- 1Medical Statistics and Analysis Center, Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing, China
- 2Department of Biostatistics, School of Public Health, Southeast University, Nanjing, China
- 3Department of Laboratory Medicine and Biomedicine Statistics, Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing, China
High vaccination coverage is essential to prevent and control the spread of the COVID-19 epidemic. Currently, the real-world acceptance of COVID-19 vaccines among adolescents aged 12–17 years in China has not been reported. We aimed to assess the acceptance rate of COVID-19 vaccination among adolescents in eastern China and to identify factors associated with the intention to get vaccinated against COVID-19. We conduct a cross-sectional questionnaire survey among adolescents from three provinces in the eastern part of China from 16 August to 28 October 2021. The questionnaires were distributed to 2,100 students, and 2,048 students completed the questionnaires. The results showed that 98.4% (2,016/2,048) of adolescents had received at least one dose of the COVID-19 vaccine and 1.6% (32/2,048) declined the vaccination. The participants from rural districts, or whose parents were vaccinated, were more likely to accept the vaccine. The main reason for declining vaccination was worry about vaccine safety (25%). The main adverse event after the vaccination was pain at the injection site. In conclusion, the vaccine coverage rate reached 98.4% among the adolescents in this study, which met the criteria for herd immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The high vaccination rate is beneficial to the prevention and control of the COVID-19 pandemic.
Introduction
First occurred at the end of 2019, the COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused a pandemic by March 2020 (1). The disease has spread worldwide, instigating a pandemic causing more than 533 million confirmed cases and leading to more than 6.3 million deaths worldwide based on the WHO weekly epidemiological update as of June 16, 2022 (2). The pandemic of COVID-19 has become a major threat to global public health, resulting in a global economic downturn and social panic (3). In addition, the pandemic has disrupted some normal medical activities such as pregnancy and childbirth (4–6). In response to the serious global impacts of COVID-19, the vaccine has become one of the most effective strategies for ending the pandemic (7, 8). There is evidence that the vaccine can not only provide direct immunity to the vaccinated individuals but also reduce infections among the unvaccinated individuals by building herd immunity from large-scale coverage of the population with immunity (9).
China has developed various COVID-19 vaccines, mainly composed of inactivated SARS-CoV-2 (10). The efficacy and safety of the vaccines have been investigated in phase 3 clinical trials (11). The vaccines have been widely used in adults in China since December 2020, and the inactivated COVID-19 vaccines have also been approved for WHO emergency use listing (12). Recently, studies showed that COVID-19 vaccination alleviated the disease severity caused by wild-type as well as variants of SARS-CoV-2 (13, 14). At the initial phase of vaccination against COVID-19, the vaccines were exclusively applied to adults (≥ 18 years old) peoples, since the disease causes severe health impacts in adults with relatively high mortality, yet it is relatively mild in adolescents and children (15). However, with the recent global COVID-19 pandemic, especially after the delta variant had become the main transmitted strain, an increasing number of infection events reminded people to be vigilant about the spread of COVID-19 among children and adolescents (15, 16). Since July 2021, the vaccination for children and adolescents has been implemented in China, and the vaccines administered for adolescents are composed of inactivated SARS-CoV-2 (Sinovac Coronavac or Sinopharm BBIBP-CorV, China).
Adolescents aged 12–17 years are of school age, and the coverage of vaccination is essential to prevent and control the spread of SARS-CoV-2 in schools. The success of herd immunity will depend on the vaccination rate. However, numerous factors could affect the vaccination rate for children and adolescents during pandemics, such as parents' opinions, school policies, consideration of vaccine safety, and others (17, 18). Hence, it is crucial to know the true vaccination rate among adolescents and to explore the associated factors with the acceptability of the COVID-19 vaccine.
The objective of this study was to assess the actual acceptance rate of the COVID-19 vaccine among adolescents in three provinces of eastern China and to identify factors associated with the intention to get vaccinated against COVID-19 in children and adolescents. Understanding the actual vaccination rate and the factors associated with the vaccination intention among adolescents may support government and public health officials to find better ways to persuade people to get vaccinated and finally reach herd immunity to SARS-CoV-2.
