Abstract
Introduction Virus particles in respiratory droplets and aerosols generated during medical/dental procedures are a potential source of SARS-CoV-2 cross infection. In the dental setting, oral decontamination could be an important adjunct to personal protective equipment and is recommended by a number of national COVID-19 guidance documents for dental settings.
Aim To assess the in vitrovirucidal activity of an oral povidone iodine (PVP-I) product against SARS-CoV-2.
Material and methods BETADINE gargle and mouthwash (1% PVP-I) was tested against SARS-CoV-2 virus under both clean and dirty conditions using a suspension assay based on EN14476 methodology. Virucidal activity of the product, undiluted and at 1:2 dilution, was tested at contact times of 15, 30 and 60 seconds. Viral titres were calculated using the Spearman-Kärber method and reported as median tissue culture infectious dose (TCID50/ml).
Results The undiluted product achieved >5 log10 reduction in viral titres compared to the control at 15, 30 and 60 seconds under both clean and dirty conditions. At a twofold dilution (0.5% PVP-I), the test product demonstrated >4 log10 kill at 15 seconds and >5 log10 kill at 30 and 60 seconds in both clean and dirty conditions.
Conclusion PVP-I gargle and mouthwash product, undiluted and at 1:2 dilution, demonstrated potent and rapid virucidal activity (≥4 log10 reduction of viral titre) in 15 seconds against SARS-CoV-2 in vitro. The PVP-I gargle and mouthwash product is widely available and could be readily integrated into infection control measures during dental treatment including pre-procedural oral decontamination.
Key points
-
Close contact and potential for aerosol generation increase risk of SARS-CoV-2 exposure during medical and dental procedures.
-
Pre-procedural mouth rinses are recommended as an additional measure to reduce cross-infection risk in dental settings.
-
PVP-I (1%) gargle and mouthwash showed 99.99% kill rate of SARS-CoV-2 in vitro within 15 seconds of contact in clean and dirty conditions.
-
The use of PVP-I-containing pre-procedural mouth rinse to reduce oral viral load could be recommended in addition to other protective measures.
Similar content being viewed by others
Introduction
Human-to-human transmission of the coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurs primarily through respiratory droplets from coughs or sneezes and/or physical contact in the community.1 In healthcare settings, besides infection through close contact and touch transfer of SARS-CoV-2 from contaminated surfaces to the nasal or oral mucosa, recent studies suggest the potential for airborne transmission of the virus through aerosols formed during medical and dental procedures.2,3,4,5 SARS-CoV-2 is known to enter and infect human cells by binding to the angiotensin-converting enzyme 2 (ACE2) receptor,6 which is abundantly expressed in the epithelia of the oral and respiratory tract; the saliva of infected individuals can contain viral loads of 107/ml or more.7,8,9 Dental procedures can be considered especially high-risk as they involve close proximity to the patient's mouth and nasopharynx, as well as exposure to saliva or even blood. Moreover, dental procedures commonly generate droplet splatter and aerosols, further increasing the risk of environmental contamination and cross infection.10
COVID-19 infection, prevention and control (IPC) measures for medical and dental care facilities11 include deferring interventions for persons with suspected or confirmed SARS-CoV-2 infection, or limiting such interventions to emergency care. However, with the potential for virus shedding known to occur during the early stages in pre-symptomatic or asymptomatic infected persons (who do not have or are yet to present any symptoms)12,13 and a variable incubation period ranging from 1-14 days,10 additional measures are needed to reduce the risk of cross infection in dental care facilities where patients with possible or confirmed COVID-19 disease may be treated.
A number of professional guidelines mention pre-procedural mouth rinses as one of such additional measures.11,14 Challacombe et al.15 have also highlighted the potential of povidone iodine (PVP-I) mouthwash and nasal spray in reducing the risk of cross infection of SARS-CoV-2 in the dental setting.
In addition to the pre-procedural use of PVP-I mouthwash and nasal spray for patients before treatment, Challacombe et al.15 also proposed that dental professionals self-administer this protocol every 2-3 hours as an adjunct to the recommended personal protective equipment (PPE).
PVP-I, a broad-spectrum antiseptic with an established safety profile,16,17 has demonstrated virucidal activity against a range of non-enveloped and enveloped viruses,17,18 including the SARS-CoV19 and MERS-CoV20 pathogenic coronaviruses - and, more recently, SARS-CoV-2.21,22 In its WHO R&D blueprint COVID-19: experimental treatments,23 the World Health Organisation lists PVP-I mouthwash alongside other drug and non-drug treatments and strategies that warrant coordinated research efforts to assess their potential usefulness against COVID-19.
