Abstract
Comparing seroprevalence and antibody kinetics in three different commercially available assays for SARS-CoV-2. Serostatus of COVID-19 patients was analyzed 5 months and 10 months after their infection, using three different assays: Diasorin LIAISON, Euroimmun, Abbott Diagnostics ARCHITECT. Seropositivity at baseline differed significantly depending on the assay (Diasorin 81%, Euroimmun 83%, Abbott 59%). At follow-up antibody levels detected in the Diasorin assay were stable, while there was a significant loss in seropositivity in the Euroimmun and Abbott assays. There are significant differences in SARS-CoV-2 antibody kinetics based on the specific assay used.
Similar content being viewed by others
Introduction
Since the beginning of the SARS-CoV-2-pandemic1 more than 112 Million cases and almost 2.5 Million2 deaths have been reported worldwide. Serum antibody testing is becoming a critical tool, both in diagnosis of COVID-19 and in seroprevalence studies.
Most patients develop detectable antibodies within 14 days after their infection3. However, there is inconsistent evidence regarding the duration of antibody persistence with studies showing only short lived antibody responses4 while others showing persistent serum levels5.
While differences in patient characteristics and disease course might explain some contradictory findings, assay dependent differences might also play a role.
We analyzed SARS-CoV-2 seroprevalence and antibody kinetics over 9 months in 109 individuals using three different commercially available assays.
Methods
Study design
Patients with PCR confirmed SARS-CoV-2 infection in Dresden (a city in Saxony/Germany with approximately 557,000 inhabitants) were invited via the local health department to participate in the AmbCoviDD19 study.
After informed consent was obtained, 5 mL of peripheral venous blood was collected from each individual to assess SARS-CoV-2 IgG antibodies at baseline and 9–12 months after their infection (follow-up). Additional information about age, comorbidities, regular medication, COVID-19-symptoms, disease course and test indication were obtained.
The AmbCoviDD19 study was approved by the Ethics Committee of the Technische Universität (TU) Dresden (BO-EK-137042020) and has been assigned clinical trial number DRKS00022549.
Laboratory analysis
We assessed SARS-CoV-2 IgG antibodies in all samples using three commercially available assays.
First, chemiluminescence immunoassay (CLIA) technology for the quantitative determination of anti-S1 and anti-S2 specific IgG antibodies to SARS-CoV-2 was used: Diasorin LIAISON SARS-CoV-2 S1/S2 IgG Assay. Antibody levels > 15.0 AU/mL were considered positive and levels between 12.0 and 15.0 AU/mL were considered equivocal.
Second, an ELISA detecting IgG against the S1 domain of the SARS-CoV-2 spike protein, Euroimmun Anti-SARS-CoV-2 ELISA, was used; a ratio < 0.8 was considered negative, 0.8–1.1 equivocal, > 1.1 positive.
Third, chemiluminescent microparticle immunoassay (CMIA) intended for the qualitative detection of IgG antibodies to the nucleocapsid protein of SARS-CoV-2, Abbott Diagnostics ARCHITECT SARS-CoV-2 IgG, was used. This assay relies on an assay-specific calibrator to report a ratio of specimen absorbance to calibrator absorbance. The interpretation of result is determined by an index (S/C) value, which is a ratio over the threshold value. An index (S/C) of < 1.4 was considered negative, ≥ 1.4 was considered positive.
Statistical analysis
Analyses were performed using IBM SPSS 25.0 and Microsoft Excel 2010. Statistical comparisons between groups were performed using the Fishers; exact test for categorical variables and T-test for means. Correlations were assessed using a Spearman’s Rank correlation coefficient (R). All tests were 2-sided, and p values < 0.05 were considered statistically significant.
Trial registration number, date of registration
DRKS00022549, 29.07.2020 “retrospectively registered”.
Ethics approval
The AmbCoviDD19 study was approved by the Ethics Committee of the Technische Universität (TU) Dresden (BO-EK-137042020) and has been assigned clinical trial number DRKS00022549.
Consent of participate
Informed consent was obtained from all individual participants included in the study.
Consent of publication
Patients signed informed consent regarding publishing their data.
Results
Overall, 109 individuals with a positive PCR in respiratory samples between March and May 2020 were enrolled in this study. 57/109 (52%) were female, median age was 46 years, 2/109 (1.8%) were younger than 19 years, 8/109 (7.3%) required hospitalization and 92/109 (84%) reported COVID-19 related symptoms (see Table 1 for full patients’ characteristics).