Materials and methods
Subjects and procedures
We performed a cross-sectional survey, from 16 August to 28 October 2021, on the real-world acceptance of COVID-19 vaccination among adolescents in the eastern region of China. Since high school students in China are usually aged 12–17 years, we distributed the questionnaires among the students in high schools. Based on the economic situation, the six provinces in the eastern part of China can be generally divided into high (Jiangsu and Zhejiang), middle (Shandong and Fujian), and low (Anhui and Jiangxi) levels of economic development. We selected one province from each level, namely Jiangsu, Fujian, and Anhui. In each province, we selected one school from urban areas and one school from rural areas. Students were selected using a cluster sampling method, and once their schools were included in the study, all students were given questionnaires. The students who participated in this survey were informed at the beginning of the questionnaires, as the statement “If you are willing to participate in this survey, this means that you like to give informed consent” is presented at the top of the questionnaire form (see Supplementary File 1). The parents/legal guardians received notices about the survey before it started. The protocol was approved by the Ethics Committee of the Nanjing Drum Tower Hospital (2021-462-01).
Survey design and pre-testing
The questionnaire was formulated based on the previous reports (17–24) and the opinion of experts in infectious diseases. Before the actual survey, we conducted a small-scale pre-survey on the target population and modified the survey questions and layout format after the feedback from the participants.
Measures
The questionnaire covered: (1) demographic characteristics, (2) attitudes toward vaccine, (3) acceptance of COVID-19 vaccine, and (4) adverse events after vaccination. The survey objective was to understand adolescents' opinions on the COVID-19 vaccine and the actual vaccination rate. There was one question designed to calculate the acceptance rate of the COVID-19 vaccine: We asked the respondents to answer the question “Have you accepted the COVID-19 vaccine?” followed by an open-ended question “Why?” or “Why not?,” with a free text box. An additional text file shows the questionnaire in more detail (see Supplementary File).
Data analysis
Basic descriptive statistics and frequencies were used to describe all variables. Univariate analysis was conducted to determine factors associated with vaccination acceptance: Mann-Whitney test for comparing non-normal continuous variables, independent t-test for comparing normally distributed continuous variables, and Chi-square test or Fisher's exact test for categorical variables. Multivariate logistic regression analysis was also used to estimate the adjusted odds ratio (OR) of vaccination, using all the variables that showed significance (p < 0.05) in the univariate analysis. All analyses were conducted with R version 4.04. p ≤ 0.05 was considered statistically significant.
Results
Characteristics of participants
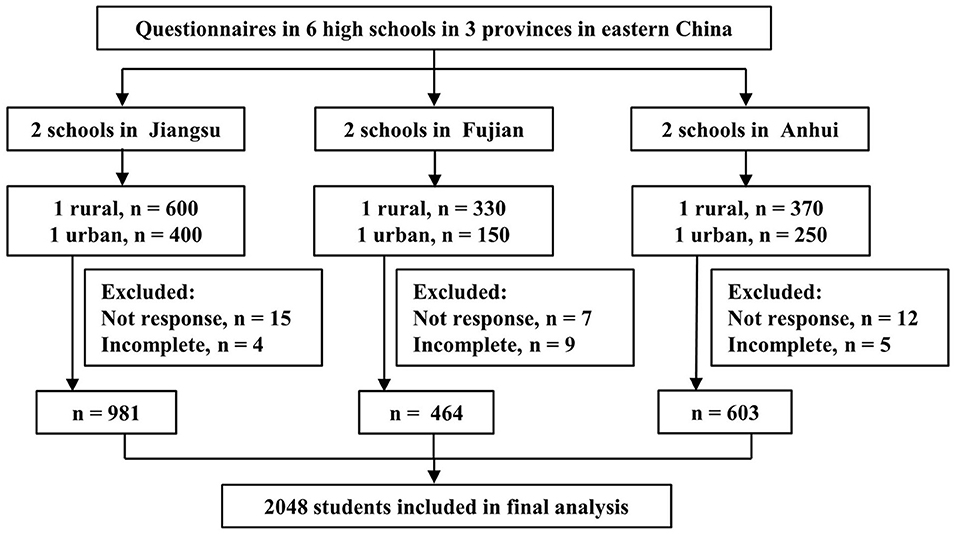
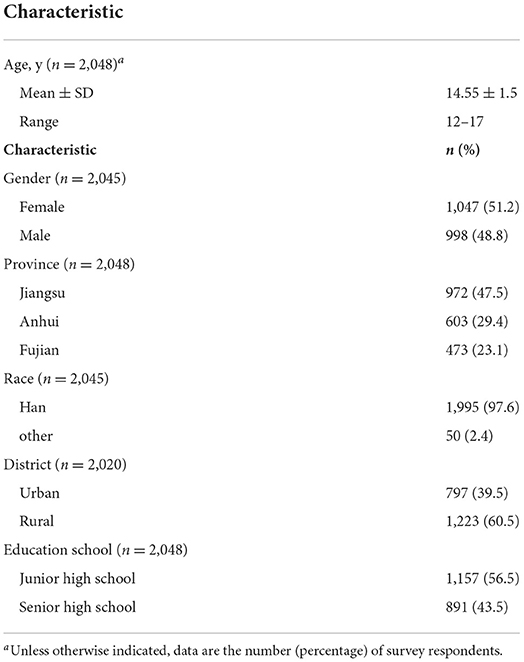
The questionnaires were distributed to 2,100 target students, and a total of 2,048 (97.5%) responded. Figure 1 shows the flow chart of study participants. All results are analyzed based on the 2,048 participants who completed the survey questionnaire. The mean age was 14.5 years (range 12–17 years), more than half (53.4%) were aged 15–17 years, and 48.8% of them were men. Most participants (60.5%) were from rural areas, and 43.5% of participants were in senior high school. The demographic information of participants is presented in Table 1.
