Abstract
SARS-CoV-2 is the virus that causes the disease called COVID-19, which has caused the worst pandemic of the century. Both, to know the immunological status of general population and to evaluate the efficacy of the vaccination process that is taking place around the world, serological tests represent a key tool. Classic serological tests, based on colorimetric techniques, such as ELISA or CLIA, continue to be the most widely used option. However, a real improvement in results is still needed. We developed a highly sensitive and specific FCM assay that allows the detection of IgG and IgA antibodies, directed against the native and functional S-protein of SARS-CoV-2 exposed on the membrane of a transfected cell line, up to 8 months after infection.
Similar content being viewed by others
SARS-CoV-2 is a Baltimore class IV RNA virus that spreads easily through the respiratory tract and causes an infectious disease called COVID-191,2. Since its detection in Wuhan in December 2019, COVID-19 has evolved into a pandemic, causing a global health emergency3.
SARS-CoV-2 infection promotes the generation of T and B cell responses, particularly against the spike glycoprotein (S-protein) blocking its ability to bind to ACE-2 protein4,5,6. However, it is still unknown whether the immune response leads to a prolonged protection against reinfection6,7,8.
The evaluation of the immune status against SARS-CoV-2 in the population is of vital importance to know the actual proportion of people who have recovered from the disease, as well as to detect individuals with active infections9. Different serological approaches to detect specific antibodies against SARS-CoV-2 S-protein (anti-SCoV2) and the functional activity directed to the formation of S-protein/ACE-2 complexes have been successfully used10,11,12. The Enzyme-Linked ImmunoSorbent Assay (ELISA) and chemiluminescence immunoassay (CLIA) have shown to detect seropositivity in the general population reaching good sensitivity and specificity10,11,12. However, it has been noted the need for improved specificity and sensitivity. In fact, several groups have developed refined techniques previously used with other coronaviruses13.
Recently, some publications highlighted the promising role of flow cytometry (FCM) in the detection of antibodies against the native S-protein from SARS-CoV-2. By contrast to other immunological techniques, FCM detects antibodies directed against the native and functional S-proteins, exposed on the membrane of a transfected cell line, allowing optimal conditions for improved sensitivity and specificity14,15.
Methods
Patients
Our study was accomplished through the analysis of 50 serum samples, collected from November 5 to December 11, 2020 by venous and capillary puncture of patients infected in the first pandemic wave (between March and April, 2020). Blood samples were centrifuged for the isolation of serum/plasma. Samples were then labelled and inactivated by heat (57 °C 50′) for a safe handling. Finally, they were stored at − 80 °C until their analysis. The median time elapsed since the first qPCR + until the FCM study was 249 days (range 220–271). In addition, we included in the study 50 pre-pandemic samples (May–June 2019) as healthy negative controls. The mean age of patients and controls was 46 years (range 22–66) and sex distribution was similar between male and female.
Cell lines
We have used a stably transfected non-adherent Jurkat cell line that expresses both the full-length native S-protein of SARS-CoV-2 and a truncated form of the human EGFR protein (S-Jurkat). These cells express simultaneously variable amounts of EGFR and S-protein, as previously described14. We also included a second wild type Jurkat cell line that was added to each test tube as negative internal fluorescence control (0-Jurkat).
Sample processing
Our strategy was based on the technique described by Horndler et al14, with the introduction of some modifications such as the addition of a viability reagent to exclude non-viable cells from the analysis.
For each individual assay, we prepared a mixture with 50.000 0-Jurkat and 150.000 S-Jurkat cells in a single tube. The cell suspension was incubated with a 1:50 dilution of serum samples in PBS14 for 20 min on ice. Then, cells were washed with PBS and stained for additional 20 min with a mixture of BD Via-Probe-FITC (BD biosciences), anti-IgG-PerCP. (Jackson ImmunoResearch), anti-IgA-Alexa Fluor 647 (Jackson ImmunoResearch) and anti-EGFR-BV421 (Biolegend), that allowed for the selection of viable cells and for the detection of antibodies bound to S-protein expressed on transfected cells. After a final wash, samples were acquired in an Omnicyt flow cytometer (Cytognos SL) and analyzed using the Infinicyt 2.0 software (Cytognos SL).
The optical filters included in the cytometer configuration were: 530/30 for BL-1 detector (FITC), 695/40 for BL-3 detector (PerCP), 670/14 for RL-1 (Alexa Fluor 647) and 440/50 for VL-1 (BV421).
A minimum of 50,000 viable events, discarding doublets and debris were considered for the analysis. To evaluate the reproducibility of the assay, each patient was tested in two independent experiments (performed in venous and capillary blood samples respectively).
Flow cytometry calibration
Flow cytometer target values were established using Rainbow beads (Cytognos SL) according to manufacturer’s instructions. The compensation matrix was created using CompBeads (BD Bioscences) for anti-EGFR and fresh blood for anti-IgG, anti-IgA and BD Via-Probe.
