, 1.09 [95% CI, 1.05–1.14]; p < 0.001) and MP > 17 J/min (HR, 1.06 [1.03–1.09]; p < 0.001) had an association with increased risk of 28–day mortality (Figure 3). In accordance, time–weighted average ΔP (HR, 1.06 [95% CI, 1.03–1.09]; p < 0.001) and time–weighted average MP (HR, 1.04 [95% CI, 1.02–1.06]; p < 0.001) also had an association with an increased risk on 28–day mortality.
Discussion
The findings of this observational study assessing the impact of time–varying ΔP and MP on 28–day mortality in invasively ventilated COVID−19 patients with ARDS, can be summarized as follows: (1) exposure to both higher ΔP and higher MP during the first 4 calendar days of ventilation had associations with increased risk of 28–day mortality in joint models; and (2) a higher cumulative exposure to ΔP > 15 cm H2O or MP > 17 J/min over the first 4 days of ventilation had associations with increased risk for 28–day mortality. These findings support the suggestion that limiting the intensity of ventilation in COVID−19 patients with ARDS by using strategies that result in a lower ΔP or MP could improve patient outcome, alike what has been suggested in patients with ARDS from another origin.
The findings of our study are in line with previous investigations of ΔP and MP in patients with ARDS from another cause than COVID−19. Indeed, in patients with ARDS from another origin, a higher ΔP and higher MP at baseline have been shown to have an association with increased mortality (7–10, 12–16). A recent study showed the adverse effect of exposure to higher cumulative doses of ΔP and MP over the duration of ventilation in critically ill patients receiving ventilation due to respiratory failure (12). We also found that a high time–varying ΔP and MP, and a higher cumulative exposure to harmful levels of ΔP and MP have an association with increased 28–day mortality, and as in the previous study these effects were more pronounced in patients with more severe forms of ARDS at baseline.
Considering all the available evidence, monitoring intensity of ventilation seems an attractive approach, both in patients with ARDS due to COVID−19 and in patients with ARDS from another origin. Yet, it remains uncertain whether the found association only reflects the relationship between respiratory system compliance and patient outcome. It is also uncertain whether measures to lower the intensity will result in better outcomes. Nevertheless, our results, corrected for baseline covariates and time–varying confounding, could represent a causal effect. Thus, ventilation strategies aiming to lower the intensity of ventilation need to be studied, preferably in randomized clinical trials.
The present cohort of COVID−19 patients with ARDS is comparable to other COVID−19 cohorts in relation to baseline characteristics and clinical outcomes (28–31). Key variables of ventilation management, like VT, RR and ΔP were comparable to what was described in previous studies in patients with COVID−19, and in line with recommendations for lung–protective ventilation in patients with ARDS (28–31). MP in our cohort was lower than what was reported in one study in COVID−19 patients, (30) suggesting a better adoption of lung–protective ventilation in our study.
Our study has strengths. We restricted the analysis to patients who were without spontaneous breathing activity for the majority of time, and ΔP and MP was only calculated for the moments a patient was passive. Calculation ΔP and MP in the presence of spontaneous breathing is not yet validated. For the main analyses we used joint models, which allows the examination of the effect of a time–varying, endogenous covariate on a time–to–event outcome, accounting for non–random dropouts due to death during follow–up.