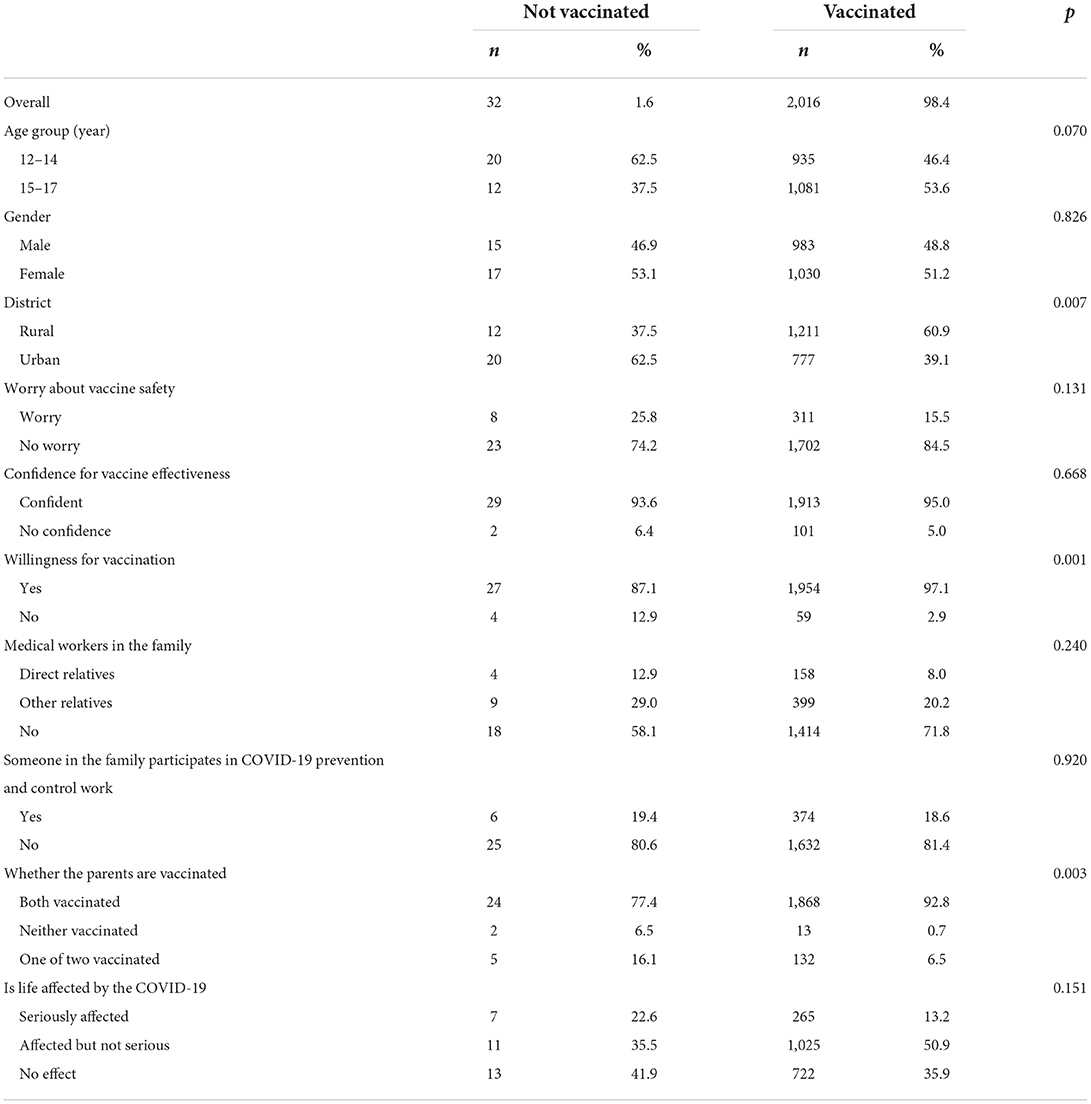
Overall, 2,016 (98.4%) participants accepted the COVID-19 vaccine, and 32 (1.6%) participants did not. Of the 2,016 vaccinated participants, 1,918 (95.1%) completed the second dose, and 98 (4.9%) did not receive the second dose because the time intervals were <2 weeks. The results of the univariate analysis between the vaccinated and unvaccinated groups were summarized in Table 2. The results showed that the acceptance of COVID-19 was associated with the district, willingness for vaccination, and whether the parents were vaccinated. Table 3 shows the results of multivariable binary logistic regression analysis on the factors associated with vaccine acceptance. The results indicated that the participants from rural districts or parents who were vaccinated were more likely to accept the vaccine.