Median time between first serological testing and positive PCR was 144 days (4.8 months).
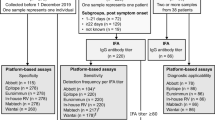
88/109 (81%) participants had detectable antibodies against SARS-CoV-2 in at least two different assays at baseline. Of these, 1/88 (1%) had no detectable antibodies in the Diasorin, 25/88 (28%) in the Abbott whereas all of these participants had at least equivocal results in the Euroimmun assay. 62/109 (57%) individuals had detectable antibodies in all three assays at baseline (Table 2, Fig. 1).
Time between infection and serological testing (146 days vs. 141 days), age (44 vs. 46 years), female gender (51% vs. 57%) hospitalization rate (8/88 (9%) vs. 0/21 (0%)) and frequency of reported symptoms (75/88 (85%) vs. 17/21 (81%)) did not differ significantly between seropositive and seronegative individuals (Table 3).
Seropositivity, however, differed significantly depending on test indication. While 44/50 (88%) participants with a defined SARS-CoV-2 positive contact were seropositive, 18/19 (95%) were positive with a travel history, only 16/27 (59%) were seropositive when tested solely based on symptoms (Fig. 2 + Supplemental Table S1).
Median time between second antibody assessment (follow-up) and infection was 295 days (9.8 months) (Table 1). 101/109 (93%) participants had detectable antibodies in at least one assay. 89/92 (97%) with detectable antibodies at baseline in at least one assay continued to have detectable antibodies in at least one assay at follow-up. 91/109 (83%) were Diasorin positive, 85/109 (78%) Euroimmun positive and 26/109 (24%) Abbott positive (Table 2).
However, while 86/88 (98%) of participants with initially detectable antibodies in the Diasorin assay continued to have detectable antibodies in this assay, numbers were significantly lower for the Euroimmun (73/90 (81%) p 0.0004) and the Abbott (19/64 (30%) p = 0.0001) (Table 4). In addition mean antibody levels were stable in the Diasorin assays over time while they dropped significantly in the Euroimmun and Abbott assays (Fig. 3).
At follow-up, 77/109 (71%) had detectable antibodies in at least two different assays (Table 2); 71/88 (81%) individuals with two positive serological tests at baseline continued to have detectable antibodies in two assays; 15/17 (88%) individuals no longer tested positive in two different assays continued to have detectable antibodies in the Diasorin, while none of these continued to have detectable antibodies in the Euroimmun or Abbott. 24/109 (22%) had detectable antibodies in all three assays at follow-up (Table 2, Fig. 1).
Five out of 21 (24%) individuals without detectable antibodies in the Diasorin assay at baseline had detectable antibodies at follow-up, 7/19 (37%) in the Euroimmun and 7/45 (16%) in the Abbott respectively. Of the 17 individuals without detectable antibodies in any assay at baseline, 12 (71%) had detectable antibodies in at least one assay at follow-up (3 Diasorin/10 Euroimmun/3 Abbott). Two individuals reported a positive SARS-CoV-2 PCR testing between baseline and follow-up. Both had initially no detectable antibodies in any assay, one had antibodies in all three assays at follow-up, one had detectable antibodies in the Diasorin and Euroimmun. Five individuals had no detectable antibodies in any assay at both times. Four out of five were tested by PCR based on symptoms alone, while 1/5 was tested due to travel history.
Discussion
The overall seroconversion rate in our study is comparable to large population based seroprevalence studies6,7, however the differences in seroprevalence detected by different assays are striking. While at baseline the Diasorin and Euroimmun provide comparable results (Fig. 1), the seroprevalence in the Abbott is 20% lower compared to the other assays. At follow-up these differences become even more pronounced. While the Diasorin shows stable persistent antibody levels, seropositive rates detected with Euroimmun and Abbott decrease significantly over time, leading to a possible underestimation of seropositivity of up to 60%. These differences might at least partly explain contradicting findings in longitudinal antibody kinetic studies.