Our study also has limitations. First, our study was conducted very early in the local outbreak, and anti–inflammatory strategies, like steroids and other treatments such as anti–IL−6, were not yet extensively used. We did not collect data regarding the use of steroids or other anti–inflammatory drugs. Also, we did not collect D–dimer levels and other laboratory tests that may have predictive value, as these were not yet routinely performed so early in the local outbreak. However, the variables used for adjustment are in line with previous studies assessing the impact of ΔP and MP in patients receiving ventilation (12, 14, 15). Our models used several important clinical variables. Of note, we only had measurements of dynamic airway ΔP as plateau or transpulmonary pressures were not routinely connected. Airway ΔP does correlate with transpulmonary ΔP, but it represents a surrogate, which might be affected by other factors. However, recent data suggested that this calculation is reliable (32). Also, we could not adjust for all possible yet unmeasured confounders and due to the observational nature, no causal relationship can be inferred or determined. A high incidence of thromboembolic complications has been found in the present study, which is in line with other reports on COVID−19 patients (33). Pulmonary embolism could cause an increase in dead space, as such affecting the intensity of ventilation. Another limitation is that participating centers did not all use the same disease severity score. Indeed, some centers reported either APACHE II or APACHE IV scores, others used the SAPS II, and some only SOFA scores. These scores cannot be used interchangeably. However, several baseline covariates were used in our models, representing all organ systems, several supportive treatments and pre–existing comorbidities. The mortality rate in our study is lower than that reported in one recent cohort of COVID−19 patients, (2) but higher than in other reports (34, 35). It remains to be determined whether the associations found here are also present in cohorts with other mortality rates. In addition, the models used had some limitations. All baseline covariates were assumed to be measured without error. The joint models assume the correct specification of random effect structure, and all interdependencies between longitudinal and time–to–event outcomes should be explained by the latent, subject–specific random effects structure. Also, there is a risk of residual confounding.
Conclusions
In conclusion, in this cohort of COVID−19 patients with ARDS, exposure to higher ΔP or MP, or to a higher cumulative exposure to a ΔP > 15 cm H2O or a MP > 17 J/min over the first 4 calendar days of ventilation had associations with increased risk for 28–day mortality. To ascertain a causal relationship between ΔP and MP with mortality, randomized clinical trials are needed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review boards of each participating center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
PRoVENT–COVID Collaborative Group
Monitored email address: provent-covid@amsterdamumc.nl.
Investigators: J.P. van Akkeren; A.G. Algera; C.K. Algoe; R.B. van Amstel; O.L. Baur; P. van de Berg; D.C.J.J. Bergmans; D.I. van den Bersselaar; F.A. Bertens; A.J.G.H. Bindels; M.M. de Boer; S. den Boer; L.S. Boers; M. Bogerd; L.D.J. Bos; M. Botta; J.S. Breel; H. de Bruin; S. de Bruin; C.L. Bruna; L.A. Buiteman–Kruizinga; O. Cremer; R.M. Determann; W. Dieperink; D.A. Dongelmans; H.S. Franke; M.S. Galek Aldridge; M.J. de Graaff; L.A. Hagens; J.J. Haringman; N.F.L. Heijnen; S.Hiel; S.T. van der Heide; P.L.J. van der Heiden; L.L. Hoeijmakers; L. Hol; M. W. Hollmann; M.E. Hoogendoorn; J. Horn; R. van der Horst; E.L.K. Ie; D. Ivanov; N.P. Juffermans; E. Kho; E.S. de Klerk; A.W.M. Koopman; M. Koopmans; S. Kucukcelebi; M.A. Kuiper; D.W. de Lange; D.M.P. van Meenen; I. Martin–Loeches, G. Mazzinari; N. van Mourik; S.G. Nijbroek; M. Onrust; E.A.N. Oostdijk; F. Paulus; C.J. Pennartz; J. Pillay; L. Pisani; I.M. Purmer; T.C.D. Rettig; J.P Roozeman; M.T.U. Schuijt; M.J. Schultz; A. Serpa Neto; M.E. Sleeswijk; M.R. Smit; P.E. Spronk; W. Stilma; A.C. Strang; A.M. Tsonas; P.R Tuinman; C.M.A. Valk; F.L. Veen; A.P.J. Vlaar; L.I. Veldhuis; P. van Velzen; W.H. van der Ven; P. van Vliet; P. van der Voort; H.H. van der Wier; L. van Welie; H.J.F.T. Wesselink; B. van Wijk; T. Winters; W.Y. Wong; A.R.H. van Zanten.
Author Contributions
MiS and AS had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. MiS, MaS, GM, FP, and AS concept and design. All authors contributed to acquisition, analysis or interpretation of data, drafting the manuscript, and critical revision of the manuscript for important intellectual content. MiS and AS statistical analysis. FP and MaS obtained funding. MaS, FP, and AS supervision. All authors contributed to acquisition, analysis or interpretation of data, drafting the manuscript, and critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
The Amsterdam University Medical Centers, location AMC funded this study. It had no role in the design, analysis, interpretation of data, writing or submission of this study.