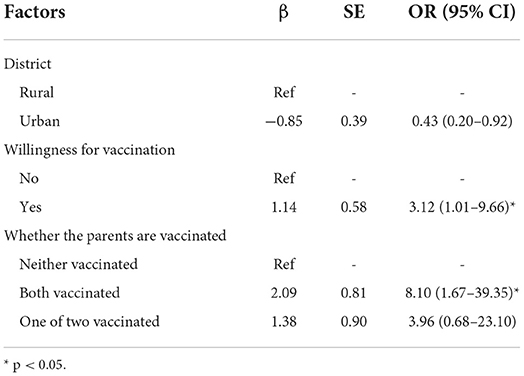
Table 3. Factors associated with COVID-19 vaccine acceptance expressed with odds ratio, in multivariate binary logistic regression.
Reasons for acceptance or decline of the COVID-19 vaccination
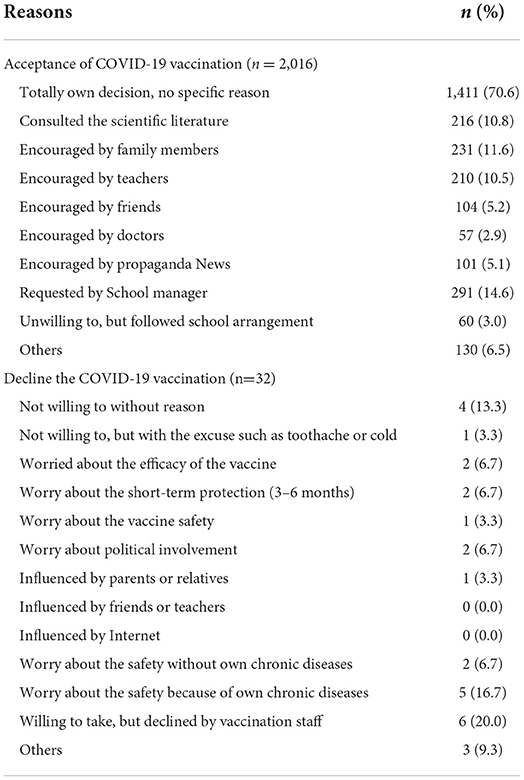
The detailed reasons for acceptance or decline of COVID-19 vaccination are presented in Table 4. The results showed that 70.6% of the vaccinated participants took the vaccine based on their own decision without a specific reason. The two other main reasons for acceptance of the COVID-19 vaccination were requested by the school manager (14.6%) and encouraged by family members (11.6%). On the other hand, worry about vaccine safety accounted for 25% (8/32) of those who declined the vaccination.