Using a standard quantitative time-kill assay, we evaluated the in vitro activity of a gargle/mouthwash containing 1% PVP-I against SARS-CoV-2 at contact times of 15, 30 and 60 seconds, to confirm the product's virucidal activity and support its use in the dental setting.
Materials and methods
The product BETADINE Gargle & Mouthwash was tested for virucidal activity at two concentrations - undiluted (PVP-I 1% w/v) and at a 50% dilution (PVP-I 0.5% w/v) - for virucidal activity against SARS-CoV-2 in both clean (0.3 g/l bovine serum albumin [BSA]) and dirty (3.0 g/l BSA + 3 ml/l human erythrocytes) conditions. The assays were performed in the Biosafety Level (BSL)-3 Laboratory at the Tropical Infectious Diseases Research and Education Centre (TIDREC), University of Malaya, Malaysia. This article does not contain any studies with human participants or animals performed by any of the authors.
Virus culture
The SARS-CoV-2 (SARS-COV-2/MY/UM/6-3; TIDREC) virus stock was prepared by infecting confluent monolayers of Vero E6 cells (American Type Culture Collection) with the virus. The Vero E6 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) and maintained at 37 °C in a 5% CO2 atmosphere. Virus-containing supernatant was harvested by centrifugation when cytopathic effects (CPEs) were observed and stored at -80 °C. Virus titres were determined by microtitration.
PVP-I cytotoxicity assay
Cytotoxicity assays were performed to determine the lowest concentration at which the PVP-I product was non-cytotoxic to the host cells. Dilutions of the PVP-I product were added to a confluent monolayer of Vero E6 cells cultured in a 96-well plate. The plate was then incubated for 72 hours before measuring the cell viability using the MTS assay and calculating the concentration of PVP-I at which no cytotoxic effects on host cells were observed. Calculations were performed using GraphPad Prism (GraphPad Software, 2365 Northside Dr, Suite 560, San Diego, CA 92108, USA). The cytotoxicity of the product to the host cells was taken into account when performing the time-kill assay.
PVP-I virus time-kill assay
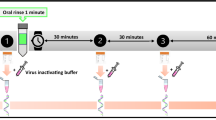
The virus time-kill assay was performed according to EN14476 standard methods for evaluating disinfectant activity.17 The PVP-I product was tested undiluted (1% w/v PVP-I) and at 50% dilution (0.5% PVP-I) against SARS-CoV-2.24 Tests were performed under clean and dirty conditions with different contact times of 15, 30 and 60 seconds to evaluate the disinfectant efficacy. The test assay comprised of 100 μl of interfering substance, 100 μl of virus suspension and 800 μl of PVP-I product (1% and 0.5% PVP-I). The virus control for this test was distilled water in place of the test product for both clean and dirty conditions.
After the specified contact times (15, 30 and 60 seconds), virucidal activity of the test product was immediately neutralised by adding DMEM with 2% FBS and then performing tenfold serial dilutions in ice-cold media (DMEM + 2% FBS). The neutralisation step ensured that there were no after effects of the test product beyond the specified contact time.17 The serial dilutions were incubated with Vero E6 cells for 72 hours until CPEs developed. The plates were then fixed with paraformaldehyde and stained with crystal violet to determine the virus titres. The virus titres for control and test conditions were calculated using the Spearman-Kärber method,25,26 and expressed as median (50%) tissue culture infectious dose (TCID50/ml). Virucidal activity was calculated as the difference in reduction in virus titre with respect to the control (Δ log10 TCID50/ml). As per European Chemicals Agency (ECHA) guidelines,24 a reduction in virus titre of ≥4 log10 (corresponding to a kill rate of ≥99.99%) is considered evidence of effective disinfectant activity.
Results
Cytotoxicity
The PVP-I (1%) gargle/mouthwash product demonstrated no cytotoxicity at a concentration of 0.63 mg/ml of PVP-I (that is, 1:16 dilution of the product) or lower. This was taken into consideration when performing the time-kill assay.