Conflict of Interest
AS reports personal fees from Dräger, outside of the submitted work. MaS reports personal fees from Hamilton and Xenios/Novalung, outside of the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.725265/full#supplementary-material
References
1. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
2. Domecq JP, Lal A, Sheldrick CR, Kumar VK, Boman K, Bolesta S, et al. Outcomes of patients with coronavirus disease 2019 receiving organ support therapies: the international viral infection and respiratory illness universal study registry. Crit Care Med. (2021) 49:437–48. doi: 10.1097/CCM.0000000000005007
3. Slutsky A, Ranieri V. Ventilator-induced lung injury. N Engl J Med. (2013) 369:2126–36. doi: 10.1056/NEJMra1208707
4. Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An official American Thoracic Society/European Society of intensive care medicine/society of critical care medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. (2017) 195:1253–63. doi: 10.1164/rccm.19511erratum
5. The ARDS. Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. (2000) 342:1301–8. doi: 10.1056/NEJM200005043421801
6. Goligher EC, Ferguson ND, Brochard LJ. Clinical challenges in mechanical ventilation. The Lancet. (2016) 387:1856–66. doi: 10.1016/S0140-6736(16)30176-3
7. Amato MBP, Meade MO, Slutsky AS, Brochard L, Costa ELV, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. (2015) 372:747–55. doi: 10.1056/NEJMsa1410639
8. Serpa Neto A, Deliberato RO, Johnson AEW, Bos LD, Amorim P, Pereira SM, et al. Mechanical power of ventilation is associated with mortality in critically ill patients: an analysis of patients in two observational cohorts. Intensive Care Med. (2018) 44:1914–22. doi: 10.1007/s00134-018-5375-6
9. Protti A, Andreis DT, Monti M, Santini A, Sparacino CC, Langer T, et al. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med. (2013) 41:1046–55. doi: 10.1097/CCM.0b013e31827417a6
10. Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. (2016) 42:1567–75. doi: 10.1007/s00134-016-4505-2
11. Marini JJ, Jaber S. Dynamic predictors of VILI risk: beyond the driving pressure. Intensive Care Medicine. (2016) 32:1597–600. doi: 10.1007/s00134-016-4534-x
12. Urner M, Jüni P, Hansen B, Wettstein MS, Ferguson ND, Fan E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Respir Med. (2020) 8:905–13. doi: 10.1016/S2213-2600(20)30325-8
13. Zhang Z, Zheng B, Liu N, Ge H, Hong Y. Mechanical power normalized to predicted body weight as a predictor of mortality in patients with acute respiratory distress syndrome. Intensive Care Med. (2019) 45:856–64. doi: 10.1007/s00134-019-05627-9
14. Tonna JE, Peltan I, Brown SM, Herrick JS, Keenan HT, Grissom CK, et al. Mechanical power and driving pressure as predictors of mortality among patients with ARDS. Intensive Care Med. (2020) 46:1941–3. doi: 10.1007/s00134-020-06130-2
15. Coppola S, Caccioppola A, Froio S, Formenti P, De Giorgis V, Galanti V, et al. Effect of mechanical power on intensive care mortality in ARDS patients. Crit Care. (2020) 24:1–10. doi: 10.1186/s13054-020-02963-x
16. Parhar KKS, Zjadewicz K, Soo A, Sutton A, Zjadewicz M, Doig L, et al. Epidemiology, mechanical power, and 3-year outcomes in acute respiratory distress syndrome patients using standardized screening: An observational cohort study. Ann Am Thorac Soc. (2019) 16:1263–72. doi: 10.1513/AnnalsATS.201812-910OC
17. Gattinoni L, Marini JJ, Collino F, Maiolo G, Rapetti F, Tonetti T, et al. The future of mechanical ventilation: Lessons from the present and the past. Crit Care. (2017) 21:1–11. doi: 10.1186/s13054-017-1750-x
18. Huhle R, Serpa Neto A, Schultz MJ, Gama de. Abreu M. Is mechanical power the final word on ventilator-induced lung injury?—no. Ann Transl Med. (2018) 6:394. doi: 10.21037/atm.2018.09.65