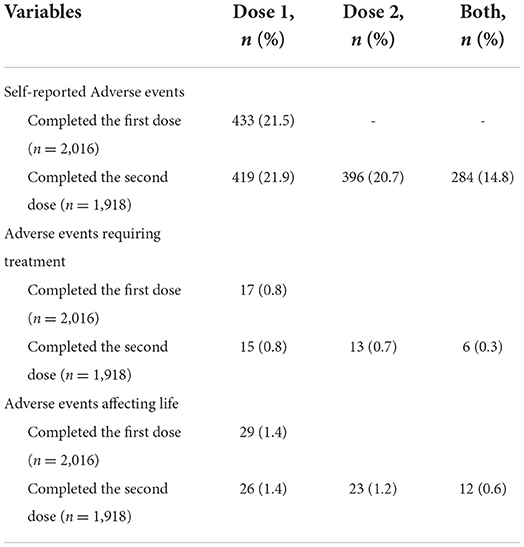
Self-reported adverse events in vaccinated participants
The self-reported adverse events in vaccinated participants are shown in Table 5. Of the 2016 vaccinated participants who completed the first vaccine dose, 433 (21.5%) reported one or more adverse events, with 0.8% (17/2,016) adverse events requiring treatment and 1.4% (29/2,016) adverse events affecting the study and life. Of the 1,918 vaccinated participants who accepted the second vaccine dose, 396 (20.6%) had adverse events. Table 6 presents the 10 common adverse events in the vaccinated participants, and the most common adverse event after either the first or second dose was pain at the injection site.
Discussion
In this cross-sectional study among adolescents between 12 and 17 years in three provinces of the eastern part of China, the majority (98.5%) of adolescents had received at least one dose of the COVID-19 vaccine, and 93.4% of adolescents took the second dose. The vaccination rate in teenagers is higher than that (70.1–86.2%) in adults in China (25–27), and that (42.4%) in adolescents in the USA (28). Independent factors associated with high uptake included the rural district, willingness for vaccination, and parents who were vaccinated.
The results showed that the vaccination rate in students is very high (98.5%), indicating that the school requirement of vaccination before school enrolling helps to increase the vaccination rate. Adolescents aged 12–17 years are in junior or senior high school age, and learning is the most important part of their life. However, their study life was once severely disrupted due to the epidemic of COVID-19. Higher levels of anxiety were reported in adolescents who had concerns about the quality of their education because of long-term online learning (17). Hence, adolescents were more likely to follow the school regulations to accept the COVID-19 vaccination.
Our study reflected that the adolescents whose parents got vaccinated were more likely to accept the vaccine. This demonstrated that parents' attitudes and behaviors have a great influence on adolescents' willingness to receive the vaccine against COVID-19 (29), which highlights the need for publicizing parents about COVID-19 vaccination for increasing the willingness of adolescents in the future. The results showed that the adolescents living in rural areas had a higher vaccination rate, compared with those living in urban areas. This could be the reason that the participants in cities were concerned more about the vaccine safety and effectiveness because they were exposed to different information about the COVID-19 vaccination from various sources, which gave them more uncertainty about the vaccination.
For increasing the vaccination rate, two of the six schools in our survey directly set up vaccination stations for COVID-19 in the school. This method has many advantages for the COVID-19 vaccination campaign. On the one hand, centralized vaccination on campus facilitates students and increases their enthusiasm for vaccination. Students who are initially afraid of vaccination may dispel their concerns when they are encouraged by classmates and teachers on-site, which improves the vaccination rates. On the other hand, the concentrated medical observation after vaccination is conducive to discovering the adverse events of the vaccine in time and can make a better assessment of the safety of the vaccine in adolescents. Previous studies also indicated that vaccine administration on-site at schools is an effective method to improve adolescent vaccination rates (28).
The main reason for vaccine hesitancy among unvaccinated participants included the concern about vaccine safety and effectiveness. Our results are in agreement with the findings in the vaccination intention surveys, in which the most critical reason for the decline of the COVID-19 vaccination is the concern for the safety and effectiveness of the vaccine (30–34). This highlights the need for the government to take measures to publicize the effectiveness and safety of vaccines. In this study, the self-reported adverse events occurred in 21.5% of vaccinated participants after the first dose and 20.6% after the second dose, lower than that reported in adults (35–37). Moreover, the vast majority of subjects with adverse events just had mild adverse events that did not affect their life and did not require treatment. Thus, this study provided evidence that inactivated COVID-19 vaccines are highly safe in adolescents.