Virucidal activity of PVP-I gargle/mouthwash
Table 1 shows the results for the time-kill assays. The undiluted test product (1% PVP-I) achieved >5 log10 reduction in viral titres at 15, 30 and 60 seconds under both clean and dirty conditions. At a 1:2 dilution (0.5% PVP-I), the test product demonstrated a >4 log10 kill at 15 seconds, and >5 log10 kill at 30 and 60 seconds under both clean and dirty conditions. We previously reported these findings in abbreviated form.27
In summary, the PVP-I product was tested at 15, 30 and 60 seconds and demonstrated ≥4 log10 reduction of SARS-CoV-2 titres (corresponding to a ≥99.99% kill rate) for all contact times, under both clean and dirty conditions. This meets the ECHA standards for disinfectant efficacy, indicating its potent and rapid virucidal activity.24
Discussion
The well-known broad-spectrum antimicrobial and virucidal activity of PVP-I (and recently reported virucidal activity against SARS-CoV-2 in clean conditions)21,22 provides some support for its use for respiratory infection control in the dental setting.28 This study further showed that the PVP-I gargle/mouthwash can reduce the SARS-CoV-2 viral load by ³99.99% in vitro within 15 seconds of contact in simulated 'clean' and 'dirty' conditions (where an interfering biological substance, erythrocytes, was included to simulate organic soiling). In an earlier study, 7% PVP-I gargle/mouthwash was found to be effective against related and deadly coronaviruses - SARS-CoV and MERS-CoV - and was able to rapidly inactivate these viruses within contact times of 15 seconds under both clean and dirty conditions.17 Likewise, in the present study, there was no discernible difference in the PVP-I virus kill rates under clean and dirty conditions, suggesting that the virucidal activity of PVP-I is unlikely to be appreciably reduced in the presence of interfering substances such as biological tissue.
Due to the potential for cross infection between dental healthcare professionals and/or patients undergoing dental procedures during the COVID-19 pandemic, all reasonable measures should be considered to reduce risk of transmission in the clinic setting.10,15 Possible transmission routes include direct contact and exposure to saliva or other biological materials, and indirect contact with contaminated instruments and/or surfaces, as well as inhalation of virus-containing droplets or aerosols generated during procedures.29 Currently recommended measures for healthcare facilities include universal source control, additional PPE use, hand and respiratory hygiene, and the establishment of work practices that minimise risk.11 Although guidelines recommend limiting the use of procedures with a high risk of aerosol production, such as ultrasonic scaling, it may be impossible to entirely eliminate aerosol generation in dental procedures.30
In view of this, we suggest taking additional preventive respiratory hygiene measures, such as the use of antimicrobial nasal sprays or gargling to reduce the possibility of transmission of SARS-CoV-2 through droplets or aerosols, even though close proximity is unavoidable during dental treatment.10 In particular, it has been suggested that the use of pre-procedural mouth rinses and gargles containing ingredients with demonstrated virucidal activity against SARS-CoV-2 could help reduce viral load in the oral cavity and thus the potential for infection during dental treatment.31 Studies are ongoing, but it remains to be seen whether these practices translate into a clinical benefit.32,33 Nevertheless, the Australian Dental Association and the US Centres for Disease Control and Prevention have recommended the use of a pre-procedural mouthwash in their COVID-19-related guidelines.11,14 Additionally, a comprehensive list of COVID-19 preventive measures against the COVID-19 disease for use by dentists have also included a suggestion for the use of 0.2% PVP-I for pre-procedural mouth rinse to reduce the viral load in saliva.34 PVP-I oral products are already widely available and can thus be readily integrated into existing infection control protocols in the dental practice during this COVID-19 pandemic.35,36,37
Some suggested potential adverse effects of oral PVP-I include oral/dental staining and irritation. It has been shown that PVP-I causes less staining of the teeth than chlorhexidine gluconate38 and that, even with prolonged use of PVP-I, this does not cause oral mucosa irritation or damage.17,39 However, as with oral rinses containing other antiseptic agents, individual patients' tolerance or taste preferences may potentially limit the use of oral products containing PVP-I.
Conclusion
Dental practice poses a potential risk of COVID-19 cross infection among patients and dental health professionals. In our study, we present direct evidence of potent virucidal activity of PVP-I gargle/mouthwash against SARS-CoV-2 in just 15 seconds in vitro. Specifically, it can potentially be used as a pre-procedural mouthwash as recommended by national dental guidelines. In today's scenario, PVP-I gargle/mouthwash may be an adjunct to PPE to help reduce the risk of COVID-19 cross infection in dental practices.