19. Vasques F, Duscio E, Pasticci I, Romitti F, Vassalli F, Quintel M, et al. Is the mechanical power the final word on ventilator-induced lung injury?—we are not sure. Ann Transl Med. (2018) 6:395–395. doi: 10.21037/atm.2018.08.17
20. Cressoni M, Cadringher P, Chiurazzi C, Amini M, Gallazzi E, Marino A, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. (2014) 189:149–58. doi: 10.1164/rccm.201308-1567OC
21. Boers NS, Botta M, Tsonas AM, Algera AG, Pillay J, Dongelmans DA, et al. PRactice of VENTilation in Patients with Novel Coronavirus Disease (PRoVENT-COVID): rationale and protocol for a national multicenter observational study in The Netherlands. Ann Transl Med. (2020) 8:1251. doi: 10.21037/atm-20-5107
22. PROVENT–COVID. Available online at: https://sites.google.com/view/provent-covid/. (accessed Mar 10, 2021)
23. Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LDJ, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med. (2021) 9:139–48. doi: 10.1016/S2213-2600(20)30459-8
24. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA - J Am Med Assoc. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
25. Rizopoulos D. The R package jmbayes for fitting joint models for longitudinal and time-to-event data using MCMC. J Stat Softw. (2016) 72:46. doi: 10.18637/jss.v072.i07
26. Rizopoulos D, Ghosh P. A bayesian semiparametric multivariate joint model for multiple longitudinal outcomes and a time-to-event. Stat Med. (2011) 30:1366–80. doi: 10.1002/sim.4205
27. Giosa L, Busana M, Pasticci I, Bonifazi M, Macrì MM, Romitti F, et al. Mechanical power at a glance: a simple surrogate for volume-controlled ventilation. Intensive Care Med Exp. (2019) 7:61. doi: 10.1186/s40635-019-0276-8
28. Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernández M, Gea A, Arruti E, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. (2020) 46:2200–11. doi: 10.1007/s00134-020-06251-8
29. Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. (2020) 180:1–12. doi: 10.1001/jamainternmed.2020.3596
30. COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. (2021) 47:60–73. doi: 10.1007/s00134-020-06294-x
31. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. (2020) 395:1763–70. doi: 10.1016/S0140-6736(20)31189-2
32. Chiumello D, Gotti M, Guanziroli M, Formenti P, Umbrello M, Pasticci I, et al. Bedside calculation of mechanical power during volume- and pressure-controlled mechanical ventilation. Crit Care. (2020) 24:417–23. doi: 10.1186/s13054-020-03116-w
33. Bompard F, Monnier H, Saab I, Tordjman M, Abdoul H, Fournier L, et al. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. (2020) 56:17–20. doi: 10.1183/13993003.01365-2020
34. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA - J Am Med Assoc. (2020) 323:1574–81.
Keywords: coronavirus disease 2019, acute respiratory distress syndrome, driving pressure, mechanical power, mortality
Citation: Schuijt MTU, van Meenen DMP, Martin–Loeches I, Mazzinari G, Schultz MJ, Paulus F and Serpa Neto A (2021) Association of Time–Varying Intensity of Ventilation With Mortality in Patients With COVID−19 ARDS: Secondary Analysis of the PRoVENT–COVID Study. Front. Med. 8:725265. doi: 10.3389/fmed.2021.725265
Received: 15 June 2021; Accepted: 14 September 2021;
Published: 18 November 2021.
Edited by:
Ata Murat Kaynar, University of Pittsburgh, United StatesReviewed by:
Huiqing Ge, Sir Run Run Shaw Hospital, ChinaIsrael Maia, Instituto de Pesquisa Hospital Coraçao São Pãulo, Brazil
Copyright © 2021 Schuijt, van Meenen, Martin–Loeches, Mazzinari, Schultz, Paulus and Serpa Neto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ary Serpa Neto, ary.serpaneto@monash.edu
 Michiel T. U. Schuijt
Michiel T. U. Schuijt David M. P. van Meenen
David M. P. van Meenen Ignacio Martin–Loeches
Ignacio Martin–Loeches Guido Mazzinari
Guido Mazzinari Marcus J. Schultz
Marcus J. Schultz Frederique Paulus
Frederique Paulus Ary Serpa Neto
Ary Serpa Neto