There are some limitations in this study. First, we only investigated students and did not survey the individuals of the same age who did not go to school; thus, it remains unclear if our findings are generalizable to general adolescents. However, the school enrollment rates of junior and senior high schools in the three provinces are all above 90% (38). Second, all the adverse events were self-reported and not real-time monitored. The survey subjects were all minors and had a low level of awareness of the adverse events in the questionnaire, which might be subject to reporting bias. Hence, the name of the proprietary adverse event was explained in a popular way in the questionnaire to facilitate the understanding of the respondents. Third, since this study only evaluated the vaccination rates during the first 2 months of the program, the vaccination coverage might have increased with the popularization of the COVID-19 vaccination. Thus, long-term follow-up surveys are required to estimate the change in actual acceptance of the COVID-19 vaccine in adolescents. Fourth, the acceptance of vaccination in this study might be influenced by the enforced regulation in the schools and this could be different in different schools. Lastly, our study only selected three provinces in the eastern part of China, and only two schools were selected in each province, which might have some selection bias.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Nanjing Drum Tower Hospital. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceptualization: TL, RQ, BX, and Y-HZ. Methodology and resources: BX and TL. Validation: BX, BC, and TL. Formal analysis: RQ. Investigation: BX, TL, RQ, YL, and WZ. Data curation: TL and RQ. Writing—original draft preparation: TL. Writing—review and editing, project administration, and supervision: BX and Y-HZ. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Jiangsu Innovative and Entrepreneurial Talent Programme (Grant Number: JSSCBS20211510).
Acknowledgments
The authors would like to thank Yikun Chen from Nanjing Foreign Language School Xianlin Campus, Lin Lu from Nanjing Zhonghua Junior High School, Xiangqing Bao from Fujian Minqing First Junior High School, and Mingchuang Liao from Fujian Datian Meishan Junior High School for their supports in the investigation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.919190/full#supplementary-material
Supplementary File 1. A questionnaire on COVID-19 vaccination among adolescents aged 12 to 17 in three provinces: Anhui, Jiangsu, and Fujian, China, 2021.
References
1. Thanh Le T, Andreadakis Z, Kumar A, Gómez RR, Tollefsen S, Saville M, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. (2020) 19:305–6. doi: 10.1038/d41573-020-00073-5
2. COVID-19 Weekly Epidemiological Update Edition 96. (2022). Available online at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-−15-june-2022 (accessed June 16, 2022).
3. Alagoz O, Sethi AK, Patterson BW, Churpek M, Safdar N. Effect of timing of and adherence to social distancing measures on COVID-19 burden in the United States: a simulation modeling approach. Ann Intern Med. (2021) 174:50. doi: 10.7326/M20-4096
4. Zhao Y, Zou L, Dong MH, Liu XX, Liu YL, Zhu JW, et al. Challenges for obstetricians and the countermeasures of COVID-19 epidemic. Matern Fetal Med. (2020) 2:68–71. doi: 10.1097/FM9.0000000000000046
5. Nieto-Calvache AJ, Padilla I, Tabares-Blanco MF, López-Girón MC, Vergara Galliadi LM. Fear is the path to the dark side: unsafe delivery, one of the consequences of fear of the SARS-CoV-2 pandemic, a case report. Matern Fetal Med. (2021) 3:292–4. doi: 10.1097/FM9.0000000000000112
6. Fahriani M, Anwar S, Yufika A, Bakhtiar B, Wardani E, Winardi W, et al. Disruption of childhood vaccination during the COVID-19 pandemic in Indonesia. Narra J. (2021) 1:e7. doi: 10.52225/narraj.v1i1.7
7. Noushad M, Nassani MZ, Alsalhani AB, Koppolu P, Niazi FH, Samran A, et al. COVID-19 vaccine intention among healthcare workers in Saudi Arabia: a cross-sectional survey. Vaccines. (2021) 9:835. doi: 10.3390/vaccines9080835
8. Goldman RD, Yan TD, Seiler M, Parra Cotanda C, Brown JC, Klein EJ, et al. Caregiver willingness to vaccinate their children against COVID-19: cross sectional survey. Vaccine. (2020) 38:7668–73. doi: 10.1016/j.vaccine.2020.09.084
9. Fine P, Eames K, Heymann DL. “Herd immunity”: a rough guide. Clin Infect Dis. (2011) 52:911–6. doi: 10.1093/cid/cir007
10. Baraniuk C. What do we know about China's covid-19 vaccines? Brit Med J. (2021) 373:n912. doi: 10.1136/bmj.n912
11. Tanriover MD, Doganay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. (2021) 398:213–22. doi: 10.1016/S0140-6736(21)01429-X
12. Anonymous. China's COVID-19 vaccine approved for WHO emergency use listing. J Biosaf Biosecur. (2021) 3:56. doi: 10.1016/j.jobb.2021.06.001
13. Li J, Jiang N, Zeng QL, Zhang Y, He X, Chu Y, et al. The epidemiological, clinical features and outcomes of imported Chinese COVID-19 patients following inactivated vaccines injection. Infect Drug Resist. (2022) 15:2115–25. doi: 10.2147/IDR.S356460
14. Zeng QL, Lv YJ, Yang J, Jiang ZY, Huang S, Li WZ, et al. Clinical characteristics of omicron SARS-CoV-2 variant infection after Non-mRNA-based vaccination in China. Front Microbiol. (2022). doi: 10.3389/fmicb.2022.901826 [Epub ahead of print].