References
World Health Organisation. Interim recommendations on obligatory hand hygiene against transmission of COVID-19. 2020. Available online at https://www.who.int/publications/m/item/interim-recommendations-on-obligatory-hand-hygiene-against-transmission-of-covid-19 (accessed November 2020).
Wax R S, Christian M D. Practical recommendations for critical care and anaesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth 2020; 67: 568-576.
Morawska L, Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ Int 2020; 139: 105730.
Wilson N M, Norton A, Young F P, Collins D W. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: a narrative review. Anaesthesia 2020; 75: 1086-1095.
Mick P, Murphy R. Aerosol-generating otolaryngology procedures and the need for enhanced PPE during the COVID-19 pandemic: a literature review. J Otolaryngol Head Neck Surg 2020; 49: 29.
Hoffmann M, Kleine-Weber H, Schroeder S et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; DOI: 10.1016/j.cell.2020.02.052.
To K K-W, Tsang O T-Y, Yip C C-Y et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis 2020; 71: 841-843.
To K K, Tsang O T-Y, Leung W-S et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020; 20: 565-574.
Yoon J G, Yoon J, Song J Y et al. Clinical Significance of a High SARS-CoV-2 Viral Load in the Saliva. J Korean Med Sci 2020; DOI: 10.3346/jkms.2020.35.e195.
Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 2020; 12: 9.
Centres for Disease Control and Prevention. Interim Infection Prevention and Control Guidance for Dental Settings During the Coronavirus Disease 2019 (COVID-19) Pandemic. 2020. Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html (accessed November 2020).
Gandhi M, Yokoe D S, Havlir D V. Asymptomatic Transmission, the Achilles' Heel of Current Strategies to Control Covid-19. N Engl J Med 2020; 382: 2158-2160.
Bai Y, Yao L, Wei T et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020; 323: 1406-1407.
Australian Dental Association. Managing COVID-19 Guidelines. 2020. Available at https://www.ada.org.au/Covid-19-Portal/Cards/Dental-Profesionals/Infection-Control/Transmission-Based-Precautions (accessed November 2020).
Challacombe S J, Kirk-Bayley J, Sunkaraneni V S, Combes J. Povidone iodine. Br Dent J 2020; 228: 656-657.
Vogt P M, Hauser J, Mueller S, Bosse B, Hopp M. Efficacy of Conventional and Liposomal Povidone-Iodine in Infected Mesh Skin Grafts: An Exploratory Study. Infect Dis Ther 2017; 6: 545-555.
Eggers M, Koburger-Janssen T, Eickmann M, Zorn J. In Vitro Bactericidal and Virucidal Efficacy of Povidone-Iodine Gargle/Mouthwash Against Respiratory and Oral Tract Pathogens. Infect Dis Ther 2018; 7: 249-259.
Kawana R, Kitamura T, Nakagomi O et al. Inactivation of human viruses by povidone-iodine in comparison with other antiseptics. Dermatology 1997; 195 Suppl 2: 29-35.
Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology 2006; 212 Suppl 1: 119-123.
Eggers M, Eickmann M, Zorn J. Rapid and Effective Virucidal Activity of Povidone-Iodine Products Against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA). Infect Dis Ther 2015; 4: 491-501.
Anderson D E, Sivalingam V, Kang A E Z et al. Povidone-iodine demonstrates rapid in-vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect Dis Ther 2020; 9: 669-675.
Bidra A S, Pelletier J S, Westover J B, Frank S, Brown S M, Tessema B. Rapid In-Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Using Povidone-Iodine Oral Antiseptic Rinse. J Prosthodont 2020; 29: 529-533.
World Health Organisation. WHO R&D Blueprint COVID 19: Experimental Treatments. 2020. Available at https://www.who.int/docs/default-source/coronaviruse/covid-classification-of-treatment-types-rev.pdf (accessed November 2020).
European Committee for Standardization. Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of virucidal activity in the medical area. Test method and requirements (Phase 2/Step 1). 2015. Available at https://infostore.saiglobal.com/preview/98702935197.pdf (accessed November 2020).
Spearman C. The method of 'right and wrong cases' ('constant stimuli') without Gauss's formulae. Br J Psychol 1908; 2: 227-242.
Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Archiv Exper Pathol Pharmakol 1931; 162: 480-483.