Flow cytometry interpretation
IgG and/or IgA antibodies specifically bound to S-proteins were identified by comparing the median fluorescence intensity (MFI) of the S-Jurkat and the 0-Jurkat cells in each sample. Specific antibody binding was estimated by determining the MFI-ratio between transfected/non transfected Jurkat cells for the fluorescent signals corresponding to anti-IgG and anti-IgA (IgG MFI-ratio and IgA MFI-ratio respectively). To further validate the specificity for S-protein of the signals generated by IgG and IgA antibodies, we studied the correlation between the MFIs corresponding to IgG or IgA bound to transfected cells and EGFR expression (as a surrogate indicator of S- protein expression14) Supplementary Fig. 1.
CLIA assay
In order to compare our strategy with conventional serological tests, we analyzed the samples with a commercial CLIA assay (MAGLUMI® SARS-CoV-2 S-RBD, Snibe diagnostic) following manufacturer’s instructions.
Neutralization test
We performed a functional analysis for the assessment of neutralizing antibodies. We evaluated the creation of syncytia between S-Jurkat and HepG2 cells expressing ACE2, the cellular receptor of CoV-2 and ligand of S-protein, through the quantification of double positive cells as previously described14.
Statistical analysis
All statistical analyses were performed using the 19.0 SPSS software. Results were expressed as medians, means, SDs and confidence intervals (CI). We used a linear regression model to correlate different levels of antibody expression. (p < 0.05).
Ethics approval
This study has been performed in accordance with all the principles included in the declaration of Helsinki, it was approved by the Ethics Committee for Drug Research of the General University Hospital of Alicante (CEIm-HGUA). An informed consent was obtained from all the participants.
Results
MFI-ratio in pre-pandemic samples
Pre-pandemic samples showed an IgG mean fluorescent intensity (IgG-MFI) ratio of 1.18 (CI 95% 0.96–1.40) and IgA-MFI ratio of 1.12 (CI 95% 1.0–1.35). No significant linear correlation between IgG/EGFR and/or IgA/EGFR. (R2 < 0.25 p > 0.1) was found in the analysis of pre-pandemic serums.
MFI-ratio in SARS-CoV-2 samples
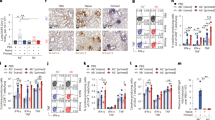
All samples from PCR + patients showed an IgG-MFI ratio > 1.4 (mean = 4.12, CI 95% 1.54–7.11) and positive IgG/EGFR correlation, so they were considered anti-S/IgG+ (Fig. 1). Using the same strategy, 88% of samples were classified as anti-S/IgA+ (IgA-MFI ratio > 1.35; mean = 2.30, CI 95% 1.5–7.8 and positive anti-IgA/EGFR correlation). Nevertheless 5/6 samples initially classified as anti-S/IgG+ /IgA− using the MFI ratio method were reclassified as anti-S/IgG+ /IgA+ using the correlation IgA/EGFR method (Fig. 2). Finally, we compared samples collected by capillary puncture versus venipuncture and we could conclude that both results were identical (Supplementary Fig. 3).
Representation of the IgA/EGFR correlation approach in a sample (grey), classified as anti-S IgG+ /IgA− by the ratio method, and a negative control (yellow). The linear regression graph shows IgA MFI versus EGFR MFI of both samples. The regression coefficient of the sample (grey) versus a negative control (yellow) demonstrate IgA seropositivity (R2 = 0.92 vs. R2 = 0.31).
Comparison with CLIA and functional analysis
All samples were evaluated by CLIA and then compared with our FCM method. Discordant results were observed in 6 patients (5 FCM+ /CLIA− ; 1 FCM− /CLIA +). These samples were subjected to a functional assay to study the presence of neutralizing antibodies that were confirmed in all five FCM+ /CLIA− cases (Fig. 3). We could not demonstrate the presence of neutralizing antibodies in the FCM−/CLIA+ case.
Neutralization assay in 6 dissenting samples: 5 FCM+ /CLIA− samples (51, 52, 61, 69 and 71) and 1 FCM− /CLIA+ sample (187). The reduction of S-protein lentiviral transduced ACE2-cells, due to the blockage of S-protein from serum antibodies, confirmed the presence of functional antibodies in all 5 FCM+ /CLIA− samples.
Discussion
In this study, we generated a highly sensitive FCM method to detect specific IgG and IgA antibodies against SARS-CoV-2 native S protein. Our results indicate that IgG antibodies remains detectable for at least 8 months since the first PCR+ in virtually all mild or asymptomatic patients. IgG is the most widely studied indicator of a SARS-CoV-2 infection and its detection has been reported since the first week after the infection16. Furthermore, previous studies performed using ELISA and/or CLIA have shown that IgG persists in up to 90% of patients for at least 6 months after acute infection17. Additionally, other remarkable studies have demonstrated that IgG exhibits significant neutralizing activity against S protein18. Nevertheless, similarly to previous studies based on FCM13,14 we have demonstrated that FCM can identify IgG/IgA antibodies in patients previously classified as negative by CLIA. This may reflect the increased ability of FCM methods to identify antibodies that recognize epitopes which may be present only in the native tridimensional configuration of S protein, not available in the solid phase based classic serological assays.