15. CDC COVID-19 Response Team. Coronavirus Disease 2019 in Children-United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:422–6. doi: 10.15585/mmwr.mm6914e4
16. Moeller KE, Meeks M, Reynoldson J, Douglass M. Implementation and outcomes of COVID-19 vaccinations at a child and adolescent psychiatric hospital. J Am Acad Child Adolesc Psychiatry. (2021) 60:1332–4. doi: 10.1016/j.jaac.2021.08.018
17. Saddik B, Hussein A, Albanna A, Elbarazi I, Al-Shujairi A, Temsah MH, et al. The psychological impact of the COVID-19 pandemic on adults and children in the United Arab Emirates: a nationwide cross-sectional study. BMC Psychiatry. (2021) 21:224. doi: 10.1186/s12888-021-03213-2
18. Scherer AM, Gedlinske AM, Parker AM, Gidengil CA, Askelson NM, Petersen CA, et al. Acceptability of adolescent COVID-19 vaccination among adolescents and parents of adolescents-United States, April 15-23, 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:997–1003. doi: 10.15585/mmwr.mm7028e1
19. Park H, Yun KW, Kim KR, Song SH, Ahn B, Kim DR, et al. Epidemiology and clinical features of myocarditis/pericarditis before the introduction of mRNA COVID-19 vaccine in Korean children: a multicenter study. J Korean Med Sci. (2021) 36:e232. doi: 10.3346/jkms.2021.36.e232
20. Lin Y, Hu Z, Zhao Q, Alias H, Danaee M, Wong LP. Understanding COVID-19 vaccine demand and hesitancy: a nationwide online survey in China. PLoS Negl Trop Dis. (2020) 14:e8961. doi: 10.1371/journal.pntd.0008961
21. Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. (2021) 27:225–8. doi: 10.1038/s41591-020-1124-9
22. Dror AA, Eisenbach N, Taiber S, Morozov NG, Mizrachi M, Zigron A, et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. (2020) 35:775–9. doi: 10.1007/s10654-020-00671-y
23. Solís Arce JS, Warren SS, Meriggi NF, Scacco A, McMurry N, Voors M, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. (2021) 27:1385–94. doi: 10.1101/2021.03.11.21253419
24. Omer SB, Benjamin RM, Brewer NT, Buttenheim AM, Callaghan T, Caplan A, et al. Promoting COVID-19 vaccine acceptance: recommendations from the lancet commission on vaccine refusal, acceptance, and demand in the USA. Lancet. (2021) 398:2186–92. doi: 10.1016/S0140-6736(21)02507-1
25. Xu B, Gao X, Zhang X, Hu Y, Yang H, Zhou YH. Real-world acceptance of COVID-19 vaccines among healthcare workers in perinatal medicine in China. Vaccines. (2021) 9:704. doi: 10.3390/vaccines9070704
26. Zheng Y, Shen P, Xu B, Chen Y, Luo Y, Dai Y, et al. COVID-19 vaccination coverage among healthcare workers in obstetrics and gynecology during the first three months of vaccination campaign: a cross-sectional study in Jiangsu province, China. Hum Vaccin Immunother. (2021) 17:4946–53. doi: 10.1080/21645515.2021.1997297
27. Huang W, Shao X, Wagner AL, Chen Y, Guan B, Boulton ML, et al. COVID-19 vaccine coverage, concerns, and preferences among Chinese ICU clinicians: a nationwide online survey. Expert Rev Vaccines. (2021) 20:1361–7. doi: 10.1080/14760584.2021.1971523
28. Murthy BP, Zell E, Saelee R, Murthy N, Meng L, Meador S, et al. COVID-19 vaccination coverage among adolescents aged 12-17 Years-United States, december 14, 2020-July 31, 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1206–13. doi: 10.15585/mmwr.mm7035e1