Hassandarvish P, Tiong V, Sazaly A B et al. Povidone iodine gargle and mouthwash. Br Dent J 2020; 228: 900.
Kanagalingam J, Feliciano R, Hah J H, Labib H, Le T A, Lin J-C. Practical use of povidone-iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections. Int J Clin Pract 2015; 69: 1247-1256.
Fallahi H R, Keyhan S O, Zandian D, Kim S G, Cheshmi B. Being a front-line dentist during the Covid-19 pandemic: a literature review. Maxillofac Plast Reconstr Surg 2020; 42: 12.
Harrel S K, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc 2004; 135: 429-437.
Gui D, Pepe G, Magalini S. Just one more hygiene practice in COVID-19. Eur Rev Med Pharmacol Sci 2020; 24: 3438-3439.
Burton M J, Clarkson J E, Goulao B et al. Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection. Cochrane Database Syst Rev 2020; DOI: 10.1002/14651858.CD013626.pub2.
Burton M J, Clarkson J E, Goulao B et al. Antimicrobial mouthwashes (gargling) and nasal sprays to protect healthcare workers when undertaking aerosol-generating procedures (AGPs) on patients without suspected or confirmed COVID-19 infection. Cochrane Database Syst Rev 2020; DOI: 10.1002/14651858.CD013628.pub2.
Ather A, Patel B, Ruparel N B, Diogenes A, Hargreaves K M. Coronavirus Disease 19 (COVID-19): Implications for Clinical Dental Care. J Endod 2020; 46: 584-595.
O'Donnell V B, Thomas D, Stanton R et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function 2020; DOI: 10.1093/function/zqaa002.
Mady L J, Kubik M W, Baddour K, Snyderman C H, Rowan N R. Consideration of povidone-iodine as a public health intervention for COVID-19: Utilization as "Personal Protective Equipment" for frontline providers exposed in high-risk head and neck and skull base oncology care. Oral Oncol 2020; 105: 104724.
Kirk-Bayley J, Challacombe S, Sunkaraneni S, Combes J. The Use of Povidone Iodine Nasal Spray and Mouthwash During the Current COVID-19 Pandemic May Protect Healthcare Workers and Reduce Cross Infection. 2020. Available online at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3563092 (accessed November 2020).
Fine P D. A clinical trial to compare the effect of two antiseptic mouthwashes on gingival inflammation. J Hosp Infect 1985; 6 Suppl A: 189-193.
Eggers M, Koburger-Janssen T, Ward L S, Newby C, Müller S. Bactericidal and Virucidal Activity of Povidone-Iodine and Chlorhexidine Gluconate Cleansers in an In Vivo Hand Hygiene Clinical Simulation Study. Infect Dis Ther 2018; 7: 235-247.
Acknowledgements
This study and editorial support for the publication of this manuscript was funded by Mundipharma Singapore Holding Pte Limited. The results of this study were reported in abbreviated form in a letter to the editor published in the British Dental Journal: P. Hassandarvish, V. Tiong, A. B. Sazaly et al., Povidone iodine gargle and mouthwash, British Dental Journal, 2020, volume 228, page 900.
Author information
Authors and Affiliations
Contributions
PH and VT are both joint first authors. All the authors contributed to the design and analysis of the experiments, and the development of the manuscript. The experiments were performed by PH and VT under the guidance of SA, who was the principal investigator for the study.
Corresponding author
Rights and permissions
About this article
Cite this article
Hassandarvish, P., Tiong, V., Mohamed, N. et al. In vitro virucidal activity of povidone iodine gargle and mouthwash against SARS-CoV-2: implications for dental practice. Br Dent J (2020). https://doi.org/10.1038/s41415-020-2402-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41415-020-2402-0
This article is cited by
-
Stability, homogeneity and measurement uncertainty estimation of PVP-I solutions for the application on oro and nasopharynx against SARS-CoV-2
Scientific Reports (2024)
-
Effectiveness of mouthwashes on reducing SARS-CoV-2 viral load in oral cavity: a systematic review and meta-analysis
BMC Oral Health (2023)
-
In vitro inactivation of SARS-CoV-2 using a povidone-iodine oral rinse
BMC Oral Health (2022)
-
Endodontics and COVID-19 - where are we now?
BDJ In Practice (2022)
-
Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: an open-label randomized clinical trial
Indian Journal of Otolaryngology and Head & Neck Surgery (2022)