Most studies have focused on the diagnostic value of IgA for early diagnosis of COVID1919. Nevertheless, this is the first study which proves that the vast majority of mild/asymptomatic patients infected with SARS-CoV-2 develop a sustained serum IgA response persisting for months after resolution of infection. IgA not only plays a crucial role in the immunological protection of mucous membranes, but also serum IgA can inhibit the inflammatory effects of other immunoglobulins helping in the regulation of systemic immune response20. Increased IgA levels in the lungs have been correlated with a reduction in SARS pathology in animal models20. These observations have not been reproduced in humans; however, recent findings strongly suggest that human IgA contributes to the neutralization of S protein18. Therefore, the detection of IgA with highly sensitive techniques may become an interesting tool to define the lifespan of the protective immune response against SARS-CoV-2.
The functional assay confirmed the presence of neutralizing anti-S antibodies in all FCM+ patients, including 5 patients classified as negative by CLIA. Therefore, our strategy can be useful to identify long-lasting antibody production as well as to determine the immune status after SARS-CoV-2 infection.
Compared to CLIA/ELISA and rapid serological tests, our FCM method requires the use of a flow cytometer and cell cultures. However, the minimal amount of sample used for FCM study along with automated analysis strategies represent significative improvements in the daily routine. In addition, the use of lentiviral vectors makes possible to transfect different mutational forms of the S protein, allowing the update of the transfected cell line as needed, for the detection of immunity against new mutational variants.
In summary, the strategy presented here confirms that FCM is a highly specific and sensitive technique for the detection of antibodies against SARS-CoV-2. FCM constitute a promising tool to study long-term protective humoral immune response in cases where antibody levels were predictably low, such as in the long-term monitoring of asymptomatic patients, immunosuppressed people or elderly patients. However, additional studies would be necessary to investigate its usefulness in the routine follow-up or in the design of specific vaccination strategies.
References
Wu, F. et al. A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269 (2020).
Lu, R. et al. Genomic characterization and epidemiology of 2019 novel coronavirus: 307 implications for virus origins and receptor binding. Lancet 395, 565–574 (2020).
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).
Walls, A. C. et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181, 281–292 (2020).
Kwiecie, I. et al. Maturation of T and B lymphocytes in the assessment of the Immune Status in COVID-19 patients. Cells 9, 2615–2631 (2020).
Schub, D. et al. High levels of SARS-CoV-2–specific T cells with restricted functionality in severe courses of COVID-19. JCI Insight 5, e142167 (2020).
Guo, L. et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin. Infect Dis. 71, 778–785 (2020).
Kim, D. S. et al. Will SARS-CoV-2 infection elicit long-lasting protective or sterilising immunity? Implications for vaccine strategies. Front. Immunol. 11 (2020).
Lou, B. et al. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur. Respir. J. 56, 2000763 (2020).
Okba, N. M. A. et al. Severe acute respiratory syndrome coronavirus 2- specific antibody responses in coronavirus disease patients. Emerg. Infect Dis. 26, 1478–1488 (2020).
Amanat, F. et al. A serologica1 assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 26, 1033–1103 (2020).
De Assis, R. R. et al. Analysis of SARS-CoV-2 antibodies in COVID-19 convalescent blood using a coronavirus antigen microarray. Nat. Commun. 12, 1–9 (2021).
Grzelak, L. et al. A comparison of four serological assays for detecting anti–SARS-CoV-2 antibodies in human serum samples from different populations. Sci. Transl. Med. 12, 559 (2020).
Horndler, L. et al. Flow cytometry multiplexed method for the detection of neutralizing human antibodies to the native SARS-CoV-2 spike protein. EMBO Mol. Med. 13, 3 (2021).
Escribano, P. et al. Detection of SARS-CoV-2 antibodies is insufficient for the diagnosis of active or cured COVID-19. Sci Rep. 10, 1–7 (2020).
Klingler, J. et al. Role of IgM and IgA antibodies in the neutralization of SARS-CoV-2. J Infect Dis. 223(6), 957–970 (2020).
Ripperger, T. J. et al. Orthogonal SARS-CoV-2 serological assay enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity 53, 925–933 (2020).
Isho, B. et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci. Immunol. 5, 52 (2020).
Holmgren, J. & Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 11, S45-53 (2005).
Du, L. et al. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 7, 226–236 (2009).
Acknowledgements
We thank Vitro S.A. (Sevilla, Spain) for their collaboration with the antibodies supply and the license of the analysis software.
Author information
Authors and Affiliations
Contributions
Cell lines production: L.H. and B.A. Methodology, software and investigation: P.P.R., F.M., H.S. Analysis and data curation: F.T. Writing and editing: P.P.R., F.T., M.U.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If mle 5aterial is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piñero, P., Marco De La Calle, F., Horndler, L. et al. Flow cytometry detection of sustained humoral immune response (IgG + IgA) against native spike glycoprotein in asymptomatic/mild SARS-CoV-2 infection. Sci Rep 11, 10716 (2021). https://doi.org/10.1038/s41598-021-90054-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90054-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.