Given the intra-individual differences, patient characteristics seem unlikely to explain the discrepancies between the different assays. Differences in antibody persistence based on targeted epitopes might play a role comparing Diasorin and Euroimmun with Abbott. However, the significant difference between Diasorin and Euroimmun at follow-up—both detecting antibodies targeting the spike protein of SARS-CoV-2—requires further investigation. The possible role and longevity of IgA antibodies need to addressed in further studies as well.
In addition, antibodies to the nucleocapsid protein of SARS-CoV-2 are thought to be useful in differentiating between seroconversion after infection and vaccination. Given the substantial loss of these antibodies over time in our sample, this approach might be less feasible than expected.
It is remarkable that 71% of individuals without any detectable SARS-CoV-2 antibodies 4–5 months after their infection had measurable levels later at 9–10 months. One possible explanation could be that their initial short lived antibody response was boostered during the study period through repeat exposure. Further immunological studies—including T-cell assays—are needed to investigate this further. The observation that the only PCR confirmed repeat infections occurred in individuals without a detectable antibody response at baseline is somewhat reassuring and might point to a correlation between antibody response and immunity.
The lack of significant associations between seropositivity and patient characteristics in our study compared to previous5,7 might be explained by the relatively long time period between infection and baseline serological testing as well as the rather homogenous study population with mild clinical courses.
The significant differences in seropositivity based on test indication is of particular interest though and most likely due to an increased rate of false positive PCRs in populations with a lower pre-test-probability. While this association is well described in the literature8 these results are an important reminder that even highly specific test as the SARS-CoV-2 RT-qPCR do have a measurable false positive rate9. This observation needs to be given special consideration when implementing population-based screening programs in asymptomatic individuals.
Conclusion
There are significant differences in SARS-CoV-2 antibody kinetics based on the specific assay used. In our cohort the Diasorin LIAISON assay performed best regarding long term detection of seropositivity. Diasorin had the lowest change in seropositivity between baseline and follow up. There is a significant difference in seropositivity in PCR-positive individuals based on the indication for PCR-testing.
Data availability
We share data if reasonable requests are received. Requests should be directed to the corresponding author at jakob.armann@uniklinikum-dresden.de.
References
Zhu, N. et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382(8), 727–733. https://doi.org/10.1056/NEJMoa2001017 (2020).
European Centre for Disease Prevention and Control. COVID-19 Situation Update Worldwide, as of Week 7, Updated 25 February 2021 (2021). https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (Accessed 28 February 2021).
Sun, B. et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 9(1), 940–948 (2020).
Carsetti, R. et al. Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front. Immunol. 11, 610300. https://doi.org/10.3389/fimmu.2020.610300 (2020).
Zhang, X. et al. Viral and antibody kinetics of COVID-19 patients with different disease severities in acute and convalescent phases: A 6-month follow-up study. Virol. Sin. 35(6), 820–829. https://doi.org/10.1007/s12250-020-00329-9 (2020).
Pollán, M. et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. The Lancet 396(10250), 535–544 (2020).
Gudbjartsson, D. F. et al. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 383(18), 1724–1734. https://doi.org/10.1056/NEJMoa2026116 (2020).
Jackson, B. R. The dangers of false-positive and false-negative test results: False-positive results as a function of pretest probability. Clin. Lab. Med. 28(2), 305–319 (2008).
Surkova, E., Nikolayevskyy, V. & Drobniewski, F. False-positive COVID-19 results: Hidden problems and costs. Lancet Respir. Med. 8(12), 1167–1168 (2020).
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by a grant by the Federal State of Saxony.
Author information
Authors and Affiliations
Contributions
E.K., L.G., A.D., R.B. and J.A. designed the study and wrote the protocol. E.K., L.G., M.U., L.H., J.B. and J.A. collected samples. A.D. and C.L. performed serological testing. E.K., L.G., R.B. and J.A. analyzed and verified the data. E.K., L.G. and J.A. wrote the manuscript. A.D., C.L. and R.B. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kahre, E., Galow, L., Unrath, M. et al. Kinetics and seroprevalence of SARS-CoV-2 antibodies: a comparison of 3 different assays. Sci Rep 11, 14893 (2021). https://doi.org/10.1038/s41598-021-94453-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94453-5
This article is cited by
-
Long-term longitudinal evaluation of the prevalence of SARS-CoV-2 antibodies in healthcare and university workers
Scientific Reports (2022)
-
Lower SARS-CoV-2 household transmission in children and adolescents compared to adults
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.