29. Bell S, Clarke R, Mounier-Jack S, Walker JL, Paterson P. Parents' and guardians' views on the acceptability of a future COVID-19 vaccine: a multi-methods study in England. Vaccine. (2020) 38:7789–98. doi: 10.1016/j.vaccine.2020.10.027
30. Gönüllü E, Soysal A, Atici S, Engin M, Yeşilbaş O, Kasap T, et al. Pediatricians' COVID-19 experiences and views on the willingness to receive COVID-19 vaccines: a cross-sectional survey in Turkey. Hum Vaccin Immunother. (2021) 17:2389–96. doi: 10.1080/21645515.2021.1896319
31. Li XH, Chen L, Pan QN, Liu J, Zhang X, Yi JJ, et al. Vaccination status, acceptance, and knowledge toward a COVID-19 vaccine among healthcare workers: a cross-sectional survey in China. Hum Vaccin Immunother. (2021) 17:4065–73. doi: 10.1080/21645515.2021.1957415
32. Chen M, Li Y, Chen J, Wen Z, Feng F, Zou H, et al. An online survey of the attitude and willingness of Chinese adults to receive COVID-19 vaccination. Hum Vaccin Immunother. (2021) 17:2279–88. doi: 10.1080/21645515.2020.1853449
33. Fan CW, Chen IH, Ko NY, Yen CF, Lin CY, Griffiths MD, et al. Extended theory of planned behavior in explaining the intention to COVID-19 vaccination uptake among mainland Chinese university students: an online survey study. Hum Vaccin Immunother. (2021) 17:3413–20. doi: 10.1080/21645515.2021.1933687
34. Rosiello DF, Anwar S, Yufika A, Adam RY, Ismaeil MIH, Ismail AY, et al. Acceptance of COVID-19 vaccination at different hypothetical efficacy and safety levels in ten countries in Asia, Africa, and South America. Narra J. (2021) 1. doi: 10.52225/narra.v1i3.55
35. Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. (2021) 326:35–45. doi: 10.1001/jama.2021.8565
36. Omeish H, Najadat A, Al-Azzam S, Tarabin N, Abu Hameed A, Al-Gallab N, et al. Reported COVID-19 vaccines side effects among Jordanian population: a cross sectional study. Hum Vaccin Immunother. (2022) 18:1981086. doi: 10.1080/21645515.2021.1981086
37. Cheng Y, Li T, Zheng Y, Xu B, Bi Y, Hu Y, et al. Self-reported adverse events among Chinese healthcare workers immunized with COVID-19 vaccines composed of inactivated SARS-CoV-2. Hum Vaccin Immunother. (2022) 22:1–7. doi: 10.1080/21645515.2022.2064134
Keywords: COVID-19 vaccine, cross-sectional, vaccination rate, adolescent, China
Citation: Li T, Qi R, Chen B, Luo Y, Zhang W, Zhou Y-H and Xu B (2022) COVID-19 vaccination coverage among adolescents aged 12–17 years in three provinces of eastern China: A cross-sectional survey, 2021. Front. Public Health 10:919190. doi: 10.3389/fpubh.2022.919190
Received: 13 April 2022; Accepted: 27 June 2022;
Published: 22 July 2022.
Edited by:
Kin On Kwok, The Chinese University of Hong Kong, ChinaReviewed by:
Harapan Harapan, Syiah Kuala University, IndonesiaTao-Hsin Tung, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, China
Qing-Lei Zeng, First Affiliated Hospital of Zhengzhou University, China
Gang Qin, Affiliated Hospital of Nantong University, China
Copyright © 2022 Li, Qi, Chen, Luo, Zhang, Zhou and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Hua Zhou, zgr03summer@126.com; Biyun Xu, biyunxu@163.com
†These authors have contributed equally to this work and share first authorship
 Taishun Li1†
Taishun Li1† Bingwei Chen
Bingwei Chen Wenjun Zhang
Wenjun Zhang Yi-Hua Zhou
Yi-Hua Zhou Biyun Xu
Biyun Xu